Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3590
Revised: January 4, 2014
Accepted: February 26, 2014
Published online: April 7, 2014
Processing time: 126 Days and 13.1 Hours
Contrast-enhanced ultrasound (CEUS) using microbubble contrast agents are useful for the diagnosis of the nodules in liver cirrhosis. CEUS can be used as a problem-solving method for indeterminate nodules on computed tomography (CT) or magnetic resonance imaging (MRI) or as an initial diagnostic test for small newly detected liver nodules. CEUS has unique advantages over CT and MRI including no renal excretion of contrast, real-time imaging capability, and purely intravascular contrast. Hepatocellular carcinoma (HCC) is characterized by arterial-phase hypervascularity and later washout (negative enhancement). Benign nodules such as regenerative nodules or dysplastic nodules are usually isoechoic or slightly hypoechoic in the arterial phase and isoechoic in the late phase. However, there are occasional HCC lesions with atypical enhancement including hypovascular HCC and hypervascular HCC without washout. Cholangiocarcinomas are infrequently detected during HCC surveillance and mostly show rim-like or diffuse hypervascularity followed by rapid washout. Hemangiomas are often found at HCC surveillance and are easily diagnosed by CEUS. CEUS can be effectively used in the diagnostic work-up of small nodules detected at HCC surveillance. CEUS is also useful to differentiate malignant and benign venous thrombosis and to guide and monitor the local ablation therapy for HCC.
Core tip: Contrast-enhanced ultrasound is a relatively new imaging technique that can be effectively used in the diagnostic algorithms for liver nodules detected in hepatocellular carcinoma surveillance. There are several unique advantages of this technique that make this imaging technique very useful.
- Citation: Kim TK, Jang HJ. Contrast-enhanced ultrasound in the diagnosis of nodules in liver cirrhosis. World J Gastroenterol 2014; 20(13): 3590-3596
- URL: https://www.wjgnet.com/1007-9327/full/v20/i13/3590.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i13.3590
Ultrasound (US) is most commonly used for imaging surveillance for hepatocellular carcinoma (HCC) in high-risk patients[1]. Once a focal hepatic nodule is detected during HCC surveillance, a diagnostic imaging test is performed. The assessment of tumor vascularity is most important in the differential diagnosis of the nodules. Doppler US techniques have been used to detect vascularity in liver tumors; however, these techniques lack sensitivity to detect tumor vascularity. Contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) is most commonly used to characterize liver masses. Contrast-enhanced ultrasound (CEUS) using a microbubble contrast agent is a relatively new imaging technique and has been proved to be useful for characterizing liver tumors. CEUS enables a real-time demonstration of continuous hemodynamic changes of liver tumors after injection of the contrast material.
Recent practice guidelines for HCC provided recommendations for the diagnostic algorithm for newly detected nodules at HCC surveillance[1-3]. The application of the imaging test varies depending on the size of the nodules. For very small lesions (< 1 cm in size), follow-up with US scan is usually recommended in 3 mo as further imaging tests may not be reliable for the diagnosis. For lesions same or larger than 1 cm, the performance of multiphasic contrast-enhanced CT, MRI or CEUS is a reasonable next step. There has been a controversy on the use of CEUS because intrahepatic cholangiocarcinoma can be misdiagnosed as HCC and CEUS has been subsequently excluded from the diagnostic tests for HCC in updated AASLD practice guidelines[1]. However, it has been argued that intrahepatic cholangiocarcinoma is relatively rare in liver cirrhosis and CEUS can depict suggestive findings of cholangiocarcinoma[4]. As the imaging diagnosis of small nodules with 1-2 cm in size can be particularly challenging, a multi-modality approach is often needed. Biopsy is performed only when imaging findings are inconclusive.
In this article, we explain the technical aspects of CEUS and its differences from CT and MRI. Then CEUS imaging findings of HCC and other cirrhosis-related nodules and the role of CEUS in diagnosing tumor thrombosis and monitoring local ablation therapy for HCC are reviewed.
CEUS uses intravenous microbubble contrast agents that are small (3-5 μm) enough to pass through the pulmonary circulation. Microbubble contrast agents are approved for radiologic use in more than 50 countries and have excellent safety record with lower rate severe adverse reactions than CT contrast[5]. There are a few different types of microbubble contrast agents that are commercially available. Presently Definity (Lantheus Medical Imaging) and SonuVue (Bracco) are most widely used. These are purely intravascular contrast agents that show strong vascular enhancement after bolus injection and slowly diffuse into the blood over about 5 min. Therefore, the contrast agent can be repeatedly injected with about 5-min intervals as needed. Sonazoid (Daiichi), which is most actively used in Japan, shows similar vascular enhancement and is taken up by Kupffer cells in the late phase.
CEUS requires a contrast specific imaging technique that is now widely available in most commercially available ultrasound systems. Low mechanical index (MI) contrast-specific mode is used to visualize the microbubbles continuously while suppressing signals from tissue. A dual-imaging mode, which enables simultaneous real-time display of gray-scale and contrast-specific mode, is essential for scanning small liver lesions. High MI frames can be used to disrupt microbubbles and the enhancement pattern of refilling the scanning plane can be evaluated. This is called disruption-replenishment technique and is useful to visualize vascular morphology within the tumor of enhancement pattern of rapidly-enhancing lesions, especially when it is used with maximum-intensity projection method[6].
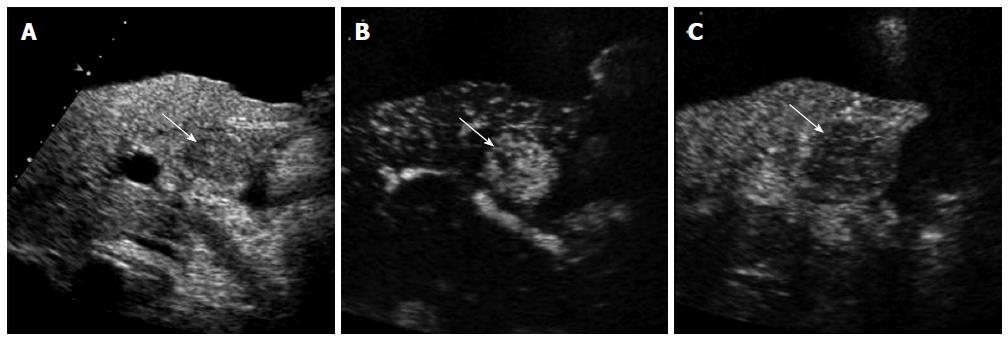
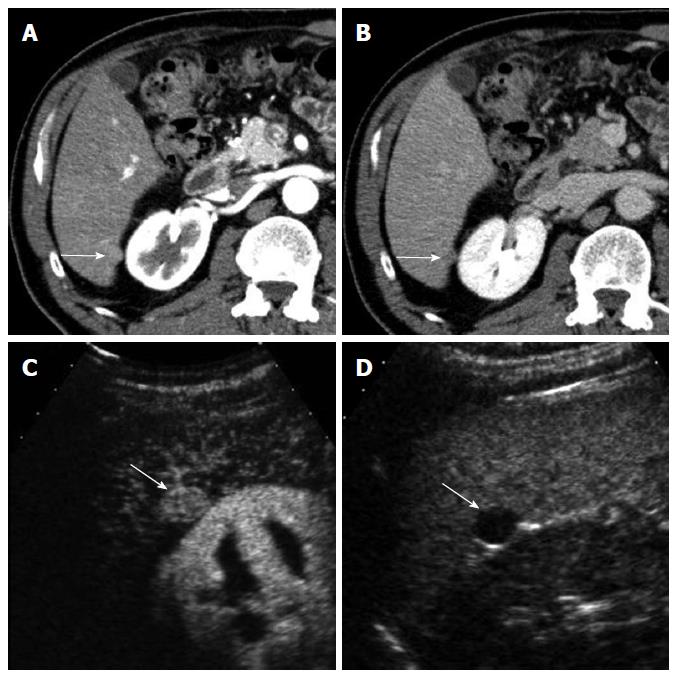
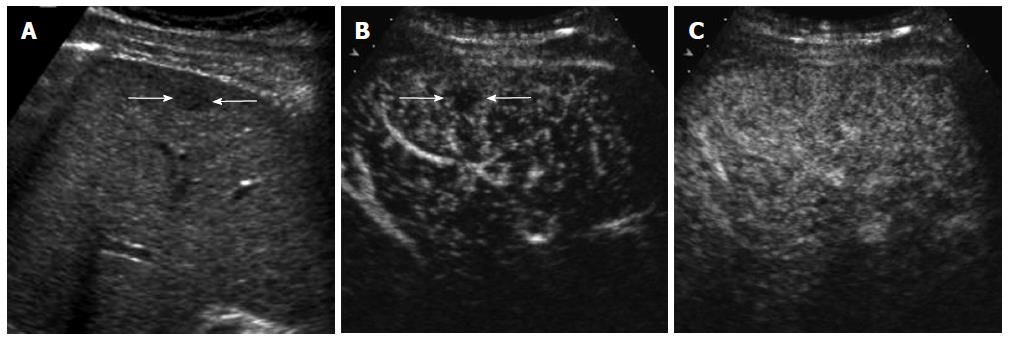
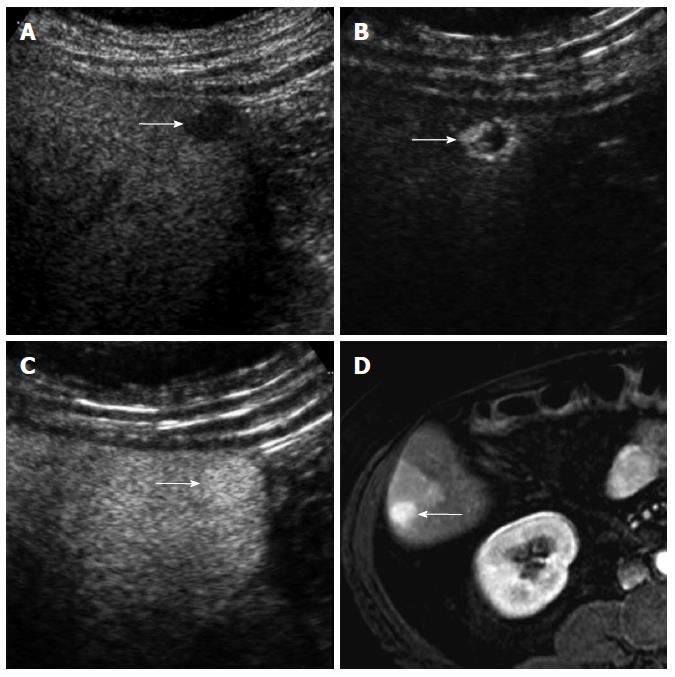
CEUS has several advantages over CT or MRI. Firstly, microbubbles can be safely injected in patients with renal failure as there is no renal excretion of the contrast[5]. CEUS is therefore a useful problem-solving method for characterizing liver masses when CT or MRI is contraindicated due to renal failure (Figure 1). There is no need of blood test for renal function before contrast injection. Secondly, CEUS allows real-time assessment of arterial-phase enhancement, eliminating the issue of appropriate arterial-phase timing. CEUS often detects arterial-phase hypervascularity when CT or MRI fails to show it because of incorrect arterial-phase timing (Figure 2)[7]. Real-time evaluation also enables a detailed assessment of arterial-phase filling pattern and vascular morphology within the liver lesion which are often critical for differential diagnosis of rapidly enhancing hypervascular liver lesions[8]. Thirdly, washout phenomenon (negative enhancement of liver lesion relative to the liver in the late phase) in malignant liver lesions is more consistently seen on CEUS than CT/MRI. This is due to the different properties of the contrast material. Microbubbles in CEUS are purely intravascular and show washout in malignant tumors in the late phase; however, CT or MRI may not show washout in malignant tumors with high vascular permeability and large extracellular interstitial space because the contrast agent can leak into the interstitium[7,9]. Fourth, CEUS is relatively inexpensive and very well tolerated by the patients who are claustrophobic. CEUS can be easily repeated in short intervals if needed without the risk of ionizing radiation. Lastly, CEUS can be performed immediately when a new liver nodule is identified, allowing an immediate characterization of benign liver nodules such as hemangiomas and avoiding additional imaging tests[10].
On the other hand, CEUS is operator-dependent and requires extensive hands-on experience. There are sonographically difficult regions in the liver such as high right subphrenic area. CEUS is not an appropriate staging modality for HCC as it only images some part of the liver with each contrast injection. CT or MRI is always needed for proper tumor staging once HCC diagnosis is made. However, CEUS can be effectively used as a useful problem-solving method utilizing its unique advantages when CT or MRI is indeterminate or contraindicated (Figures 1 and 2)[10].
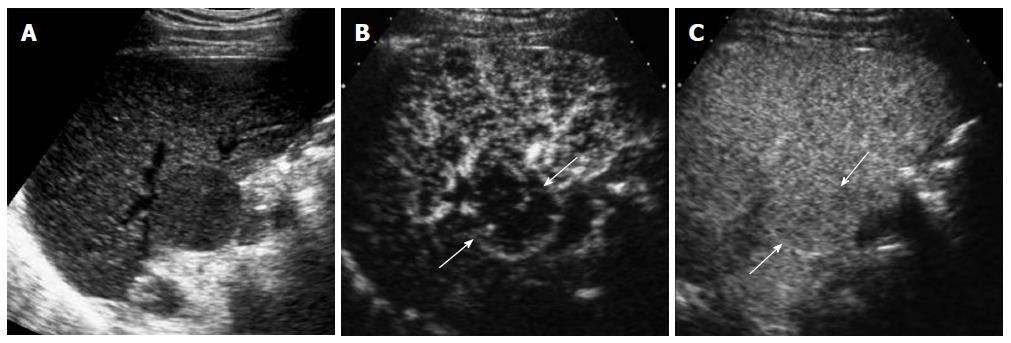
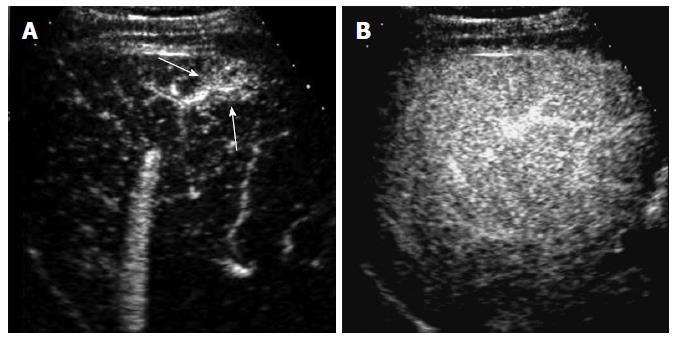
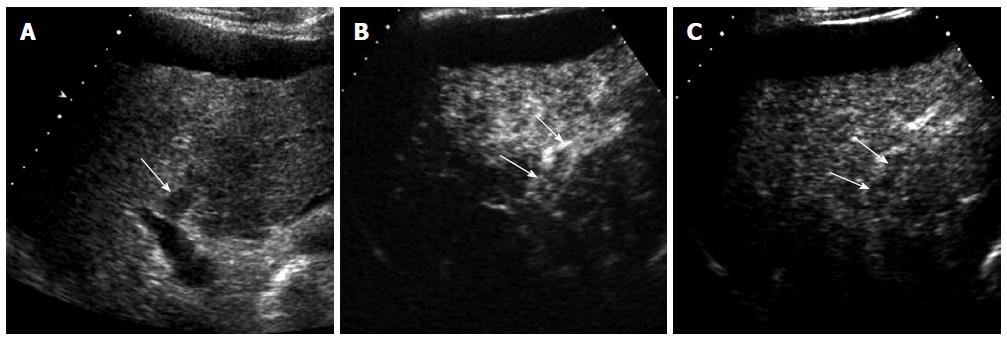
HCC is characterized by arterial-phase hypervascularity and later washout on CEUS (Figures 1 and 2). The arterial-phase enhancement is often diffuse or heterogeneous. Peripheral rim-like enhancement is unusual in HCC and is commonly seen in metastasis or intrahepatic cholangiocarcinoma[11]. Large HCCs often show non-enhancing areas due to necrosis or internal hemorrhage. Maximum intensity projection images can visualize vascular morphology in HCC which is typically very irregular and dysmorphic[6]. Washout in HCC tends to be late and often begins later than 90 s after injection whereas metastases or intrahepatic cholangiocarcinomas consistently show rapid washout (< 60 s)[9]. Most of small cholangiocarcinomas that are infrequently detected during HCC surveillance can be characterized by CEUS by demonstrating rim-like arterial phase enhancement and rapid washout in our experience (Figure 3). Biopsy should be performed when these unusual enhancement patterns for HCC are observed on CEUS.
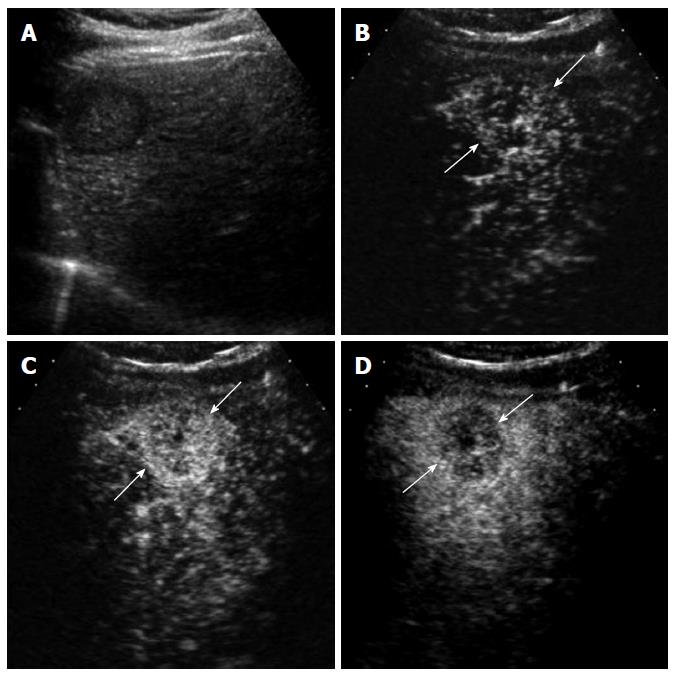
Washout may not be seen in occasional cases of well-differentiated HCC. Washout timing is related to the pathologic differentiation of HCC: well-differentiated HCC tends to show later washout or no washout whereas poorly-differentiated HCC tends to show rapid washout[12]. It is important to understand that most new hypervascular nodules on CEUS detected during HCC surveillance are HCC regardless of washout if the nodules do not show the appearance of hemangioma[13]. However, a biopsy is needed to confirm HCC for hypervascular nodules without washout. CEUS is superior to CT or MRI for detecting hypervascularity of HCC because of real-time evaluation of arterial-phase enhancement[14-16]. Therefore, CEUS can be effectively used for characterizing non-hypervascular, indeterminate lesions on CT and MRI (Figure 2). There is a small subset of hypovascular HCC which is mostly of well-differentiated pathology. These lesions usually show a transient hypovascularity followed by gradual enhancement and the lesions become isoechoic relative to the normal in the portal venous phase and late phase (Figure 4).
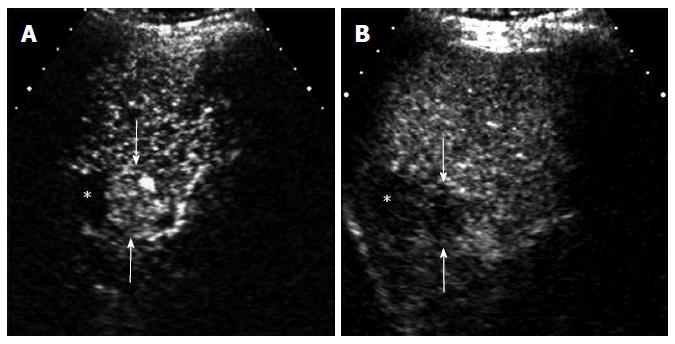
Regenerative nodules or dysplastic nodules (DNs) are usually non-hypervascular. CEUS may show a transient hypoenhancement relative to the liver in the arterial phase, reflecting the step-wise changes of nodule perfusion during the hepatocarcinogenesis (Figure 5). Therefore, there is an overlap of imaging findings on CEUS between DNs and well-differentiated HCCs[17]. The differentiation is difficult not only on imaging but also on histological examination. Recent practice guidelines recommend a biopsy for all new liver nodules ≥ 1 cm that are indeterminate on imaging[18]; however, many of these nodules are borderline lesions, in which the differential diagnosis in small needle biopsy specimens is challenging due to histological heterogeneity within the nodules. In the setting of a competing potentially fatal disease (i.e., cirrhosis), the intensive identification, biopsy, and treatment of early HCCs has yet to be justified. Khalili et al[19], in their study of 93 indeterminate 1-2-cm nodules on dynamic CT, MRI, and CEUS, suggested a strategy of close imaging follow-up for most indeterminate 1-2-cm nodules, with selective application of biopsy for nodules with arterial hyperenhancement or in the presence of a synchronous typical HCC.
Hemangiomas are frequently detected during HCC surveillance. Gray-scale ultrasound findings, such as diffuse hyperechogenicity or hyperechoic peripheral rim, can help suggest the diagnosis of hemangioma. In fatty liver, hemangiomas are often hypoechoic relative to the echogenic liver parenchyma. In our recent study[20], 43/184 (23%) of newly detected nodules at HCC surveillance were hemangiomas. Immediate performance of CEUS can achieve a confident diagnosis by demonstrating the characteristic enhancement pattern that includes peripheral nodular enhancement, gradual central fill-in, and sustained enhancement and avoid further imaging tests such as CT or MRI. CEUS is also useful to demonstrate the characteristic enhancement pattern in fast filling hemangioma which often shows homogeneous enhancement in the arterial phase of CT or MRI (Figure 6)[21].
Nontumorous arterioportal shunting is a common mimicker of malignancy in cirrhotic liver. It is typically wedge-shaped, peripherally located, and homogeneous hypervascular in the arterial phase. The lesion becomes isointense to the liver in the venous phase and never shows washout (Figure 7). Focal fat deposits or focal fat sparing areas with nodular appearance can mimic the appearance of focal liver masses. There lesions are mostly isoechoic to the liver in the arterial and late phases on CEUS. The presence of normal portal veins within the lesion can confirm the benign lesion[21].
US is most commonly used for imaging guidance for radiofrequency ablation (RFA) of HCC because of its real-time imaging capability and no ionizing radiation. It is occasionally difficult to visualize the HCC lesion on US when the lesion is isoechoic to the liver or the underlying liver is severely cirrhotic with markedly heterogeneous echotexture. CEUS can help localize the lesion before RFA procedure by demonstrating a focal hypervascular lesion. Marginal recurrence adjacent to the ablation zone may not be easily located on gray-scale US because of the pre-existing abnormality. CEUS can easily identify the exact location of the recurrent HCC that can provide excellent guidance for RFA needle positioning (Figure 8)[22].
For post-RFA monitoring for HCC, CT or MRI is primarily used in our institution. CEUS is often performed when there is an indeterminate lesion on CT or MRI and often clarifies the abnormality with high confidence.
Malignant thrombosis in the portal veins or hepatic veins is a critical determinant of HCC staging as it directly influences treatment strategy. CEUS is highly accurate to differentiate malignant and benign venous thrombosis. CEUS demonstrates enhancement within the malignant thrombosis in the arterial phase and subsequent washout similar to the parenchyma HCC masses (Figure 9)[23]. The presence of formed vessels within the thrombus is another useful feature to diagnose malignant thrombosis. However, care should be taken in assessing formed vessels within the thrombus on CEUS as recanalized portion within chronic benign thrombosis may mimic the appearance of formed vessels in malignant thrombus. Occlusive thrombosis and expansion of the venous lumen are more frequently seen in malignant than benign thrombosis, but these are not specific features of malignancy.
CEUS is an excellent imaging technique with several unique advantages over CT or MRI, including safe usage in renal failure patients, better detection of arterial-phase hypervascularity due to real-time imaging capability, consistent demonstration of washout in malignant lesions, and excellent patient’s compliance. Therefore CEUS can be effectively used in the diagnostic algorithms of new liver nodules detected during HCC surveillance and pre- or post-RFA evaluation for HCC.
| 1. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6628] [Article Influence: 441.9] [Reference Citation Analysis (1)] |
| 2. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 844] [Article Influence: 52.8] [Reference Citation Analysis (2)] |
| 3. | European Association For The Study Of The Liver1; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] |
| 4. | Barreiros AP, Piscaglia F, Dietrich CF. Contrast enhanced ultrasound for the diagnosis of hepatocellular carcinoma (HCC): comments on AASLD guidelines. J Hepatol. 2012;57:930-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology. 2010;257:24-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 6. | Wilson SR, Jang HJ, Kim TK, Iijima H, Kamiyama N, Burns PN. Real-time temporal maximum-intensity-projection imaging of hepatic lesions with contrast-enhanced sonography. AJR Am J Roentgenol. 2008;190:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Wilson SR, Kim TK, Jang HJ, Burns PN. Enhancement patterns of focal liver masses: discordance between contrast-enhanced sonography and contrast-enhanced CT and MRI. AJR Am J Roentgenol. 2007;189:W7-W12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Kim TK, Jang HJ, Burns PN, Murphy-Lavallee J, Wilson SR. Focal nodular hyperplasia and hepatic adenoma: differentiation with low-mechanical-index contrast-enhanced sonography. AJR Am J Roentgenol. 2008;190:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Bhayana D, Kim TK, Jang HJ, Burns PN, Wilson SR. Hypervascular liver masses on contrast-enhanced ultrasound: the importance of washout. AJR Am J Roentgenol. 2010;194:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Lanka B, Jang HJ, Kim TK, Burns PN, Wilson SR. Impact of contrast-enhanced ultrasonography in a tertiary clinical practice. J Ultrasound Med. 2007;26:1703-1714. [PubMed] |
| 11. | Jang HJ, Kim TK, Wilson SR. Imaging of malignant liver masses: characterization and detection. Ultrasound Q. 2006;22:19-29. [PubMed] |
| 12. | Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Jang HJ, Kim TK, Wilson SR. Small nodules (1-2 cm) in liver cirrhosis: characterization with contrast-enhanced ultrasound. Eur J Radiol. 2009;72:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Takahashi M, Maruyama H, Shimada T, Kamezaki H, Sekimoto T, Kanai F, Yokosuka O. Characterization of hepatic lesions (≤ 30 mm) with liver-specific contrast agents: a comparison between ultrasound and magnetic resonance imaging. Eur J Radiol. 2013;82:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Maruyama H, Takahashi M, Ishibashi H, Yoshikawa M, Yokosuka O. Contrast-enhanced ultrasound for characterisation of hepatic lesions appearing non-hypervascular on CT in chronic liver diseases. Br J Radiol. 2012;85:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Sugimoto K, Moriyasu F, Shiraishi J, Saito K, Taira J, Saguchi T, Imai Y. Assessment of arterial hypervascularity of hepatocellular carcinoma: comparison of contrast-enhanced US and gadoxetate disodium-enhanced MR imaging. Eur Radiol. 2012;22:1205-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Kim TK, Lee KH, Khalili K, Jang HJ. Hepatocellular nodules in liver cirrhosis: contrast-enhanced ultrasound. Abdom Imaging. 2011;36:244-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3258] [Article Influence: 130.3] [Reference Citation Analysis (1)] |
| 19. | Khalili K, Kim TK, Jang HJ, Yazdi LK, Guindi M, Sherman M. Indeterminate 1-2-cm nodules found on hepatocellular carcinoma surveillance: biopsy for all, some, or none? Hepatology. 2011;54:2048-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Kim TK, Lee KH, Jang HJ, Haider MA, Jacks LM, Menezes RJ, Park SH, Yazdi L, Sherman M, Khalili K. Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1-2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology. 2011;259:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Kim TK, Jang HJ, Wilson SR. Benign liver masses: imaging with microbubble contrast agents. Ultrasound Q. 2006;22:31-39. [PubMed] |
| 22. | Kim YJ, Lee MW, Park HS. Small hepatocellular carcinomas: ultrasonography guided percutaneous radiofrequency ablation. Abdom Imaging. 2013;38:98-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Raza SA, Jang HJ, Kim TK. Differentiating malignant from benign thrombosis in hepatocellular carcinoma: contrast-enhanced ultrasound. Abdom Imaging. 2014;39:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
P- Reviewers: Chan KM, Lin ZY, Wang K S- Editor: Wen LL L- Editor: A E- Editor: Wang CH