Published online Mar 28, 2014. doi: 10.3748/wjg.v20.i12.3369
Revised: November 18, 2013
Accepted: January 6, 2014
Published online: March 28, 2014
Processing time: 187 Days and 1.8 Hours
AIM: To understand the clinicopathological and prognostic features of gastric cancer in younger and older patients.
METHODS: Between January 2002 and December 2008, 1667 patients underwent curative gastric surgery. For comparative purposes, the patients were divided into two groups: younger patients who were less than 40 years old (112 patients), and older patients who were 40 years old and older (1555 patients). In both groups, propensity scoring methods were used to select patients with similar disease statuses. A total of 224 matched cases, with 112 patients in each group, were included in the final analysis.
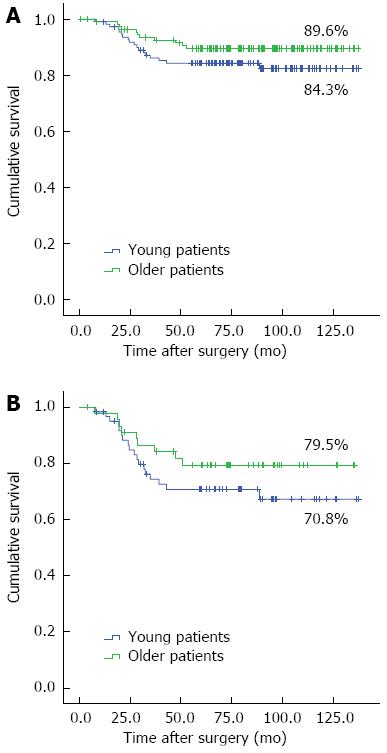
RESULTS: Compared to the older group, the younger group with gastric cancer had a significantly higher percentage of females (P = 0.007), poorly differentiated or signet ring cell carcinoma (P < 0.001), advanced T stage gastric cancer (P = 0.045), and advanced tumor-node-metastasis stage cancer (P = 0.036). The older group with gastric cancer had more comorbidities (P < 0.001). With the exception of the number of lymph node dissection (P < 0.001) and retrieved lymph node (P = 0.010), there were no statistically significant differences between the postoperative outcomes of the two groups. During the follow-up period, there were 19 recurrences in the younger group and 11 recurrences in the older group. The overall five-year survival rates in the younger and older groups were 84.3% and 89.6%, respectively (P = 0.172). There were no significant differences (P = 0.238) in the overall survival of patients with advanced T stage gastric cancer in the two groups, with five-year survival rates of 70.8% in the younger group and 79.5% in the older group. With regard to the age-adjusted survival rate, there was significant difference between the two groups (P = 0.225).
CONCLUSION: In spite of aggressive cancer patterns in the younger group with gastric cancer, the younger group did not have a worse prognosis than the older group in our study.
Core tip: In this study, propensity scoring methods were used to select patients with similar disease statuses. A total of 224 matched cases (112 patients in each group) were included in the analysis. The younger group with gastric cancer had more aggressive patterns than did the older group. The overall five-year survival rates between the younger and older groups were not significantly different. While there were more cases of aggressive cancer patterns in the younger group, early diagnosis and curative resections improved the prognosis and patient survival; the younger group with gastric cancer did not show a worse prognosis than the older group.
- Citation: Kim KH, Kim YM, Kim MC, Jung GJ. Analysis of prognostic factors and outcomes of gastric cancer in younger patients: A case control study using propensity score methods. World J Gastroenterol 2014; 20(12): 3369-3375
- URL: https://www.wjgnet.com/1007-9327/full/v20/i12/3369.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i12.3369
Gastric cancer is one of the most common digestive cancers in the world. In addition, gastric cancer is the fourth most common cancer, and the second leading cause of cancer-related death, with approximately 700000 deaths annually[1]. The prevalence of gastric cancer remains high in some Asian countries, especially in South Korea and Japan. While the incidence of advanced gastric cancer is decreasing in developed countries as a result of recent developments in medical screening, routine screening does not include people younger than 40 years of age, so gastric cancer in younger patients is a disturbing problem. In addition, gastric cancer is difficult to detect in younger patients who are asymptomatic, even in the advanced stages. The proportion of patients with gastric cancer ranges from 6% to 15% in patients younger than 41 years of age[2-4]. There is controversy about whether gastric cancer differs between younger and older patients. Several reports have suggested that younger patients are often diagnosed with advanced-stage disease, and younger patients have been observed to have a markedly worse prognosis than their older counterparts[5-10]. However, another recent report showed the prognosis for younger patients with gastric cancer to be equal or better than that of older patients[11-17].
The proportion of younger patients with gastric cancer is smaller than that of older patients. Therefore, most studies have used small cases series of younger patients and large cases series of older patients for their comparisons[2-15]. In this case-control study, matching was performed with the aim of selecting subsets of case and control groups with similar distributions of observed covariates (operation date and type of gastrectomy), thereby increasing the robustness of this retrospective observational study by reducing the bias that can be introduced by unbalanced groups. We used propensity scoring methods to evaluate whether the prognosis for younger patients with gastric cancer is equal to or poorer than that of older patients.
From a prospectively collected gastric cancer database, we identified 1945 patients who underwent gastric surgery between January 2002 and December 2008. Among them, 1667 patients who had no peritoneal seeding or distant liver metastasis underwent R0 resection. We defined the younger group with gastric cancer as being less than 40 years old, which is similar to the cut-off that has been used in previous reports[3,5-6,13-15,17-19]. We divided the 1667 gastric cancer patients into two groups: younger patients who were less than 40 years old (112 patients), and older patients who were at least 40 years old (1555 patients). To reduce potential confounders in this retrospective observational study, the values of the propensity scores were used to adjust for differences between the two groups with regard to operation date and type of gastrectomy. A total of 224 matched cases, with 112 patients in each group, were included in the final analysis. The data were prospectively retrieved from operative and pathological reports, and follow-up data were obtained from the outpatient clinical database. Clinicopathological characteristics, postoperative outcomes, postoperative morbidities and mortalities, and survival rates were retrospectively compared between the two groups. The following data were obtained for each patient: age, gender, body mass index (BMI), comorbid disease, tumor size, histologic type, tumor location, tumor-node-metastasis (TNM) stage, and postoperative outcomes. Postoperative outcomes included operative time, hospital stay, type of gastrectomy, reconstruction, extent of lymph node dissection, number of retrieved lymph nodes, recurrence, and survival.
In this study, the gastric cancer stage was classified according to the 7th edition of the American Joint Committee on Cancer staging criteria[20]. Standard lymph node dissection (D2) was performed according to the 2010 Japanese gastric cancer treatment guidelines[21].
Adjuvant chemotherapy was performed in patients with pathologically identified advanced gastric cancer who had provided their informed consent. Adjuvant chemotherapy was not performed for patients with stage I gastric cancer, but adjuvant chemotherapy was performed for patients within stage IIA or higher gastric cancer. Follow-up results were obtained from patient hospital records and telephone calls, and recurrence was determined by endoscopy, computed tomography, and positron emission tomography. All patients who received follow-up were monitored postoperatively with routine blood tests, tumor markers (carcinoembryonic antigen and carbohydrate antigen 19-9), chest radiography, endoscopy, and computed tomography. In patients with early gastric cancer, follow-up studies were performed every six months for two years and annually for three years. For patients with advanced gastric cancer, follow-up studies were performed every three months for the first year, every six months for the second year, and annually for following three years.
We classified recurrence patterns into four categories[22]: locoregional, hematogenous, peritoneal, and distant lymph nodes. Locoregional recurrence was defined as the presence of tumors in the adjacent organs, which includes gastric bed, anastomosis, gastric stump, and regional lymph nodes. Hematogenous recurrence included recurrence in the liver, lung, bone, brain, or other distant sites. Peritoneal recurrence was defined as peritoneal seeding or ovarian metastases (Krukenberg’s tumor). Recurrence in distant lymph nodes was defined as extraabdominal lymph nodes.
To reduce bias, a propensity scoring approach was used to match the younger and older patients according to the operation date and type of gastrectomy. Patients less than 40 years of age (case) were matched to patients at least 40 years old (control) by using patient identifiers and operation dates (± 15 d). Matching was performed to select subsets of case and control groups with similar distributions of the observed covariates operation date and type of gastrectomy. The matching increased the robustness of the retrospective observational design by reducing the bias that can be introduced from unbalanced groups. The data were summarized using frequencies and percentages for categorical variables and means and standard deviations for continuous variables. After descriptive analyses were performed, a Fisher’s test was used to compare categorical variables between the groups, and a Mann-Whitney U test was used to compare continuous variables between the groups. P values of less than 0.05 were considered to be statistically significant. Survival curves were calculated using the Kaplan-Meier method. All statistical analyses were conducted using SPSS version 18.0 (SPSS, Chicago, IL).
The proportion of females in the younger group was greater than that in the older group (P = 0.007). The older group had more comorbidities than did the younger group (P < 0.001), and the younger group had more poorly differentiated or signet ring cell gastric carcinomas (P < 0.001). In addition, using the Lauren classification, a diffuse form of cancer was found in 64.3% of patients in the younger group (P < 0.001). Compared to the older group, the younger group had more aggressive T stage gastric cancer (P = 0.045) and more cases of advanced TNM stage cancer (P = 0.036). There were no significant differences in the BMI, tumor size, tumor location, and N stage between the two groups (Table 1).
| Clinicopathological feature | Younger patients (n = 112) | Older patients (n = 112) | P-value |
| Gender | 0.007 | ||
| Male | 49 | 70 | |
| Female | 63 | 42 | |
| BMI (mean ± SD, kg/m2) | 22.4 ± 3.4 | 23.1 ± 2.7 | 0.086 |
| Comorbidity | < 0.001 | ||
| No | 100 | 74 | |
| Yes | 12 | 38 | |
| Size of main lesion (mean ± SD, mm) | 4.1 ± 3.1 | 3.9 ± 3.3 | 0.604 |
| Histologic type | < 0.001 | ||
| Well differentiated | 7 | 29 | |
| Moderate differentiated | 19 | 34 | |
| Poorly differentiated | 70 | 41 | |
| Signet ring cell | 14 | 5 | |
| Other | 2 | 3 | |
| Lauren classification | < 0.001 | ||
| Intestinal | 18 | 58 | |
| Diffuse | 72 | 34 | |
| Mixed | 22 | 20 | |
| Tumor location | 0.154 | ||
| Upper | 15 | 15 | |
| Middle | 40 | 25 | |
| Lower | 55 | 70 | |
| Whole | 2 | 2 | |
| T stage1 | 0.045 | ||
| EGC | 50 | 66 | |
| AGC | 62 | 46 | |
| N stage1 | 0.132 | ||
| N0 | 67 | 72 | |
| N1 | 12 | 16 | |
| N2 | 11 | 14 | |
| N3 | 22 | 10 | |
| Stage1 | 0.036 | ||
| I | 57 | 75 | |
| II | 21 | 11 | |
| III | 34 | 26 |
Table 2 shows the postoperative outcomes of the two groups; there were statistically significant differences in the extent of lymph node dissection (P < 0.001) and the number of retrieved lymph nodes (P = 0.010). There were no significant differences in operative time, hospital stay, operative method, type of gastrectomy, type of anastomosis, or cancer-related organ resection. Complications in the younger group were found in 10 (8.9%) of the 112 patients, and one (0.9%) major complication required endoscopic control of intraluminal bleeding. Nine minor complications in the younger group were treated with conservative management. In the older patients, there were 16 (14.3%) complications, 3 (2.7%) of which were major; one patient required endoscopic control of intraluminal bleeding, and two patients required re-operation to repair duodenal stump leakage. Other complications in older patients were also treated with conservative management. There were no statistically significant differences between the two groups in postoperative complications (P = 0.297).
| Postoperative outcomes | Younger patients (n = 112) | Older patients (n = 112) | P-value |
| Operative time (min, mean ± SD) | 207.3 ± 57.1 | 205.7 ± 56.7 | 0.832 |
| Hospital stay (d, mean ± SD) | 7.9 ± 2.6 | 10.1 ± 13.0 | 0.087 |
| Operative method | 0.268 | ||
| Laparoscopy | 46 | 37 | |
| Open | 66 | 75 | |
| Type of gastrectomy | 1.000 | ||
| Total | 28 | 28 | |
| Subtotal | 84 | 84 | |
| Type of anastomosis | 0.856 | ||
| B-I | 48 | 52 | |
| B-II | 35 | 32 | |
| R-Y | 29 | 28 | |
| Lymph node dissection | < 0.001 | ||
| D2 | 43 | 79 | |
| Over D2 | 69 | 33 | |
| Number of retrieved lymph nodes (mean ± SD) | 41.4 ± 16.4 | 36.3 ± 13.2 | 0.010 |
| Cancer-related combined resection | 0.409 | ||
| No | 107 | 103 | |
| Yes (number of patients) | 5 | 9 | |
| Spleen1 | 4 | 6 | |
| Pancreas1 | 0 | 3 | |
| Liver1 | 0 | 1 | |
| Adrenal gland1 | 0 | 1 | |
| Small bowel1 | 1 | 0 | |
| Ovary1 | 1 | 0 | |
| Median follow-up duration (mo, range) | 79.2 (8.0-137.7) | 80.3 (0.7-137.5) | 0.523 |
| Postoperative complications | 0.297 | ||
| No | 102 | 96 | |
| Yes | 10 | 16 | |
| Wound problem | 3 | 5 | |
| Intra-abdominal bleeding | 1 | 1 | |
| Intra-luminal bleeding | 3 (12) | 4 (12) | |
| Ileus | 1 | 1 | |
| Duodenal stump leakage | 0 | 22 | |
| Acute pancreatitis | 0 | 1 | |
| Pulmonary disease | 0 | 2 | |
| Hepatic disease | 1 | 0 | |
| Dumping syndrome | 1 | 0 | |
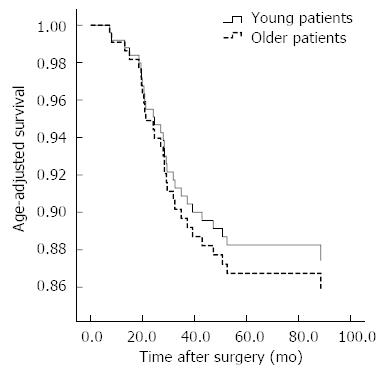
The median follow-up period was 79.2 mo in the younger group and 80.3 mo in the older group. We performed adjuvant chemotherapy for patients within stage II or higher. In the 55 patients in the younger group with stage II or higher cancer, 49 patients received with adjuvant chemotherapy; in the 37 older patients with stage II or higher, 36 patients adjuvant chemotherapy. There was no significant difference in the use of adjuvant chemotherapy between the two groups (P = 0.235). During the median follow-up period, tumor recurrences occurred in 19 cases in the younger group and 11 cases in the older group. The median time to recurrence after surgery was 17.8 mo in the younger group and 18.5 mo in the older group, but the difference between the groups was not statistically significant (P = 0.169). In the younger group, peritoneal recurrence was the most common recurrence pattern. However, in the older group, the most common recurrences were locoregional and hematogenous (Table 3). The overall five-year survival rates in the younger and older groups were 84.3% and 89.6%, respectively. There were no significant differences in the survival rates between the two groups (P = 0.172). In cases of advanced T stage gastric cancer, the overall five-year survival rates were 70.8% in the younger group and 79.5% in the older group (P = 0.238) (Figure 1). There was no significant difference in the age-adjusted survival rate between the two groups (P = 0.225) (Figure 2).
| Postoperative outcomes | Younger patients (n = 112) | Older patients (n = 112) | P-value |
| Median follow-up duration (mo, range) | 79.2 (8.0-137.7) | 80.3 (0.7-137.5) | 0.523 |
| Chemotherapy (stage II or higher) n (%) | 49/55 (89.1) | 36/37 (97.3) | 0.235 |
| Tumor recurrence | 0.169 | ||
| No | 93 | 101 | |
| Yes | 19 | 11 | |
| Time to recurrence after surgery (mo, mean ± SD) | 17.8 ± 9.8 | 18.5 ± 12.6 | 0.862 |
| Recurrence patterns1 | |||
| Locoregional | 6 | 5 | |
| Hematogenous | 6 | 5 | |
| Peritoneal | 10 | 3 | |
| Distant lymph nodes | 2 | 2 |
Gastric cancer is generally considered to be an age-related disease, and more than half of gastric cancer patients are older than 60 years of age. Gastric cancer rates have been consistently decreasing as a result of recent medical screening systems. However, gastric cancer in younger group populations remains a serious problem in some countries, such as South Korea, where routine screening does not occur in people under 40 years of age. Therefore, in the absence of gastrointestinal symptoms, gastric cancer is difficult to diagnoses in young people, even in the advanced stages of the disease. This retrospective matched case-control study was performed to determine the clinicopathological and prognostic features of gastric cancer in younger and older patients.
Studies have generally been limited by a small number of patients, the inclusion of historical data, a lack of comparison with similar control groups, and a limited ability to account for disease survival. Thus, most studies have made comparisons between small cases series of younger patients and large cases series of older patients with gastric cancer[2-15]. These unbalanced comparisons may generate biased and inconsistent results. Therefore, in this study, we matched the younger and older groups with increasing robustness to reduce the bias that can be caused by unbalanced groups.
Many studies have shown interesting clinical differences between younger and older patients with gastric cancer. Most notably, the gender ratio is different between younger and older patients with gastric cancer, which means that gender-associated differences can be amplified in different age group. For example, younger groups are comprised of more female patients than older groups[4,5,11,12,14,15], and in male patients, malignant neoplasms often occur in the stomach, esophagus, liver, colon, or rectum[23]. There is currently no widely accepted explanation for the reversal of the male/female ratio in younger patients with gastric cancer. Several reports have suggested that the predominance of females may be caused by hormonal factors, such as the amount of estrogen and the higher percentage of estrogen receptor-positive cells in younger females[15,24]. However, the relationship between hormones and the prognosis of gastric cancer remains controversial. In our study, there was a significantly higher proportion of females in the younger group compared to the older group (P = 0.007).
Another interesting clinical difference between younger and older patients with gastric cancer is the histologic aggressiveness of the cancer. Several studies have reported similar clinicopathological features in younger patients with gastric cancer compared to older patients[2-6,11-14,23]. While there are some differences among the reports, younger patients with gastric cancer generally have more diffuse gastric cancer than older patients (following the Lauren classification); younger patients also tend to have a more poorly differentiated carcinoma or signet ring cell carcinoma, a more advanced T stage, a greater likelihood to have lymph node metastasis, and a greater proportion of tumors in the body of the stomach. These features include poorly differentiated diffuse adenocarcinomas, which are associated with genetic abnormalities[25-27]. Genetic susceptibility is one major factor that is associated with the development of gastric cancer. Diffuse types of gastric cancer are reported to be common in younger groups that have a genetic predispositions, and these diffuse gastric cancers lead to a poorer prognosis, the intestinal type of gastric cancer, which is associated with a better prognosis than diffuse types, is more common in older patients[28,29]. In younger compared to older patients, gastric cancer is significantly more likely to be located in the body of the stomach[17]. In the present study, poorly differentiated and signet ring cell type cancers were more predominant in the younger group than in the older group (P < 0.001). Following the Lauren classification, the diffuse type was more prevalent in the younger group (P < 0.001). Although there were no significant differences in tumor location, there was a higher frequency of tumors in the middle portion of the stomach in the younger group compared to the older group. Tumor staging is a crucial factor for determining whether and how to perform surgery and tumor stage correlates closely with prognosis. As already noted, the younger group with gastric cancer had more aggressive T and N stages than did the older group. Although there were significant differences in the N stage, the T and TNM stages were more advanced in the younger group compared to the older group (P = 0.045 and P = 0.036, respectively).
The prognosis of young patients with gastric cancer has been debated for years. Compared to older patients, younger patients have more aggressive patterns of gastric cancer, and several reports have shown that younger patients with gastric cancer have worse prognoses than older patients[5-10]. However, another recent report showed the prognosis in the younger group to be equal to or better than that of the older group[11-17]. The good prognosis and improved survival rate that have recently been found in younger patients may be a result of improved surgical techniques and extended lymph node dissections for gastric cancers with aggressive patterns of the disease. In our study, more extensive lymph node dissections (over D2) were performed in the younger group with gastric cancer (P < 0.001), and more lymph nodes were retrieved in the younger group than in the older group. The overall five-year survival rates in the younger and older groups were 84.3% and 89.6%, respectively, but the difference was not statistically significant (P = 0.172). For advanced T stage gastric cancer, the overall five-year survival rates were 70.8% in the younger group and 79.5% in the older group, and the difference was also not statistically significant (P = 0.238). After adjusting for age, there was no significant differences in the survival curve between the two groups (P = 0.225) (Figure 2).
In our study, tumor recurrence occurred in 19 cases in the younger group and 11 cases in the older group. Kong et al[17] reported that peritoneal metastasis occurred more often in younger patients than older patients with gastric cancer. Peritoneal metastasis is predominantly seen in gastric cancer with ascites, which is often poorly differentiated. The link to poorly differentiated gastric cancer may partially explain why peritoneal metastasis is more prevalent in younger patients. While there was no significant difference in tumor recurrence between the two groups (P = 0.169), there were more peritoneal metastases in the younger group than in the older group.
The lower comorbidity and postoperative complication rates are advantages for young patients with gastric cancer. Comorbidity is an important factor that affects postoperative complications[30]. Yoo et al[31] reported that the presence of postoperative complications such as leakage, are negative prognostic factors in patients with advanced gastric cancer. Therefore, in our study, a lower comorbidity and a lack of major postoperative complications such as leakage, may have improved the prognosis in the younger group with gastric cancer, which may have counteracted the aggressive patterns of gastric cancer that were present in the younger group.
A delay in gastric cancer diagnosis may be a factor in the poor prognosis that is observed in younger groups with gastric cancer. If a younger patient has a suspicious lesion or a familial history of gastric cancer, physicians should carefully observe this patient and perform regular endoscopic evaluations. Early diagnosis of younger patients with gastric cancer will improve their surgical prognosis.
In our case-control matched study, the younger group with gastric cancer had more advanced and aggressive patterns, which is consistent with previous studies. While the younger group with gastric cancer tended to have aggressive patterns, early diagnosis and curative resection improved the prognosis of the younger group with gastric cancer.
There is controversial about whether younger patients with gastric cancer have a worse prognosis than older patients. The proportion of younger patients with gastric cancer is smaller than that of older patients. Therefore, most studies have used small case series of younger patients and large cases series of older patients for their comparisons.
In this case-control study, matching was performed to select subsets of case and control groups with similar distributions of the observed covariates operation date and type of gastrectomy, which increased the robustness of the retrospective observational study by reducing the bias that can be introduced by unbalanced groups.
In this matched case-control study, the authors used propensity scoring methods to evaluate whether the prognosis for younger patients with gastric cancer is equal to or poorer than that of older patients.
While there were more cases of aggressive cancer patterns in the younger group with gastric cancer, early diagnosis and curative resection improved the prognosis and patient survival.
This study aimed at analyse the prognostic outcomes of gastric cancer in younger patients compared to older subject using propensity score methods. The manuscript is well written and the results are interesting.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25604] [Article Influence: 1706.9] [Reference Citation Analysis (10)] |
| 2. | Kitamura K, Yamaguchi T, Yamamoto K, Ichikawa D, Taniguchi H, Hagiwara A, Sawai K, Takahashi T. Clinicopathological analysis of gastric cancer in young adults. Hepatogastroenterology. 1996;43:1273-1280. [PubMed] |
| 3. | Theuer CP, de Virgilio C, Keese G, French S, Arnell T, Tolmos J, Klein S, Powers W, Oh T, Stabile BE. Gastric adenocarcinoma in patients 40 years of age or younger. Am J Surg. 1996;172:473-46; discussion 473-46;. [PubMed] |
| 4. | Matley PJ, Dent DM, Madden MV, Price SK. Gastric carcinoma in young adults. Ann Surg. 1988;208:593-596. [PubMed] |
| 5. | Eguchi T, Takahashi Y, Yamagata M, Kasahara M, Fujii M. Gastric cancer in young patients. J Am Coll Surg. 1999;188:22-26. [PubMed] |
| 6. | Smith BR, Stabile BE. Extreme aggressiveness and lethality of gastric adenocarcinoma in the very young. Arch Surg. 2009;144:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Theuer CP, Kurosaki T, Taylor TH, Anton-Culver H. Unique features of gastric carcinoma in the young: a population-based analysis. Cancer. 1998;83:25-33. [PubMed] |
| 8. | Chung HW, Noh SH, Lim JB. Analysis of demographic characteristics in 3242 young age gastric cancer patients in Korea. World J Gastroenterol. 2010;16:256-263. [PubMed] |
| 9. | Lawrence W, Menck HR, Steele GD, Winchester DP. The National Cancer Data Base report on gastric cancer. Cancer. 1995;75:1734-1744. [PubMed] |
| 10. | Nakamura K, Ueyama T, Yao T, Xuan ZX, Ambe K, Adachi Y, Yakeishi Y, Matsukuma A, Enjoji M. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer. 1992;70:1030-1037. [PubMed] |
| 11. | Kim DY, Ryu SY, Kim YJ, Kim SK. Clinicopathological characteristics of gastric carcinoma in young patients. Langenbecks Arch Surg. 2003;388:245-249. [PubMed] |
| 12. | Santoro R, Carboni F, Lepiane P, Ettorre GM, Santoro E. Clinicopathological features and prognosis of gastric cancer in young European adults. Br J Surg. 2007;94:737-742. [PubMed] |
| 13. | Choi JH, Chung HC, Yoo NC, Lee HR, Lee KH, Kim JH, Roh JK, Park CS, Min JS, Lee KS. Gastric cancer in young patients who underwent curative resection. Comparative study with older patients. Am J Clin Oncol. 1996;19:45-48. [PubMed] |
| 14. | Lai JF, Kim S, Li C, Oh SJ, Hyung WJ, Choi WH, Choi SH, Wang LB, Noh SH. Clinicopathologic characteristics and prognosis for young gastric adenocarcinoma patients after curative resection. Ann Surg Oncol. 2008;15:1464-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Isobe T, Hashimoto K, Kizaki J, Miyagi M, Aoyagi K, Koufuji K, Shirouzu K. Characteristics and prognosis of gastric cancer in young patients. Oncol Rep. 2013;30:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Al-Refaie WB, Hu CY, Pisters PW, Chang GJ. Gastric adenocarcinoma in young patients: a population-based appraisal. Ann Surg Oncol. 2011;18:2800-2807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Kong X, Wang JL, Chen HM, Fang JY. Comparison of the clinicopathological characteristics of young and elderly patients with gastric carcinoma: a meta analysis. J Surg Oncol. 2012;106:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Lee JH, Ryu KW, Lee JS, Lee JR, Kim CG, Choi IJ, Park SR, Kook MC, Kim YW, Bae JM. Decisions for extent of gastric surgery in gastric cancer patients: younger patients require more attention than the elderly. J Surg Oncol. 2007;95:485-490. [PubMed] |
| 19. | Park YK, Kim JC, Koh YS, Joo JK, Ryu SY, Kim YJ, Kim SK, Kim DY. Early gastric carcinoma in young patients. Int Surg. 2006;91:316-319. [PubMed] |
| 20. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York: Springer 2010; . |
| 21. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1911] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 22. | Song J, Lee HJ, Cho GS, Han SU, Kim MC, Ryu SW, Kim W, Song KY, Kim HH, Hyung WJ. Recurrence following laparoscopy-assisted gastrectomy for gastric cancer: a multicenter retrospective analysis of 1,417 patients. Ann Surg Oncol. 2010;17:1777-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Kitamura K, Taniguchi H, Yamaguchi T, Takahashi T. Early gastric cancer in young adults. Tohoku J Exp Med. 1996;179:93-100. [PubMed] |
| 24. | Kojima O, Takahashi T, Kawakami S, Uehara Y, Matsui M. Localization of estrogen receptors in gastric cancer using immunohistochemical staining of monoclonal antibody. Cancer. 1991;67:2401-2406. [PubMed] |
| 25. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 26. | Antonioli DA, Goldman H. Changes in the location and type of gastric adenocarcinoma. Cancer. 1982;50:775-781. [PubMed] |
| 27. | Sipponen P, Järvi O, Kekki M, Siurala M. Decreased incidences of intestinal and diffuse types of gastric carcinoma in Finland during a 20-year period. Scand J Gastroenterol. 1987;22:865-871. [PubMed] |
| 28. | Wu MS, Chen CJ, Lin JT. Host-environment interactions: their impact on progression from gastric inflammation to carcinogenesis and on development of new approaches to prevent and treat gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1878-1882. [PubMed] |
| 29. | Oliveira C, Seruca R, Carneiro F. Genetics, pathology, and clinics of familial gastric cancer. Int J Surg Pathol. 2006;14:21-33. [PubMed] |
| 30. | Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, Lee HJ, Cho GS, Han SU, Hyung WJ. Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale korean multicenter study. Ann Surg Oncol. 2008;15:2692-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Yoo HM, Lee HH, Shim JH, Jeon HM, Park CH, Song KY. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer. J Surg Oncol. 2011;104:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
P- Reviewers: Franceschi F, Hiraki M, Nozaki I, Tsuda H S- Editor: Zhai HH L- Editor: A E- Editor: Zhang DN