INTRODUCTION
Sepsis is a systemic inflammatory response caused by infections, trauma, and various diseases. It has become the most common cause of death in modern clinics[1]. Severe pathological changes associated with sepsis are present throughout the development process from the systemic inflammatory response syndrome to the multi-organ dysfunction syndrome[2]. Despite extensive studies of the inflammatory changes on the occurrence and development of sepsis, less is known regarding the changes in energy metabolism in vital organs associated with sepsis.
Sepsis is generally considered to involve physiological changes in the body towards high metabolism. Characteristic changes include increased energy consumption, excessive protein and fat metabolism, negative nitrogen balance, high blood glucose (hyperglycemia) levels, and excessive production of glycogen[3,4]. To date, there is no consensus on the cause of high metabolism. This complex response is jointly influenced and determined by a series of factors, including endotoxin, interleukins (IL-1 and IL-6), platelet-activating factor, tumor necrosis factor, arachidonic acid (metabolite of the cyclooxygenase and lipoxygenase pathways) metabolism, reactive oxygen species, neutrophil adhesive material, nitric oxide, complement, and the coagulation cascade[5]. Overall, high blood glucose is an important causative factor of enhanced metabolism in sepsis. Early onset and sustained development of hyperglycemia are possibly important factors responsible for the high mortality seen in sepsis[6].
Studies have shown that oxidative decomposition of glycogen is restricted in patients with sepsis, and the resulting hyperglycemia is closely related to the manifestations of septic patients regarding disease progression, immune dysfunction, poor wound healing, and enhanced muscle protein breakdown[6-11]. In addition, the survival rate of patients with severe acute sepsis can be improved significantly by strengthening the application of insulin to control blood glucose to near normal levels[12]. However, a recent large randomized study showed that the control of blood glucose levels by intensive insulin therapy cannot effectively reduce the mortality rate of patients in intensive care units (ICU), and instead increases the risk of low blood glucose (hypoglycemia) and hypoglycemia-induced complications in critically ill patients[13-15]. Therefore, it is crucial to find a new and effective mode of treatment as a secondary or even alternative option to insulin therapy in maintaining normal blood glucose levels in septic patients.
Carbon monoxide (CO) is one of the metabolic products of heme oxygenase (HO), and regulates inflammation. Our previous studies have demonstrated that endogenous CO has a protective effect on vital organs (including liver, lung, and intestine) by suppressing multiple organ inflammatory responses and reducing oxidative stress[16-20]. No other study has thus far assessed the regulatory effect of CO on glucose metabolism in sepsis.
In the present study, an exogenous CO intervention was used for the first time and 18F-fluorodeoxyglucose (FDG)/micro-positron emission tomography (PET) was employed to detect hepatic glucose metabolism in vivo in septic mice. The protective effect of CO on hepatic mitochondria in septic mice was examined, and its regulatory effect on abnormal glucose metabolism was explored. The results will provide new evidence for potentially improving outcomes as a consequence of exogenous CO on the survival rate in septic patients.
MATERIALS AND METHODS
Materials
Tricarbonyldichlororuthenium (II) dimer (CORM-2) was obtained from Sigma Aldrich (St Louis, MO, United States) and solubilized in dimethyl sulfoxide (DMSO) to obtain a 40 mmol/L stock. The inactive form of CORM-2 (negative controls) was used in the experiments and was prepared as follows: CORM-2 was “inactivated” (iCORM-2) by leaving the CORM-2 stock at 37 °C in a 5% CO2 humidified atmosphere for 24 h to liberate CO. The iCORM-2 solution was also bubbled with nitrogen to remove the residual CO present in the solution. An adenosine triphosphate assay kit and a bicinchoninic acid protein assay kit were obtained from Beyotime (Jiangsu, China). A lactate assay kit was obtained from Jiancheng (Jiangsu, China). A blood glucose meter was purchased from ACCU-CHEK Performa (Roche, Germany). 18F-FDG was obtained from Jiangsu Institute of Nuclear Medicine (Jiangsu, China). A small animal PET device and 3D OSEM MAP imaging system were purchased from Inveon Dedicate (Siemens, Germany). A WIZARD Gamma Counter was obtained (PE/1470 Wizard, United States).
Animal model of cecal ligation and puncture
C57BL/6 male mice (20 ± 2 g) were randomly divided into four groups, including the sham group which received no treatment, the cecal ligation and puncture (CLP) group which underwent CLP surgery, the CLP + CORM-2 and CLP + iCORM-2 group which underwent CLP surgery, followed by tail vein injection of 8 mg/kg CORM-2 and iCORM-2, respectively. The concentration of CORM-2 injected into mice was obtained from the literature and relevant experience of our research group[16,17,21]. The Council on Animal Care and Use at Jiangsu University approved the experimental protocol on animal protection and welfare. Anesthesia consisted of the spontaneous inhalation of isoflurane-N2O in a 60% oxygen/40% nitrogen mixture, which was performed when necessary.
Measurement of mouse survival rate after CLP
A total of 48 C57BL/6 male mice (aged 6-8 wk and weighing 20 ± 2 g) were fed in the laboratory for 1 wk. The mice were divided into four groups (n = 12) according to a random number table as described above. Mouse survival was monitored six times daily for up to 72 h.
Blood glucose measurements
A total of 24 C57BL/6 male mice (aged 6-8 wk and weighing 20 ± 2 g) were randomly divided into four groups (n = 6) and treated as described above. Blood glucose levels were assayed in all groups of mice preoperatively, and at 1, 2, 4, 6, 8, 10, 12, 16, 20, 24 and 36 h postoperatively. During glucose measurement, the tail tip (2-3 mm) was excised and massaged to harvest a small volume of blood (1.0-10 μL) which was placed into the hole of a blood glucose test strip. Glucose measurements were obtained using a rapid blood glucose meter. For continuous monitoring, blood flow was obtained by scrubbing the end of the tail with sterile-clean alcohol wipes.
Preparation of liver tissue homogenates
A total of 36 C57BL/6 male mice were randomly divided into four groups (n = 9) and treated as described above. All mice were sacrificed 24 h postoperatively. Plasma and complete liver tissue specimens were collected and stored in liquid nitrogen at -70 °C before use. To prepare tissue homogenates, 0.1 g specimens were weighed and added to 1 mL of phosphate buffered saline solution and processed by ultrasonic lysis on ice twice (30 s each)[16,17]. The homogenates were centrifuged at 12000 g and 4 °C. The supernatants were collected and stored at -70 °C before use.
Histopathological examination
Liver tissue specimens (approximately 0.4 g) were taken from the left lobe of the liver 24 h postoperatively and fixed in 10% formalin, followed by routine paraffin embedding and sectioning. After hematoxylin/eosin (HE) staining, the sections were observed under a light microscope (× 400 magnification) to examine sinusoidal congestion, cellular edema, and inflammatory cell infiltration in liver specimens[22].
Micro-PET imaging and 18F-FDG biodistribution
During the testing procedures, continuous anesthesia was achieved by mask inhalation of isoflurane with a VMR small animal anesthesia machine. 18F-FDG was administered by tail vein injection at a dose of 20 μCi. Micro-PET scanning was performed after 60 min of continuous anesthesia, during which attention was paid to temperature control and anesthetic dose. Continuous scanning was performed with a small animal PET device for 10 min, and images were reconstructed using a 3D OSEM MAP imaging system. The obtained data were processed by free attenuation correction using the β-smoothing parameter of 0.1. The maximum uptake value of FDG in the liver of each mouse was calculated by measuring the 18F radioactivity in the marked contour area of the liver. The standardized radioactive uptake value (SUV) in each mouse was obtained by standardization of tissue weight and calculation of the injection decay time of the radioisotope[23]. Mice were euthanized immediately after micro-PET scanning. Complete liver specimens were weighed and the radiation level was then detected with a γ-ray counter. The calculated results were standardized to obtain the final SUV values[24,25].
Transaminase, glucose-metabolizing enzyme, and lactic acid assays
Blood specimens were collected by right ventricular puncture. Plasma was separated and stored at 4 °C in heparin tubes. Plasma alanine transaminase (ALT) and aspartate transaminase (AST) activities were assayed using an automatic blood biochemical analyzer. Hepatic glucokinase (GK) activities and lactic acid levels were assayed by enzyme-linked immunosorbent assay kits following the manufacturer’s instructions.
Detection of mitochondrial function
Hepatic mitochondria were isolated by the Aprille method[26]. Mitochondria were resuspended in the assay buffer which was supplemented with 125 mmol/L sucrose, 50 mmol/L KCl, 2 mmol/L KH2PO4, 5 μmol/L rotenone, 10 mmol/L HEPES, and 5 mmol/L succinate with a protein content of 1.0 mg/mL. The extent of mitochondrial swelling was assayed by measuring the decrease in absorbance (A540) every 30 s for 30 min following the addition of 50 μmol/L Ca2+ at 37 °C.
Statistical analysis
Measured data are presented as a mean value ± SD. Statistical analysis of the experimental data was performed by single-factor one-way analysis of variance using SPSS 17.0 software and independent specimen Student’s t test. Survival analysis was performed by log-rank sum test. An alpha value of P < 0.05 was considered a statistically significant difference between measures and outcomes.
RESULTS
Survival of septic mice
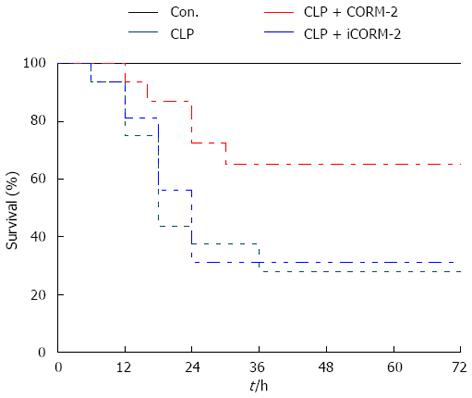
In this study, the 72 h survival rate of mice in each treatment group was examined postoperatively. The results showed that the survival rate of mice in the CLP group was substantially low, and was only 42% at 24 h and 26% at 48 h postoperatively. In contrast, the survival rate of mice in the CORM-2 group was significantly increased after the CORM-2 intervention, and was 73% at 24 h and 66% at 48 h postoperatively (P < 0.05). Moreover, at 72 h postoperatively, 66% of mice were still alive (Figure 1).
Figure 1 Effect of carbon monoxide-releasing molecule II on survival of septic mice.
Mice were challenged with cecal ligation and puncture (CLP) and treated with tricarbonyldi- chlororuthenium (II) dimer (CORM-2) or iCORM-2 as described in the Methods section. All mice had access to water and food. Mouse survival was monitored six times daily for up to 72 h after surgery. Data are shown as the percentage of surviving animals. The survival rate of mice in the CLP group was substantially low, and was only 42% at 24 h and 26% at 48 h postoperatively. In contrast, the survival rate of mice in the CORM-2 group was significantly increased after the CORM-2 intervention, and was 73% at 24 h and 66% at 48 h postoperatively (P < 0.05). Moreover, at 72 h postoperatively, 66% of the mice were still alive.
Effect of CORM-2 on blood glucose in septic mice
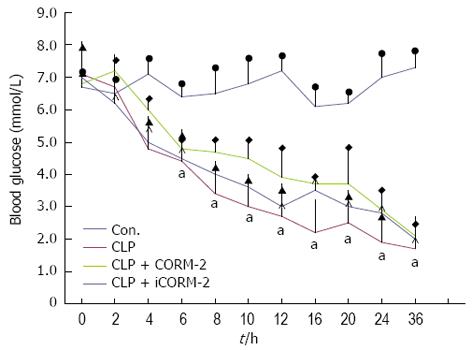
In each experimental group, blood glucose levels decreased to 30%-50% of that found in normal controls, and remained at these low levels for more than 36 h postoperatively (P < 0.05). In addition, substantial changes in blood glucose levels were not found in the CLP mice after CORM-2 intervention (P > 0.05, Figure 2).
Figure 2 Effect of carbon monoxide-releasing molecule II on blood glucose in septic mice.
Mice were challenged with cecal ligation and puncture (CLP) and treated with tricarbonyldichlororuthenium (II) dimer (CORM-2) or iCORM-2 as described in the Methods section. Serum glucose concentrations were assessed in all groups of mice preoperatively, and at 1, 2, 4, 6, 8, 10, 12, 16, 20, 24 and 36 h postoperatively. Blood glucose levels in the CLP group decreased to 30%-50% of the normal controls, and remained at these low levels for more than 36 h postoperatively. However, no changes were found in blood glucose levels in the CLP mice after intervention with CORM-2. Results are mean ± SD, aP < 0.05 vs sham mice.
Histopathological changes in the liver of septic mice
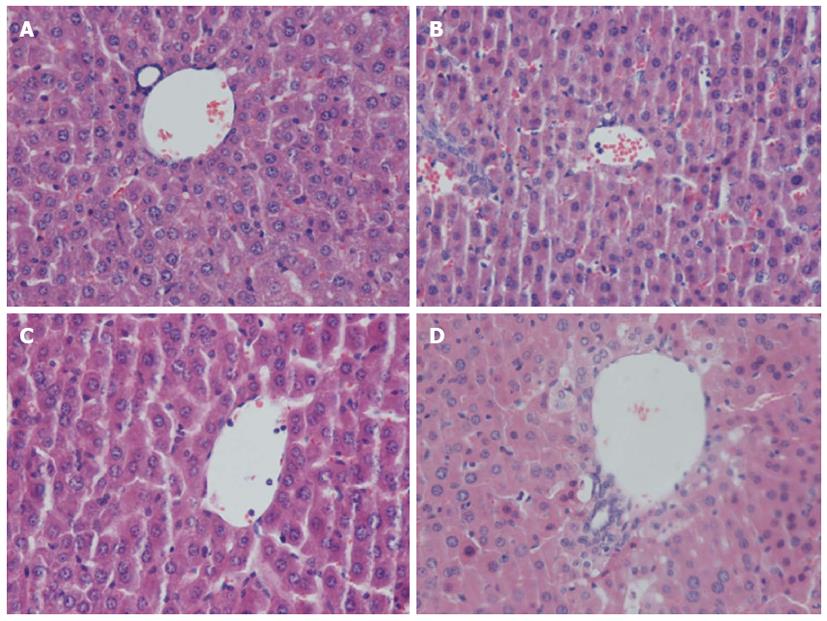
Under light microscopy, liver specimens from mice in the CLP group showed sinusoidal dilatation and congestion, hepatocellular swelling, vacuolar changes in partial hepatocytes, and neutrophil infiltration. In the CLP + CORM-2 group, sinusoidal congestion and hepatocellular swelling remained in certain areas of the liver specimens, however, hepatocellular damage was less severe and the extent of hepatic neutrophil infiltration was reduced (Figure 3).
Figure 3 Effect of carbon monoxide-releasing molecule II on liver injury in septic mice.
Mice were challenged with cecal ligation and puncture (CLP) and treated with tricarbonyldichlororuthenium (II) dimer (CORM-2) or iCORM-2 as described in the Methods section. Morphologic characteristics at 24 h after CLP were observed under light microscopy with hematoxylin and eosin (HE) staining. Liver specimens from mice in the CLP group showed sinusoidal dilatation and congestion, hepatocellular swelling, vacuolar changes in partial hepatocytes, and neutrophil infiltration. However, in the CLP + CORM-2 group, hepatocellular damage was obviously less severe and the extent of hepatic neutrophil infiltration was reduced. A: Sham; B: CLP; C: CLP + CORM-2; D: CLP + iCORM-2.
Effect of CORM-2 on hepatic glucose metabolism in septic mice
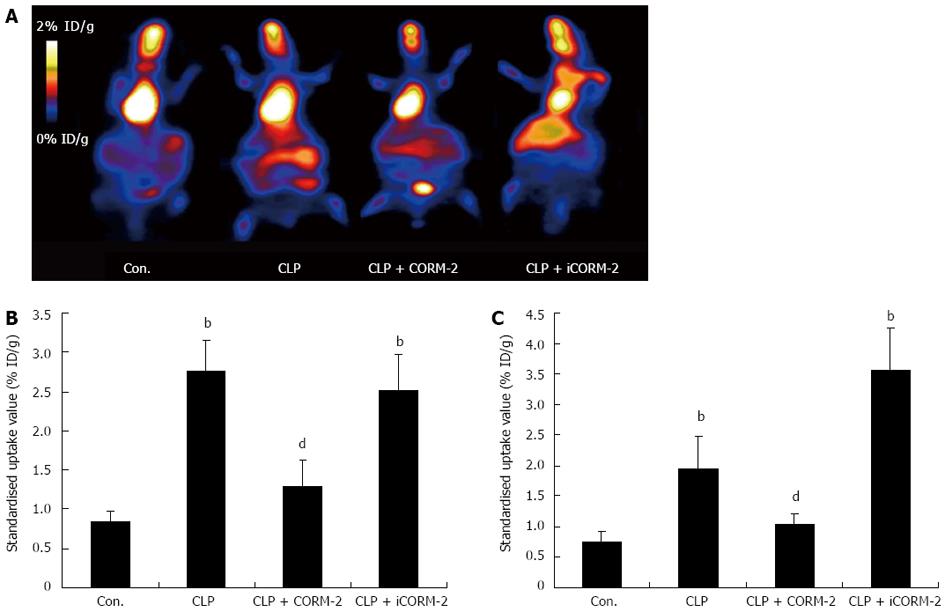
In vivo FDG/micro-PET imaging showed the uptake of the radioactive tracer in the experimental groups 1 h after tail vein injection. The marked area represents the coronal section of a liver from a septic mouse. Moreover, the greater the brightness of this area, the higher the FDG uptake value of the liver, and thus the greater the ability to take up glucose. Compared with the normal control group, the CLP group of mice showed significant increases in the level of hepatic glucose metabolism, and these increases were suppressed by CORM-2 intervention (Figure 4A and B). After micro-PET scanning, complete liver specimens were extracted for evaluation of 18F-FDG biodistribution and the data obtained quantified the experimental results (Figure 4C).
Figure 4 Effect of carbon monoxide-releasing molecule II on fludeoxyglucose uptake in the liver of septic mice.
Mice were challenged with cecal ligation and puncture (CLP) and treated with tricarbonyldichlororuthenium (II) dimer (CORM-2) or iCORM-2 as described in the Methods section. 18F-fludeoxyglucose (FDG) was administered by tail vein injection at a dose of 20 μCi followed by micro-positron emission tomography scanning and measurement of 18F-FDG biodistribution. Compared with the normal control group, the CLP group of mice showed significant increases in the level of hepatic glucose metabolism, and these increases were suppressed by CORM-2 intervention. A: Representative images of liver FDG uptake; B: The mean standardized radioactive uptake values in liver tissue; C: Quantification of 18F-FDG biodistribution. bP < 0.01 vs sham mice; dP < 0.01 vs CLP mice.
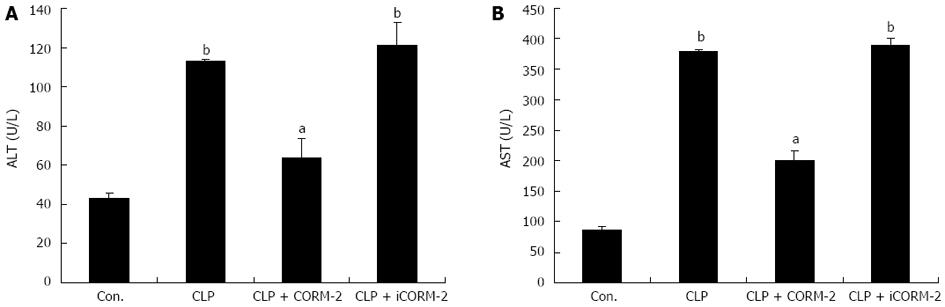
Effect of CORM-2 on plasma ALT and AST activities in septic mice
Plasma ALT and AST activities are important indicators commonly used to determine the extent of hepatocellular damage. The experimental results showed that the CLP group of mice had significantly increased plasma ALT and AST activities 24 h postoperatively as compared with the sham group (P < 0.05). However, the CLP + CORM-2 group had minor increases in plasma ALT and AST activities, and the differences were statistically significant compared with the CLP group (P < 0.05, Figure 5). Together, these results indicate that CORM-2 reduces plasma ALT and AST activities in septic mice, thereby mitigating hepatic damage.
Figure 5 Effect of carbon monoxide-releasing molecule II on plasma aspartate aminotransferase and alanine aminotransferase activities in septic mice.
Mice were challenged with cecal ligation and puncture (CLP) and treated with tricarbonyldichlororuthenium (II) dimer (CORM-2) or iCORM-2 as described in the Methods section. Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities at 24 h after surgery were determined. Plasma activities of ALT (A) and AST (B) were higher in CLP-challenged mice compared with sham mice, whereas ALT and AST activities were markedly decreased after administration of CORM-2. Results are mean ± SD, bP < 0.01 vs sham mice; aP < 0.05 vs CLP mice.
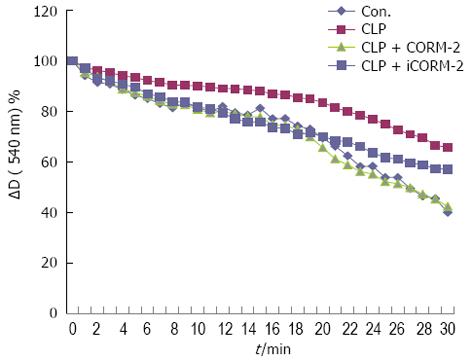
Effect of CORM-2 on hepatic mitochondrial function in septic mice
The degree of hepatic mitochondrial swelling was examined to determine sepsis-induced changes in hepatic mitochondrial function. The CLP group of mice had significant hepatic mitochondrial swelling with severely damaged mitochondrial function. After CORM-2 intervention, the extent of hepatic mitochondrial swelling was reduced and mitochondrial function was clearly protected (P < 0.05, Figure 6).
Figure 6 Effect of carbon monoxide-releasing molecule II on hepatic mitochondrial function in septic mice.
Mice were challenged with cecal ligation and puncture (CLP) and treated with tricarbonyldichlororuthenium (II) dimer (CORM-2) or iCORM-2 as described in the Methods section. Isolated mitochondria were resuspended in the assay buffer. Ca2+-induced mitochondrial swelling was assayed by the decrease in absorbance at 540 nm. The CLP group of mice had significant hepatic mitochondrial swelling with severely damaged mitochondrial function. Following CORM-2 intervention, the extent of hepatic mitochondrial swelling was reduced and mitochondrial function was clearly protected (P < 0.05 vs CLP mice). The curves represent typical recordings from experiments of at least three different mitochondrial preparations.
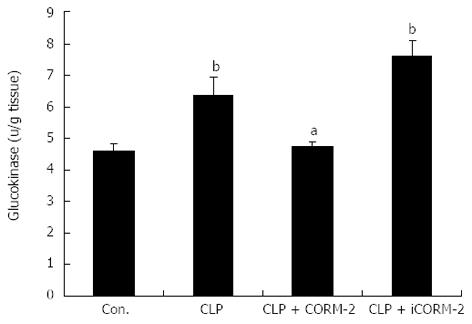
Effect of CORM-2 on hepatic GK activity level in septic mice
GK is a type of hexokinase (HK), and is referred to as HK IV which exists predominantly in the insulin cells of the hepatocyte nucleus. GK is one of the key enzymes involved in hepatic glucose metabolism, and its activity reflects hepatic glucose metabolism. Compared with the normal control group, the CLP group of mice had significantly increased hepatic GK activity. In contrast, the CLP + CORM-2 group had significantly reduced hepatic GK activity (Figure 7).
Figure 7 Effect of carbon monoxide-releasing molecule II on hepatic glucokinase activity level in septic mice.
Mice were challenged with cecal ligation and puncture (CLP) and treated with tricarbonyldichlororuthenium (II) dimer (CORM-2) or iCORM-2 as described in the Methods section. Hepatic glucokinase activities were assayed by enzyme-linked immunosorbent assay. Compared with the sham group, the CLP group of mice showed a significant increase in hepatic glucokinase (GK) activity. In contrast, the CLP + CORM-2 group showed significantly reduced hepatic GK activity. Results are mean ± SD, bP < 0.01 vs sham mice; aP < 0.05 vs CLP mice.
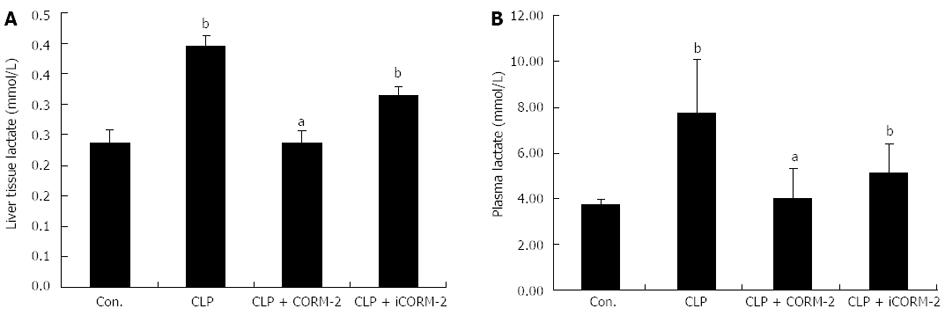
Effect of CORM-2 on lactic acid levels in septic mice
The levels of lactic acid in plasma and hepatic homogenates of septic mice were determined by enzyme-linked immunosorbent assay. When compared with the sham group, the CLP and CLP + iCORM-2 groups had significantly higher levels of lactic acid (P < 0.05). However, after CORM-2 intervention, lactic acid levels in plasma and hepatic homogenates declined significantly (Figure 8).
Figure 8 Effect of carbon monoxide-releasing molecule II on hepatic lactate production in septic mice.
Mice were challenged with cecal ligation and puncture (CLP) and treated with tricarbonyldichlororuthenium (II) dimer (CORM-2) or iCORM-2 as described in the Methods section. Lactate production in hepatic homogenates (A) and plasma (B) were assessed 24 h after CLP injury. When compared with the sham group, the CLP groups had significantly higher levels of lactic acid. After CORM-2 intervention, lactic acid levels in plasma and hepatic homogenates declined significantly. Results are presented as mean ± SD. bP < 0.01 vs sham mice; aP < 0.05 vs CLP mice.
DISCUSSION
Sepsis is now regarded as one of the leading causes of death in clinics[27]. According to the hospital statistical records, millions of septic patients are hospitalized each year in the United States, and there are more than 200000 deaths as a consequence of septic shock[28]. Sepsis occurs across all age groups in the human population[29]. It is recognized as the second leading cause of death in ICU patients[30-32]. The main feature of sepsis is that excessive proinflammatory mediators are released and exceed the ability of the body to physiologically regulate the inflammatory response according to normal homeostatic mechanisms[33-35]. The reason why a large number of inflammatory factors are produced during sepsis is that multiple vital organs and tissues undergo a variety of pathological changes, including abnormal tissue or cellular metabolism[36].
The tissue inflammatory response leads to local vasodilation and increases microvascular permeability, with the systemic manifestation in a highly dynamic state. The resulting demand for substrates of energy metabolism (including oxygen, glucose, protein, and fat) is relatively high, which increases energy metabolism and causes a series of clinical manifestations, such as hyperglycemia, lactic acidosis, hyperlipidemia, high protein catabolism, and a negative nitrogen balance. One of the major pathological changes is hyperglycemia. This is caused by an increase in anaerobic glycolysis of muscle tissues and fat metabolism, and a reduction in hepatic glycogen synthesis[36-38].
In septic patients, hyperglycemia not only means severe illness and a generally poor prognosis[39,40], but also exerts significant harmful effects on multiple vital organs. Hyperglycemia weakens the immune system, decreases resistance to infection, and reduces neutrophil function (including a reduction in chemotactic ability, the formation of oxygen radicals, and a decreased capacity for phagocytosis of bacteria, despite the actual increase in peripheral vascular leakage). In contrast, hyperglycemia influences the formation of cytokines in tissues, including an increase in proinflammatory cytokine production, such as tumor necrosis factor and IL-6, which are released at an early stage[41].
Recent studies have shown that blood glucose is not an independent factor which increases the mortality rate in septic patients[42,43]. However, blood glucose plays a crucial role in severe sepsis and this disease itself has a significant impact on the changes in blood glucose[44]. Therefore, maintaining stable blood sugar levels is critical in the treatment of sepsis. It is for this reason that a number of studies have attempted to stabilize blood sugar levels in septic patients through intensive insulin therapy at an early stage of treatment, with the aim of improving the survival of patients or reducing their duration of hospitalization[45]. However, a number of studies have found that such intensive insulin therapy frequently induces hypoglycemia in septic patients, and serious clinical consequences may arise[46,47]. In such a dilemma, there is no doubt that a new treatment is needed as a secondary or even alternative approach for the control of blood glucose.
CO is a metabolic product of HO and has a remarkable anti-inflammatory capacity. To date, no study has reported on the effects of CO on the in vivo metabolic capacity of glucose or other similar biological agents. Among the metabolic products from the heme portion of HO-1, CO and bilirubin both inhibit apoptosis, necrosis, inflammation, and oxidative stress. Moreover, iron ions enhance the synthesis of anti-oxidants and ferritin. Recent research suggests that the HO system may have a role in insulin sensitivity and cellular metabolism[48,49].
Recently, transition metal carbonyls have been identified as potential CO-releasing molecules (CORMs) with the potential to facilitate the pharmaceutical use of CO by delivering it to the tissues and organs of interest[50]. Studies have shown that CORM-2 suppresses LPS-induced inflammatory responses in human umbilical vein endothelial cells (HUVECs), peripheral blood mononuclear cells and macrophages[51,52]. Similarly, much results have confirmed that CO derived from CORMs protects mice from lethal endotoxemia and sepsis induced by LPS or cecal ligation and puncture (CLP)[53-55].
Our previous studies showed that CORM-2 inhibited over-expression of adhesion molecules, attenuated leukocyte sequestration in the organs of CLP or burn-induced septic mice, and decreased intracellular oxidative stress and NO production in LPS-stimulated HUVECs[16-20]. In the present study, we investigated the changes in hepatic glucose metabolism in septic mice after CLP treatment with or without CORM-2 intervention, and further explored the potential effects of CO on energy metabolism.
The experimental data showed that at 1-2 h after operation, blood glucose levels in the liver of CLP mice significantly decreased to 30%-50% of the normal level, and stabilized for more than 48 h until death. However, following CORM-2 intervention, blood glucose levels were seen to decrease slightly, but the changes were not statistically significant.
The main reason for this phenomenon may be that the feeding behavior of mice was substantially reduced postoperatively. Since mice are rodents with an inherently active metabolism, the energy required by them after trauma is significantly enhanced. Thus, short-term fasting may cause significant hypoglycemia. Barkhausen et al[56] reported this phenomenon in 2009. Is this possibly caused by a reduction in metabolic ability after serious infection due to sepsis? If so, then is it contrary to the theory of systemically high metabolism in septic patients studied previously?
Here we investigated in vivo hepatic glucose metabolism in mice using 18F-FDG/micro-PET. To date, the application of PET technology has enabled remarkable research observations in cancer and neurobiology. In addition, PET technology has been used in gene expression, pharmacological studies, and energy metabolism. However, the application of PET technology in the field of inflammation is restricted, and this is primarily due to the limitations of tracers and resolution. Moreover, research reports to date, have not been available on the application of PET technology in sepsis. This study used micro-PET, which is presently the most advanced molecular imaging technology. To meet the research requirements for model animals of small sizes, PET technology has been improved significantly in terms of spatial resolution and sensitivity.
An increasing number of radiolabels have been used for in vivo imaging of small animals. 18F-FDG is a glucose-like radioactive tracer, which has been used for the in vivo study of changes in glucose metabolic ability in local tissues and organs in humans and animals, as influenced by various diseases or drugs[57]. 18F-FDG is transported into cells by the glucose transporter which is located on the cell membrane and then phosphorylated to 18F-2’-FDG-6 by HK. Unlike G-6-P, 18F-2’-FDG-6 does not continue glucose metabolism, but is retained in cells. Therefore, the 18F-FDG levels represent the ability of cells to transport and metabolize glucose. Evidence shows that high FDG uptake not only occurs in malignancies, but is also commonly present in benign inflammatory responses[58].
In this study, 18F-FDG/micro-PET was used for the first time to investigate changes in hepatic glucose metabolism in septic mice after CORM-2 intervention. The results showed that the level of hepatic glucose metabolism in septic mice significantly increased, and exceeded normal levels. In contrast, CLP mice showed no significant difference when compared with the sham group following CO intervention. In addition, the 18F-FDG biodistribution analysis showed that CO inhibited the abnormal increase in hepatic glucose metabolism in septic mice.
To determine the potential cause of the above phenomena, we determined hepatic GK expression levels in septic mice. GK is primarily found in animal hepatocytes and pancreatic islet B-cells. GK is the key enzyme involved in glucose metabolism and an increase in GK level generally means an elevation in glucose metabolism. In the present study, hepatic GK expression levels in CLP septic mice 24 h after operation, was significantly higher than that seen in the sham group. These changes were obviously reduced after CO intervention. We speculate that CO inhibited the expression of GK, thereby reducing the level of hepatic glucose metabolism in septic mice.
Another important change related to abnormal metabolism in sepsis is lactic acidosis. This process, also known as acid shuttle, results from an increase in anaerobic glycolysis of muscle and other tissues caused by reverse dysregulation of hormones and cytokines. The duration and extent of lactic acidosis directly affects the formation of late onset organ dysfunction, while acidosis caused by lactic acid accumulation is extremely unfavorable in the prognosis of sepsis[16,36,44].
Interestingly, our experimental data showed that CORM-2 suppressed the abnormal increase in plasma lactic acid levels in septic mice. Lactic acidosis has a significant impact on energy metabolism in the body, and the increase in plasma lactic acid levels is an exact basis for measuring the impairment of mitochondrial function. In this context, we wondered whether CO has a protective effect on hepatocellular mitochondria as it suppressed increases in the levels of plasma lactic acid in septic mice. Hence, we determined the degree of impairment of mitochondrial function by analyzing hepatic mitochondrial swelling in septic mice. The results showed that CO effectively protected hepatic mitochondrial function in septic mice. It is possible that CO regulates blood glucose and plasma lactic acid levels by protecting mitochondrial function.
The protective effect of CO on liver function was confirmed by observations of hepatic pathological sectioning and serum ALT/AST measurements. CO reduced sinusoidal dilatation and congestion, hepatocellular swelling and deformation, and infiltration of inflammatory cells. In addition, the rise in plasma ALT levels was markedly suppressed after CO intervention.
Liver is the most important organ in the body to regulate blood glucose, and has a substantial ability to regulate glucose metabolism. CO possibly protects liver function in a variety of ways, and thus effectively assists the body to maintain blood glucose metabolism at a normal physiological level. This will significantly favor improvement in the functions of vital organs and tissues, and thus translate to improved prognosis in sepsis. Indeed, this was reflected in our experimental survival analysis data in experimental mice. On the basis of our previous study on the anti-inflammatory effects of CO, the present study opens up a new research direction for exploration of the effects of CO on energy metabolism in the body. This study primarily focused on CO regulation of glucose metabolism and its preliminary mechanism in sepsis. Exogenous CO has significantly physiological functions and could be developmed as a potential therapeutic agent. More detailed molecular mechanisms need to be studied further and clarified from a more comprehensive perspective.