Published online Mar 28, 2014. doi: 10.3748/wjg.v20.i12.3327
Revised: November 8, 2013
Accepted: November 28, 2013
Published online: March 28, 2014
Processing time: 201 Days and 10 Hours
AIM: To analyze the prevalence of thiopurine-methyltransferase (TPMT) genotypes and their association with drug toxicity in inflammatory bowel disease (IBD) patients from southeastern Brazil.
METHODS: A total of 219 consecutive patients with IBD, of which 146 had Crohn’s disease and 73 had ulcerative colitis, regularly seen at the outpatient unit of the Division of Gastroenterology at the University Hospital Pedro Ernesto of the State University of Rio de Janeiro, a tertiary referral center, were enrolled in this study from February 2009 to January 2011. We analyzed the presence of major TPMT genetic variants (TPMT*2, *3A, *3C) in IBD patients by means of a specific allele and RFLP-PCR. Genomic DNA was isolated from peripheral blood leukocytes by proteinase-K/Sodium Dodecyl Sulfate digestion and phenol-chloroform extraction. TPMT*2 (C238G), TPMT*3A (G460A/A719G), and TPMT*3C (A719G) genotypes were detected by real-time polymerase chain reaction followed by direct sequencing with specific primers. Clinical data were systematically recorded, and correlated with the genotype results.
RESULTS: The distribution of the selected TPMT gene polymorphism TPMT*2 (C238G), TPMT*3A (G460A/A719G), and TPMT*3C (A719G) genotypes was 3.6%, 5.4%, and 7.7% of the patients, respectively. Among the side effects recorded from patients taking azathioprine, 14 patients presented with pancreatitis and/or an elevation of pancreatic enzymes, while 6 patients had liver toxicity, and 2 patients exhibited myelosuppression/neutropenia. TPMT polymorphisms were detected in 37/219 patients (8 heterozygous for *2, 11 heterozygous for *3A, and 18 heterozygous for *3C). No homozygotic polymorphisms were found. Despite the prevalence of the TPMT*3C genotype, no differences among the genotype frequencies were significant. Although no association was detected regarding myelotoxicity or hepatotoxicity, a trend towards the elevation of pancreatic enzymes was observed for TPMT*2 and TPMT*3C genotypes.
CONCLUSION: The prevalence of TPMT genotypes was high among Brazilian patients. Variants genes *2 and *3C may be associated with azathioprine pancreatic toxicity in a IBD southeastern Brazilian population.
Core tip: Although commonly used to treat patients with inflammatory bowel disease, the potentially severe side effects of azathioprine remain a concern. To determine a patient’s predisposition to azathioprine toxicity, the thiopurine-methyl-transferase genotype must be determined prior to azathioprine administration.
- Citation: Carvalho ATP, Esberard BC, Fróes RSB, Rapozo DCM, Grinman AB, Simão TA, Santos JCVC, Carneiro AJV, Ribeiro-Pinto LF, Souza HSP. Thiopurine-methyltransferase variants in inflammatory bowel disease: Prevalence and toxicity in Brazilian patients. World J Gastroenterol 2014; 20(12): 3327-3334
- URL: https://www.wjgnet.com/1007-9327/full/v20/i12/3327.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i12.3327
The purine analogs azathioprine (AZA) and 6-mercaptopurine (6-MP) are the most common immunosuppressant drugs used to treat inflammatory bowel disease (IBD) and have been shown to be effective in inducing and maintaining remission[1-5]. Recent evidence has indicated that azathioprine can control active inflammation and prevent relapse in Crohn’s disease (CD) and ulcerative colitis (UC), as well as reduce steroid dependence and induce and maintain remission in CD. Many of these beneficial effects have been attributed to the ability of AZA to induce T cell apoptosis[6,7]. However, in one third of patients, treatment with AZA is withdrawn due to either toxicity (gastrointestinal intolerance, pancreatitis, and bone marrow suppression in 9%-28% of patients) or a lack of a clinical response (15% of cases)[6,8].
The metabolism of AZA and 6-MP is complex. AZA is a pro-drug, which is absorbed into the plasma and rapidly converted to 6-MP via a glutathione-dependent process. 6-MP can be inactivated by xanthine oxidase (XO) and thiopurine-methyl-transferase (TPMT) or converted to cytotoxic 6-thioguanine nucleotides (6-TGNs) via a multi-enzymatic process[7,9,10] TPMT or XO deficiency results in greater conversion of 6-MP to 6-TGNs, which are the predominant active metabolites related to drug efficacy, but this conversion also induces toxicity. Patients with low TPMT activity display higher 6-TGN levels when treated with standard doses of AZA and are at increased risk of myelosuppression. However, patients with high TPMT activity are usually resistant to thiopurines or require a higher dose to achieve efficacy, which increases the risk of hepatotoxicity[7,9-12].
However, the large variability in the activity of TPMT among patients has been attributed to TPMT gene polymorphisms[13,14]. The TPMT gene has been localized to chromosome 6p22.3. A total of 24 TPMT genetic polymorphisms have been identified; these polymorphisms are associated with decreased levels of enzymatic activity and/or thiopurine drug-induced toxicity. These activities are genetically determined and demonstrate a trimodal distribution in the Caucasian population: 88.6% of individuals carry two allele types resulting in normal or high TPMT activity (TPMT *1), 0.3% are homozygous for low activity alleles with no detectable TPMT activity, and 11.1% are heterozygous and display intermediate activity[13,14]. The overall concordance rate between TPMT genetic polymorphisms and specific phenotypes is 98.4%[7,9,10,12,15].
Three mutant alleles, TPMT *2, *3A, and *3C, account for the majority of the variant alleles in the entire human population studied to date. Intermediate or low TPMT activity is most frequently associated with these allele polymorphisms[13,14]. The distribution of these alleles differs significantly among ethnic populations, particularly the *3A and *3C genotypes[7,9,11,12]. However, a number of studies have shown that the frequency of these alleles does not differ in IBD patients compared to controls within the same populations.
The aim of this study was to determine the prevalence of TPMT gene polymorphisms in a group of Brazilian patients with IBD and to investigate the relationship between these polymorphisms and thiopurine related-toxicity and treatment response.
A total of 219 consecutive patients with IBD, of which 146 had CD and 73 had UC, regularly seen at the outpatient unit of the Division of Gastroenterology and Gastrointestinal Endoscopy at the University Hospital Pedro Ernesto of the State University of Rio de Janeiro, a tertiary referral center, were enrolled in this study from February 2009 to January 2011. The diagnosis of IBD was based on established diagnostic criteria, including clinical, imaging, endoscopic, and histological parameters.
The following data were collected from all patients: gender, age, age at diagnosis, disease activity, history of IBD-related surgery, chronic steroid use including steroid-dependent or steroid-refractory disease, and the presence of side effects from medical treatment. For patients with CD, the disease location was characterized as the terminal ileum (L1), colon (L2), ileocolon (L3), or upper gastrointestinal tract (L4). In addition, the predominant disease behavior was defined as non-stricturing/non-penetrating (B1), stricturing (B2), and perforating (B3), according to the Montreal classification[16]. Perianal disease was considered separately as an additional feature. CD activity was based on the Crohn’s Disease Index[17]. For patients with UC, disease extension was based on the Montreal classification using a modified criteria that combined ulcerative proctitis and left-sided UC (E1 + E2) and included extensive UC (pancolitis; E3). Disease activity was assessed using the Truelove index[18] (Table 1).
| Diagnosis | CD (n = 146) | UC (n = 73) |
| Gender | ||
| Male:female | 61:85 | 30:43 |
| Age at diagnosis | ||
| < 40 (A1):40 (A2) | 111:35 | 38:35 |
| Disease location CD | ||
| Terminal ileum (L1) | 30 (20.5) | |
| Colon (L2) | 42 (28.8) | |
| Ileocolon (L3) | 65 (44.5) | |
| Upper GI (L4) | 9 (6.2) | |
| Disease behavior | ||
| NS/NP (B1) | 53 (36.3) | |
| Stricturing (B2) | 45 (30.8) | |
| Penetrating (B3) | 48 (32.9) | |
| Perianal disease | ||
| Yes:no | 38:108 (26.1) | |
| Disease location UC | ||
| Pancolitis | 38 (52.1) | |
| Non-pancolitis | 35 (47.9) | |
| Disease activity | ||
| Moderate/severe | 28 (19.2) | 15 (20.5) |
| Mild/Remission | 118 (80.8) | 58 (79.5) |
| Surgery because of IBD | ||
| Yes:no | 48:98 (32.9) | 0:73 (0) |
| Chronic steroid use | ||
| Yes:no | 26:120 (17.8) | 10:63 (13.7) |
| Side effects of medication | ||
| Yes:no | 43:103 (29.4) | 18:55 (24.6) |
| Azathioprine | ||
| Yes:no | 138:8 (94.5) | 44:29 (60.3) |
After a questionnaire containing clinical information was completed for each patient, peripheral blood samples were obtained from all participants by venipuncture and collection in EDTA tubes. Genomic DNA was isolated from peripheral blood leukocytes by proteinase-K/Sodium Dodecyl Sulfate digestion and phenol-chloroform extraction as previously described[19]. The TPMT gene polymorphisms most commonly described in the literature, TPMT*2 (C238G), TPMT*3A (G460A/A719G), and TPMT*3C (A719G) genotypes, were detected by real-time polymerase chain reaction (PCR) followed by direct sequencing with specific primers (Table 2) for each region of interest. These regions corresponded to exons 12, 21, and 26 of the TPMT gene. The genotype frequencies of the TPMT gene polymorphisms were analyzed in the study population of CD and UC patients. Genotype-phenotype associations with major clinical features were established, and the estimated risks for the mutations were calculated.
| Name | Sequence | Product size |
| P2W reverse | 5’ GTA TGA TTT TAT GACGGT TG 3’ | 254 |
| P2M reverse | 5’ GTA TGA TTT TATGCA GGT TTC 3’ | |
| P2C forward | 5’ TAA ATAGGAACC ATCGGA CAC 3’ | |
| 460 forward | 5’ TCC CCA AAT CAT AAC AGA GTG 3’ | 375 |
| 460 reverse | 5’ CTAGAACCCAGAAAAAGTATAG3’ | |
| 719 forward | 5’ CGT TGT CTT GAG AAG GTT GA 3’ | 175 |
| 719 reverse | 5’ CAT TAC ATT TTC AGG CTT TAG CAT A 3’ |
Briefly, PCR was performed using a buffer containing 0.75 mmol/L MgCl2, 0.2 mmol/L dNTPs, 1.0 U Platinum Taq DNA polymerase (all from Invitrogen, Life Technologies, Carlsbad, CA, United States), 20 pmol each primer, 200 ng genomic DNA, and sterile ultrapure water to a final volume of 50 μL. For amplification, the DNA was first denatured for 5 min at 94 °C, followed by 35 cycles of denaturation for 30 s at 92 °C, annealing for 30 s at 60 °C (exons 12 and 21) or 58 °C (exon 26), and extension for 1 minute at 72 °C. At the end of the 35 cycles, an additional 10-min cycle at 72 °C was performed. The PCR products were then purified with the Illustra GFX™ PCR DNA and Gel Band Purification kit according to the manufacturer’s protocol (GE Healthcare, Buckinghamshire, United Kingdom).
The sequencing reactions were performed using the ET Dye Terminator Cycle Sequencing Kit (GE Healthcare, Buckinghamshire, United Kingdom) according to the manufacturer’s protocol. The primers used were the same as those used for the PCR reaction (Table 2). For each product, eight sequencing reactions were performed; four contained sense oligonucleotides, and four contained antisense oligonucleotides.
To determine the G238C, G460A, and A719G polymorphisms, we performed PCR amplification in a final volume of 50 μL. The PCR reaction contained 100 ng genomic DNA, 1 U Platinum Taq polymerase, 1X reaction buffer, 10 mmol/L deoxynucleoside triphosphates (dNTP) (all from Life Technologies, United States) and primers (Table 2).
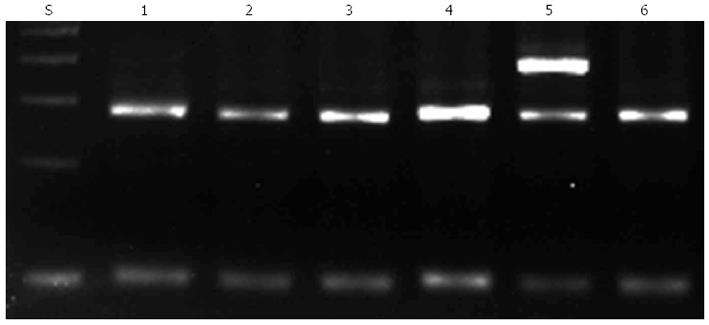
To detect the G238 polymorphism, a PCR assay was performed as previously described. Unpurified PCR products with a length of 256 base pairs were analyzed by agarose gel electrophoresis followed by staining with ethidium bromide. A DNA fragment was amplified with primers P2M and P2C when C238 (mutant) was present, whereas a DNA fragment was amplified with primers P2W and P2C when G238 (wild-type) was present (Figure 1).
To detect the G460A polymorphism, a PCR assay was performed as previously described. The PCR product was digested with Mwo I (New England Biolabs, Ipswich, MA, United States) for 1 h at 60 °C. The digested products were analyzed by gel electrophoresis. Mwo I digestion of wild-type DNA yielded fragments of 267 and 98 base pairs, whereas DNA containing the G460A polymorphism was not digested, resulting in an uncleaved fragment of 365 base pairs.
To detect the A719G polymorphism, a PCR assay was performed as previously described. The PCR product was digested with Acc I (New England Biolabs, Ipswich, MA, United States) for 2 h at 37 °C and analyzed by electrophoresis. The A719G polymorphism generates an Acc I restriction site in the amplified fragment and yields fragments of 207 and 86 base pairs upon digestion. Wild-type DNA yielded an uncleaved fragment of 293 base pairs.
Tests for Hardy-Weinberg equilibrium were performed using the Genepop (Genepop web version 3.1) software. For all other data evaluations, we used SPSS 17.0 software (SPSS Inc., Chicago, IL, United States). The distribution of the individual characteristics was evaluated using simple descriptive statistics. Differences among the distributions of the selected variables were evaluated using either the chi-square test or the Fisher exact test for categorical data. All of the tests were two-tailed, and statistical significance was established at P-values of less than 0.05.
This study was approved by the Ethical Committee of the University Hospital Pedro Ernesto of the State University of Rio de Janeiro, and informed consent was obtained from all subjects (2310/2008). The study protocol was in accordance with the ethical principles for medical research involving human subjects statement of the Helsinki Declaration.
The prevalence of the selected TPMT gene polymorphism TPMT*2 (C238G), TPMT*3A (G460A/A719G), and TPMT*3C (A719G) genotypes was 3.6%, 5.4%, and 7.7% of the patients, respectively. TPMT polymorphisms were detected in 37/219 patients, being 8 heterozygous for *2 (21.6%), 11 heterozygous for *3A (32.4%), and 18 heterozygous for *3C (46.0%). Despite the prevalence of the TPMT*3C genotype, no differences among the genotype frequencies were significant. No homozygous polymorphism was found in this study. The allele frequencies in the study population are shown in Table 3. No significant difference was observed in the allele frequencies of the TPMT gene variants according to the predicted Hardy-Weinberg equilibrium.
| Polymorphism | CHz | HTz | RHz | n | Allelic frequency | χ2 | P-value | |
| CD | ||||||||
| C238G | C:C | C:G | G:G | C | G | |||
| Observed | 141 | 5 | 0 | 146 | 0.98 | 0.02 | 0.04 | 0.83 |
| Expected | 141 | 5 | 0 | |||||
| G460A/A719G | G:G/A:A | G:A/A:G | A/A:G/G | G/A | A/G | |||
| Observed | 137 | 9 | 0 | 146 | 0.97 | 0.03 | 0.14 | 0.70 |
| Expected | 137 | 9 | 0 | |||||
| A719G | A:A | A:G | G:G | A | G | |||
| Observed | 128 | 18 | 0 | 146 | 0.94 | 0.06 | 0.63 | 0.42 |
| Expected | 128 | 16 | 1 | |||||
| UC | ||||||||
| C238G | C:C | C:G | G:G | C | G | |||
| Observed | 71 | 2 | 0 | 73 | 0.99 | 0.01 | 0.01 | 0.90 |
| Expected | 71 | 2 | 0 | |||||
| G460A/A719G | G:G/A:A | G:A/A:G | A/A:G/G | G/A | A/G | |||
| Observed | 70 | 3 | 0 | 73 | 0.98 | 0.02 | 0.03 | 0.85 |
| Expected | 70 | 3 | 0 | |||||
| A719G | A:A | A:G | G:G | A | G | |||
| Observed | 66 | 7 | 0 | 73 | 0.95 | 0.05 | 0.18 | 0.66 |
| Expected | 66 | 7 | 0 | |||||
Next, we investigated the prevalence of the TPMT gene variants and analyzed the distribution of each polymorphism with respect to the specific type of IBD (Table 4). For all three TPMT gene polymorphisms analyzed in this study, the frequencies of the wild-type (common homozygous), heterozygous, and homozygous polymorphic genotypes were similar among CD and UC patients (P = 0.722; P = 0.750; and P = 0.612, respectively). Importantly, no homozygous polymorphic types were observed.
| TPMT SNP | CHz | HTz | RHz | P-value |
| C238G | C:C | C:G | G:G | |
| CD (n = 143) | 137 (95.8) | 6 (4.2) | 0 | 0.722 |
| UC (n = 71) | 69 (97.2) | 2 (2.8) | 0 | |
| G460A/A719G | G:G/A:A | G:A/A:G | A/A:G/G | |
| CD (n = 126) | 118 (93.7) | 8 (6.3) | 0 | 0.750 |
| UC (n = 69) | 66 (95.7) | 3 (4.3) | 0 | |
| A719G | A:A | A:G | G:G | |
| CD (n = 135) | 122 (90.4) | 13 (9.6) | 0 | 0.612 |
| UC (n = 71) | 66 (93.0) | 5 (7.0) | 0 |
Next, we investigated the potential role of genotype in specific phenotypic variables. The associations between the sub-phenotypic categories and the genotype frequencies of the single nucleotide polymorphism (SNPs) in different subgroups of patients with CD and UC are shown in Table 5.
| C238G | G460A/A719G | A719G | ||||
| Adverse effect | CHz | HTz | CHz | HTz | CHz | HTz |
| Crohn’s disease | 35/143 (24.5) | 30/126 (23.8) | 33/135 (24.4) | |||
| Myelosuppression | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 3 (2.1) | 0 | 2 (1.6) | 0 | 2 (1.5) | 0 |
| Flu-like symptoms | 3 (2.1) | 0 | 3 (2.4) | 0 | 3 (2.2) | 0 |
| Nausea/vomiting | 12 (8.4) | 0 | 9 (7.1) | 1 (0.8) | 11 (8.1) | 1 (0.7) |
| Allergy/dermatitis | 1 (0.7) | 0 | 2 (1.6) | 0 | 2 (1.5) | 0 |
| Hepatotoxicity | 5 (3.5) | 1 (0.7) | 5 (4.0) | 0 | 5 (3.7) | 0 |
| Amylase/lipase elevation | 6 (4.2) | 2 (1.4) | 6 (4.7) | 0 | 3 (2.2) | 4 (2.9) |
| Pancreatitis | 1 (0.7) | 1 (0.7) | 2 (1.6) | 0 | 2 (1.5) | 0 |
| Ulcerative colitis | 14/71 (19.7) | 18/68 (26.5) | 14/71 (19.7) | |||
| Myelosuppression | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 |
| Flu-like symptoms) | 1 (1.4) | 0 | 1 (1.5) | 0 | 1 (1.4) | 0 |
| Nausea/vomiting | 4 (5.6) | 0 | 3 (4.4) | 0 | 3 (4.2) | 0 |
| Allergy/dermatitis | 3 (4.2) | 2 (2.8) | 3 (4.4) | 0 | 2 (2.8) | 0 |
| Hepatotoxicity | 0 | 0 | 0 | 0 | 0 | 0 |
| Amylase/lipase elevation | 2 (2.8) | 1 (1.4) | 3 (4.4) | 0 | 3 (4.2) | 0 |
| Pancreatitis | 0 | 1 (1.4) | 1 (1.5) | 0 | 1 (1.4) | 0 |
Among the side effects recorded from patients taking azathioprine, 14 patients presented with pancreatitis and/or an elevation of pancreatic enzymes, while 6 patients had liver toxicity, and 2 patients exhibited myelosuppression/neutropenia. No associations between the TPMT*3A (G460A/A719G) polymorphism and specific CD or UC sub-phenotypes were observed. By contrast, we observed a trend towards a positive association between the TPMT*2 (C238G) and TPMT*3C (A719G) genotypes and the development of pancreatitis and/or abnormal levels of pancreatic-related enzymes in patients with IBD. For the TPMT*2 (C238G) genotype, amylase/lipase tended to be more elevated in patients with CD, while pancreatitis appeared to be more common in patients with UC. For the TPMT*3C (A719G) genotype, the levels of amylase/lipase tended to be higher among patients with CD. Other adverse effects, including myelossuppression/neutropenia and hepatotoxicity presented an overall low prevalence and were not associated with TPMT polymorphisms (Table 5).
This is the first study to investigate the TPMT*2, TPMT*3A and TPMT*3C genotypes in Brazilian patients with IBD. We determined that the prevalence of TPMT gene polymorphisms is relatively high among Brazilian patients, including two genetic variants, TPMT*2 and TPMT*3C, that may be associated with pancreatic toxicity in IBD patients taking azathioprine. Nevertheless, the distributions of wild-type, heterozygous, and homozygous genotypes were similar among CD and UC patients, and no homozygous polymorphic type was observed.
Immunosuppressant agents such as Azathioprine and 6-MP are essential to promote and maintain remission in IBD patients. Unfortunately, major side effects have been associated with the use of these drugs, potentially limiting their utility. TPMT functions as a crucial enzyme in the metabolism of thiopurine drugs (including azathioprine and 6-MP) by methylating (i.e., inactivating) these drugs[10,20]. In IBD, genetic polymorphisms of TPMT have been associated with low or no enzyme activity, resulting in higher levels of metabolite concentrations and enhancing pharmacological activities and the risk of side effects[21].
TPMT deficiency can also be measured by its activity in red blood cells or by genotypic determination. Although the methods are different, they yield similar results. Genotyping has greater clinical significance because it is not subject to external influences such as blood transfusions[10,22,23] TPMT gene polymorphisms can be easily identified, enabling the identification of patients with potential risk for drug toxicity. This is of particular interest in Brazil, which features a heterogeneous population and has been considered a low prevalence area for IBD, although the incidence has increased rapidly in the last decade[15].
This study identified a TPMT gene polymorphism prevalence of 16.9% among patients with IBD, which is relatively high compared to reports in the literature for Caucasian populations (10% and 11%)[7,20,21]. In other studies, the analysis of different populations has yielded more variable results, but the data on IBD patients did not differ from controls within the same populations. Studies in North American and European Caucasians indicate a predominance of the 3A genotype, which represents nearly 85% of all polymorphisms[9,24]. Indjova et al[25] also observed a predominance of the 3A genotype in a healthy Bulgarian population, although with a lower prevalence (30.4%). In a cohort of ninety-seven thiopurine-treated paediatric IBD patients, 18 (18.56%) were heterozygous, while 2 (2.06%) were homozygous for a mutated TPMT gene[26]. However, studies of African and Eastern Asian patients have indicated a predominance of the 3C genotype. In Asiatic studies, analyses of TPMT gene polymorphisms have detected only the 3C allele among a healthy Chinese population[27] as well as among South Korean patients with IBD[28]. By contrast, in western Asia, studies in Jordan[15] and Iran[29] detected different patterns of polymorphisms, and the occurrence of the 3C genotype was not exclusive.
In the present study, the distribution of genotypes among the 37 heterozygotes within the IBD population was 21.6% TPMT*2, 32.4% TPMT*3A, and 46% TPMT*3C. With regard to the entire population in the study, this distribution corresponded to 3.6%, 5.4%, and 7.7% of the population, respectively, in contrast to previous studies of Caucasian (*3A) and Asian (*3C) populations, which usually report a clear predominance of a single gene polymorphism. Nevertheless, compared to other Brazilian data obtained from different regions and with a non-IBD population[30-32], we observed an overall higher prevalence of TPMT gene polymorphisms. However, we observed a similar *3A and *3C allele distribution compared to other studies involving non-IBD patients from Rio de Janeiro[30,32]. This finding is supported by the heterogeneous and highly mixed nature of the population in southeastern Brazil, particularly Rio de Janeiro, where European and African immigrants constitute a majority of the population background[33].
Similar to other studies, we did not identify any significant association between TPMT gene polymorphisms and hepatotoxicity[21] or myelotoxicity[9,12]. It is possible that the sample size and/or the absence of polymorphic homozygotic patients may have influenced these results. However, interestingly, Gazouli et al[26] did not find any association between TPMT polymorphisms and the occurrence of thiopurine-related adverse events, even having detected a relatively high rate of polymorphic homozygotic among IBD patients. In this study, we observed a potential association of the *2 and *3C genotypes with pancreatic changes. An elevation of pancreatic enzymes was associated with the TPMT*2 and TPMT*3C genotypes in CD, while pancreatitis was more frequently observed in UC patients with the TPMT*2 genotype. A possible association between specific TPMT genotypes and the pancreatic changes combined with the use of azathioprine in this study may support the dependence of these changes on genetic polymorphisms rather than idiosyncratic responses, as previously suggested[21,34]. Thus, future studies with larger sample sizes and different populations will be necessary to confirm these findings.
In conclusion, our investigation of TPMT gene polymorphisms identified that the prevalence of TPMT gene polymorphisms is relatively high among Brazilian patients and a specific genetic profile among IBD patients from Rio de Janeiro. The possibility of specific TPMT genotypes be associated with adverse events, including potentially severe pancreatic toxicity, should alert physicians to the potential need to perform genetic testing prior to the initiation of therapy with thiopurine agents. Such measures could facilitate the selection of appropriate medications and personalized doses of thiopurine agents to minimize toxic responses while maintaining full treatment efficacy.
The purine analogs azathioprine and 6-mercaptopurine (6-MP) are the most common immunosuppressant drugs used to treat inflammatory bowel disease (IBD). Thiopurine-methyl-transferase (TPMT) is a crucial enzyme in the metabolism of azathioprine and 6-MP. TPMT genetic polymorphisms are associated with decreased levels of enzymatic activity and/or thiopurine drug-induced toxicity.
Evidence indicates the existence of TPMT activity differences between wild-type and heterozygous and homozygous mutated subjects. The carriers of at least one variant allele and with both intermediate and absent TPMT activity have an increased risk for developing thiopurine-induced myelotoxicity compared with individuals with normal genotype and TPMT activity. A high degree of concordance was demonstrated between TPMT genotype and phenotype.
In Caucasians, approximately 11% of the population harbour heterozygous and 0.3% homozygous TPMT mutations, leading to an intermediate or low TPMT activity, respectively. More than 24 mutations are now indexed but the clinical relevance of some of them remains unclear. TPMT*3A, TPMT*3C, and TPMT*2 represent the most prevalent mutant alleles in Caucasians and African-Americans and account for 80%-95% of intermediate or deficient methylator phenotypes.
In IBD patients from Rio de Janeiro the distribution of genotypes were predominantly TPMT*3A, and TPMT*3C, in contrast to previous studies of Caucasian (*3A) and Asian (*3C) populations, which usually report a clear predominance of a single gene polymorphism. This finding is supported by the heterogeneous and highly mixed nature of the population in southeastern Brazil, where European and African immigrants constitute a majority of the population background.
Thiopurine-methyltransferase polymorphisms are relatively common among Brazilian patients with inflammatory bowel disease. In contrast to most studies, the rates of myelossuppression and hepatotoxicity were low, but a trend towards pancreatic toxicity may be associated with TPMT*2 and TPMT*3C genetic variants in IBD patients taking azathioprine.
The authors firstly investigated the TPMT*2, TPMT*3A and TPMT*3C genotypes in Brazilian patients with IBD and demonstrated that the prevalence of TPMT gene polymorphisms is relatively high among Brazilian patients, including two genetic variants, TPMT*2 and TPMT*3C, that have been associated with pancreatic toxicity in IBD patients taking azathioprine. These results were interesting and important in clinical pretreatment of IBD.
| 1. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 798] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 2. | Loftus EV. Progress in the diagnosis and treatment of inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:3-16. [PubMed] |
| 3. | Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: a review of medical therapy. World J Gastroenterol. 2008;14:354-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 165] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465-83; quiz 464, 484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 596] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 5. | Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501-23; quiz 524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 955] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 6. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2770] [Article Influence: 115.4] [Reference Citation Analysis (3)] |
| 7. | Roblin X, Peyrin-Biroulet L, Phelip JM, Nancey S, Flourie B. A 6-thioguanine nucleotide threshold level of 400 pmol/8 x 10(8) erythrocytes predicts azathioprine refractoriness in patients with inflammatory bowel disease and normal TPMT activity. Am J Gastroenterol. 2008;103:3115-3122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Bär F, Sina C, Fellermann K. Thiopurines in inflammatory bowel disease revisited. World J Gastroenterol. 2013;19:1699-1706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Ansari A, Hassan C, Duley J, Marinaki A, Shobowale-Bakre EM, Seed P, Meenan J, Yim A, Sanderson J. Thiopurine methyltransferase activity and the use of azathioprine in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:1743-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Gisbert JP, Niño P, Rodrigo L, Cara C, Guijarro LG. Thiopurine methyltransferase (TPMT) activity and adverse effects of azathioprine in inflammatory bowel disease: long-term follow-up study of 394 patients. Am J Gastroenterol. 2006;101:2769-2776. [PubMed] |
| 11. | Gearry RB, Barclay ML. Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Dubinsky MC, Yang H, Hassard PV, Seidman EG, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology. 2002;122:904-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 360] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 13. | González-Del Angel A, Bermúdez-López C, Alcántara-Ortigoza MA, Vela-Amieva M, Castillo-Cruz RA, Martínez V, Torres-Espíndola L. Thiopurine S-methyltransferase (TPMT) genetic polymorphisms in Mexican newborns. J Clin Pharm Ther. 2009;34:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Hakooz N, Arafat T, Payne D, Ollier W, Pushpakom S, Andrews J, Newman W. Genetic analysis of thiopurine methyltransferase polymorphism in the Jordanian population. Eur J Clin Pharmacol. 2010;66:999-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Souza MH, Troncon LE, Rodrigues CM, Viana CF, Onofre PH, Monteiro RA, Passos AD, Martinelli AL, Meneghelli UG. [Trends in the occurrence (1980-1999) and clinical features of Crohn’s disease and ulcerative colitis in a university hospital in southeastern Brazil]. Arq Gastroenterol. 2002;39:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2443] [Article Influence: 122.2] [Reference Citation Analysis (2)] |
| 17. | Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1940] [Cited by in RCA: 2240] [Article Influence: 48.7] [Reference Citation Analysis (1)] |
| 18. | Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1066] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 19. | Miller DN, Bryant JE, Madsen EL, Ghiorse WC. Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl Environ Microbiol. 1999;65:4715-4724. [PubMed] |
| 20. | Gearry RB, Barclay ML, Roberts RL, Harraway J, Zhang M, Pike LS, George PM, Florkowski CM. Thiopurine methyltransferase and 6-thioguanine nucleotide measurement: early experience of use in clinical practice. Intern Med J. 2005;35:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Dong XW, Zheng Q, Zhu MM, Tong JL, Ran ZH. Thiopurine S-methyltransferase polymorphisms and thiopurine toxicity in treatment of inflammatory bowel disease. World J Gastroenterol. 2010;16:3187-3195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Payne K, Newman W, Fargher E, Tricker K, Bruce IN, Ollier WE. TPMT testing in rheumatology: any better than routine monitoring? Rheumatology (Oxford). 2007;46:727-729. [PubMed] |
| 23. | Daperno M, Sostegni R, Canaparo R, Serpe L, Lavagna A, Crocellà L, Castagno F, Vernetto A, Rigazio C, Ercole E. Prospective study of the effects of concomitant medications on thiopurine metabolism in inflammatory bowel disease. Aliment Pharmacol Ther. 2009;30:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Yates CR, Krynetski EY, Loennechen T, Fessing MY, Tai HL, Pui CH, Relling MV, Evans WE. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997;126:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 529] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 25. | Indjova D, Atanasova S, Shipkova M, Armstrong VW, Oellerich M, Svinarov D. Phenotypic and genotypic analysis of thiopurine s-methyltransferase polymorphism in the bulgarian population. Ther Drug Monit. 2003;25:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Gazouli M, Pachoula I, Panayotou I, Mantzaris G, Syriopoulou VP, Goutas N, Vlachodimitropoulos D, Anagnou NP, Roma-Giannikou E. Thiopurine S-methyltransferase genotype and the use of thiopurines in paediatric inflammatory bowel disease Greek patients. J Clin Pharm Ther. 2010;35:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Cao Q, Zhu Q, Shang Y, Gao M, Si J. Thiopurine methyltransferase gene polymorphisms in Chinese patients with inflammatory bowel disease. Digestion. 2009;79:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Cheon JH, Kim JH, Kim BY, Kim SW, Hong SY, Eun CS, Hong SS, Byeon JS, Kim TI, Han DS. Allele frequency of thiopurine methyltransferase and inosine triphosphate pyrophosphatase gene polymorphisms in Korean patients with inflammatory bowel diseases. Hepatogastroenterology. 2009;56:421-423. [PubMed] |
| 29. | Bahari A, Hashemi M, Bari Z, Moazeni-Roodi A, Kaykhaei MA, Narouie B. Frequency of thiopurine S-methyltransferase (TPMT) alleles in southeast Iranian population. Nucleosides Nucleotides Nucleic Acids. 2010;29:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Reis M, Santoro A, Suarez-Kurtz G. Thiopurine methyltransferase phenotypes and genotypes in Brazilians. Pharmacogenetics. 2003;13:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Silva MR, de Oliveira BM, Viana MB, Murao M, Romanha AJ. Thiopurine S-methyltransferase (TPMT) gene polymorphism in Brazilian children with acute lymphoblastic leukemia: association with clinical and laboratory data. Ther Drug Monit. 2008;30:700-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Boson WL, Romano-Silva MA, Correa H, Falcão RP, Teixeira-Vidigal PV, De Marco L. Thiopurine methyltransferase polymorphisms in a Brazilian population. Pharmacogenomics J. 2003;3:178-182. [PubMed] |
| 33. | Parra FC, Amado RC, Lambertucci JR, Rocha J, Antunes CM, Pena SD. Color and genomic ancestry in Brazilians. Proc Natl Acad Sci USA. 2003;100:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 644] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 34. | Weersma RK, Peters FT, Oostenbrug LE, van den Berg AP, van Haastert M, Ploeg RJ, Posthumus MD, Homan van der Heide JJ, Jansen PL, van Dullemen HM. Increased incidence of azathioprine-induced pancreatitis in Crohn’s disease compared with other diseases. Aliment Pharmacol Ther. 2004;20:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
P- Reviewers: Gazouli M, Hokama A, Trkulja V, Yamakawa M S- Editor: Cui XM L- Editor: A E- Editor: Zhang DN