Published online Mar 28, 2014. doi: 10.3748/wjg.v20.i12.3059
Revised: November 26, 2013
Accepted: January 6, 2014
Published online: March 28, 2014
Processing time: 180 Days and 7.8 Hours
With the increasing clinical use of cytostatic and novel biologic targeted agents, conventional morphologic tumor burden assessments, including World Health Organization criteria and Response Evaluation Criteria in Solid Tumors, are confronting limitations because of their difficulties in distinguishing viable tumor from necrotic or fibrotic tissue. Therefore, the investigation for reliable quantitative biomarkers of therapeutic response such as metabolic imaging or functional imaging has been desired. In this review, we will discuss the conventional and new approaches to assess tumor burden. Since targeted therapy or locoregional therapies can induce biological changes much earlier than morphological changes, these functional tumor burden analyses are very promising. However, some of them have not gone thorough all steps for standardization and validation. Nevertheless, these new techniques and criteria will play an important role in the cancer management, and provide each patient more tailored therapy.
Core tip: Accurate tumor burden assessment is a critical component of patient management and the investigation of new therapies. With the increasing clinical use of novel biologic targeted agents or locoregional therapies, morphological analysis confronted limitations, and new methods to assess tumor burden were desired. Advances in imaging technique enable us to assess tumor functions such as viability, vascular physiology, or metabolism, which can be new approaches to assess tumor burden.
- Citation: Hayano K, Fuentes-Orrego JM, Sahani DV. New approaches for precise response evaluation in hepatocellular carcinoma. World J Gastroenterol 2014; 20(12): 3059-3068
- URL: https://www.wjgnet.com/1007-9327/full/v20/i12/3059.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i12.3059
Accurate assessment of tumor burden is an important component of cancer patient management and the investigation of new therapies. Traditionally, therapeutic response has been assessed by serial tumor size measurements according to World Health Organization (WHO) criteria or Response Evaluation Criteria in Solid Tumor (RECIST)[1-3]. These criteria, which are based on anatomical measurement, are well established tool, and easy to apply for assessment of tumor burden. However, these morphological evaluations have substantial limitations, including the presence of tumors that cannot be measured, poor measurement reproducibility and mass lesions of unknown activity that persist following therapy[3]. They also have a difficulty in distinguishing viable tumor from necrotic or fibrotic tissue and recognizing the delay between cell kill and tumor shrinkage. Faced with these limitations, more sophisticated measurements (including tumor volume and lesion regression rates) have been applied to the evaluation of the tumor response to therapy.
With the increasing clinical use of cytostatic and novel biologic targeted agents or locoregional therapies (LRTs) such as ablation and transarterial chemoembolisation (TACE) in the management of hepatocellular carcinoma (HCC), it has become increasingly recognized that new methods of therapy assessment need to be developed urgently. For example, antiangiogenic agents are known to rapidly decrease contrast enhancement on computed tomography (CT)/magnetic resonance imaging (MRI) scans that occur within days of initiation of reduced vascular permeability to contrast agents rather than a true antitumor effect[4]. Faced with these limitations, the investigation for reliable quantitative biomarkers to assess tumor burden and therapeutic response including blood surrogate parameters, metabolic imaging and functional imaging based on CT, MRI, or positron emission tomography (PET) has been desired[4-7]. In this review, we discuss various conventional and new approaches to determine tumor burden in the current clinical practice of HCC.
In 1981, the WHO first published tumor response criteria, mainly for use in trials where tumor response was the primary endpoint. The WHO criteria introduced the concept of an overall assessment of tumor burden by summing the products of bidimensional lesion measurements and determined response to therapy by evaluation of change from baseline while on treatment. Subsequently, RECIST was introduced and approved for clinical use in 2000[1]. RECIST criteria were primarily conceived to provide specific guidelines for tumor burden measurement. After extensive experience and validation in several chemotherapeutic trials in solid tumors, it was revised in 2009 as RECIST 1.1[8]. RECIST 1.1 relies on the measurement of a maximum of five target lesions, not exceeding two per organ; subsequently, the sum of the greatest diameters is recorded followed by a final classification[3]. On the other hand, it has been questioned if these unidimensional measurements can reflect total tumor burden accurately. With the advent of imaging technologies such as workstation and 3D software, longitudinal or oblique measurements readily can be determined, and tumor volumes can be computed algorithmically. Sohaib et al[9] reported that CT volumetric measurements were accurate and reproducible in their phantom study. Welsh et al[10] reported that RECIST might overestimate tumor burden compared with volumetric measurements in HCC and pancreatic cancer, and they concluded that volumetric analysis might be the preferred method to detect tumor progression. However, the practical clinical value of tumor volumetric analysis remains controversial. There is no consensus about the recommended volume equivalents converted from diameter thresholds, which can be effectively applied without sacrificing either reproducibility or sensitivity to tumor progression or partial response.
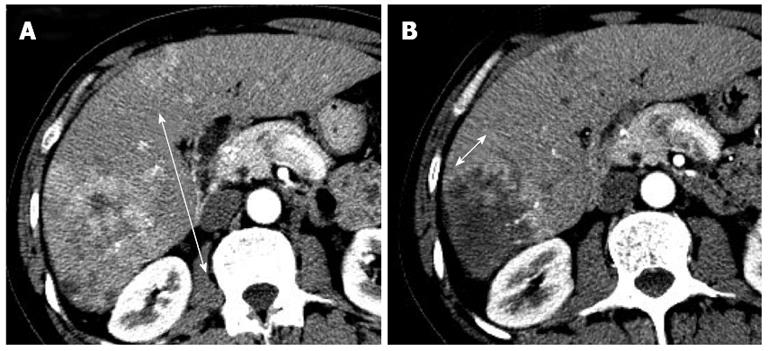
Recent studies have demonstrated poor correlations between the clinical benefit provided by targeted therapy agents or LRTs and conventional morphologic tumor burden analysis[11-14]. Unlike cytotoxic agents that may induce rapid tumor shrinkage, targeted therapy agents are acknowledged to yield sustained tumor stabilization and delay tumor progression. For example, antiangiogenic agents can reduce tumor vascularization, provoke areas of necrosis, and sometimes cause cavitation in solid tumors. These peculiar features have been reported with bevacizumab, sorafenib, and sunitinib in HCC[11,15-18]. In addition, the main objective of all effective LRTs is to induce necrosis of the tumor regardless of the shrinkage of the lesion. Therefore, in 2000, a panel of experts on HCC of the European Association for the Study of Liver (EASL) amended the response criteria to take into account tumor necrosis induced by treatment[19]. In 2008, The American Association for the Study of Liver Disease developed a set of guidelines that included a formal modification of the response assessment based on the RECIST criteria and aimed to translate into the concept of viable tumor (tumoral tissue showing arterial uptake in the arterial phase of the contrast-enhanced imaging techniques), which are referred to as modified RECIST (mRECIST) criteria (Figure 1)[20,21]. These criteria are summarized in Table 1. Forner et al[13] reported that overall response rates of 21.8% for RECIST criteria and 81.8% for EASL in 55 HCC patients treated with a variety of LRTs. Simillar findings about overall response rates were reported by Keppke et al[22] (RECIST 23%, WHO 26%, and necrotic area 59%), Riaz et al[23] (RECIST 42.4%, WHO 42.4%, and EASL 70.2%), and Prajapati et al[24] (RECIST 10.8%, WHO 4.1%, EASL 39.2%, and mRECIST 52.5%).
| WHO | RECIST 1.1 | EASL | mRECIST | |
| Complete response (CR) | Disappearance of all lesions | Disappearance of all lesions and pathologic lymph nodes | Disappearance of intratumoral areterial enhancement | Disappearance of all lesions and pathologic lymph nodes |
| Partial response (PR) | ≥ 50% decrease in the sum of the area (longest diameters multiplied by longest perpendicular diameters) | ≥ 30% decrease in the sum of the longest diameters | ≥ 50% decrease in the sum of the arterial enhancing areas (longest diameters multiplied by longest perpendicular diameters) | At least a 30% decrease in the sum of diameters viable (enhancing) target lesions, taking as reference the baseline sum of the target lesions |
| Stable disease (SD) | Neither PR nor PD | Neither PR nor PD | Neither PR nor PD | Neither PR nor PD |
| Progressive disease (PD) | ≥ 25% increase in the sum of the area | ≥ 20% increase in the sum of the longest diameters and ≥ 5 mm absolute increase in the sum of the longest diameters | ≥ 25% increase in the size of the arterial enhancing areas or development of a new lesions | ≥ 20% increase in the sum of diameters of viable target lesions recorded since treatment started or development of new lesions |
A question then arises which response criteria have the strong association with survival. Previous reports have shown that WHO, RECIST, and EASL responses are associated with improved survival[23,25], but these studies didn’t make the comparison at a single time point. In the phase II study of brivanib in advanced HCC, mRECIST was able to demonstrate a higher response and disease control rate and longer time to progression than the WHO criteria[26]. In a recent retrospective study of HCC patients treated with sorafenib, patients categorized as responder according to mRECIST had a longer overall survival (OS) than non-responder[27]. Prajapati et al[24] reported that mRECIST and EASL had significant correlation with survival, whereas WHO and RECIST 1.1 had poor correlation. Another key issue is that radiological assessments with EASL and mRECIST can be carried out at an early time point, in comparison with WHO and RECIST[12,22,23]. Therefore, response evaluation based on the concept of viable tumor may be valuable for making early decisions regarding further therapy.
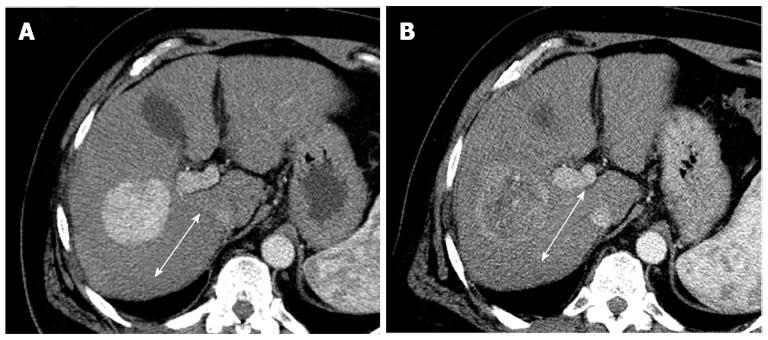
The tumor density analysis based on contrast enhanced CT attenuation measurement can serve as an additional method for response assessment in solid tumors[28]. Choi et al[28] reported that gastrointestinal stromal tumors treated with imatinib mesylate, reduced tumor density on the portal venous phase CT, which had a correlation with the tumor necrosis, or cystic or myxoid degeneration without changes in tumor size. The tumor density is measured by drawing a region of interest (ROI) circumscribing the boundary of the tumor in the portal venous phase[29]. In gastrointestinal stromal tumors, a decrease in tumor Housefield units > 15% correlated with progression free survival[30]. In HCC, a recent studies showed that tumor density measurement on the portal venous phase CT images was more sensitive than RECIST in detecting patients with longer time to progression after sunitinib therapy (Figure 2)[31].
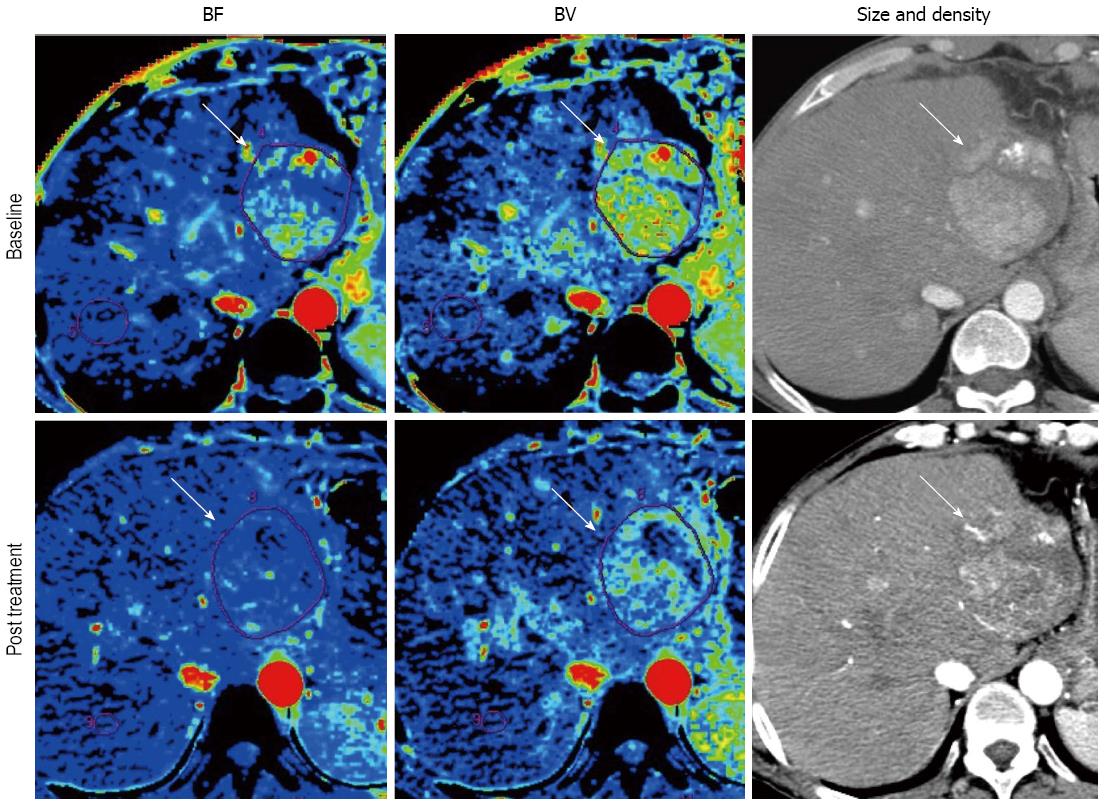
As discussed earlier, the morphologic tumor burden assessment has a difficulty in distinguishing viable tumor from necrotic or fibrotic tissue because molecular targeted agents suppress tumor growth by downregulating angiogenesis without causing much morphologic change. In this sense, the investigation for reliable quantitative assessment of therapeutic response including blood surrogate parameters, metabolic imaging and functional imaging has been desired[4,5]. Perfusion technique, which enables quantification of tumor vascularity by measuring the temporal changes in tissue density following intravenous contrast administration, are readily incorporated into the existing CT and MRI protocols that continue to provide the mainstay for anatomical imaging in oncology[32]. Most scanners now come equipped with sophisticated hardware platforms coupled with powerful and user-friendly software packages for tissue perfusion analysis. Perfusion parameters are dependent on the scan protocol and the mathematical model for perfusion analysis[33,34], but the commonly described perfusion CT parameters include blood flow (BF), blood volume (BV), mean transit time (MTT), and permeability surface area product[15,16,34]. Similarly for dynamic contrast-enhanced (DCE)-MRI, transfer constant (Ktrans) is the most accepted quantitative surrogate end point from compartment models[35-38]. Several studies have demonstrated the value of perfusion imaging for monitoring the effect of antiangiogenic agents advocating various imaging tools in various solid tumors[14-17,39-43]. Several papers reported that BF or BV decreased even after 2 wk of antiangiogenic therapy (Figure 3)[15,16]. Moreover, perfusion imaging has a potential to be a biomarker of antiangiogenic therapy[14,16,41-43]. In perfusion CT, Jiang et al[16] demonstrated that HCC with higher baseline MTT correlated with favorable clinical outcome. In DCE-MRI study of renal cell carcinoma, high baseline Ktrans and reduction in Ktrans after treatment were related to progression free survival (PFS)[41,42]. In advanced HCC, DCE-MRI demonstrated reduction in Ktrans during antiangiogenic treatment and the change of Ktrans during treatment was related to better PFS and OS in clinical trials of tyrosine kinase inhibitors[14,17,43].
Considering the accessibility and availability, Perfusion CT is superior to DCE-MRI. However, relatively high radiation dose and limited coverage of the anatomy are two major draws backs of perfusion CT. Therefore, several efforts are being made with low dose scanning technique[34]. In addition, there is no consensus on a scanning protocol or a mathematical model in abdominal lesion. The definition of the tumor ROI and the acquisition time is also a subject to similar consideration[44,45].
On the other hand, DCE-MRI has the advantage of lack of ionizing radiation, good spatial resolution and soft-tissue contrast. However, it is one of the most expensive and still technically challenging imaging modalities, requiring longer image acquisition times and provides smaller interscan reproducibility, as compared with CT[46,47]. DCE-MRI also lacks the standard protocol and the established response evaluation criteria.
Regardless of these limitations, perfusion technique must be a potentially powerful tool for HCC patient management, which may enable prediction or early detection of therapy responder.
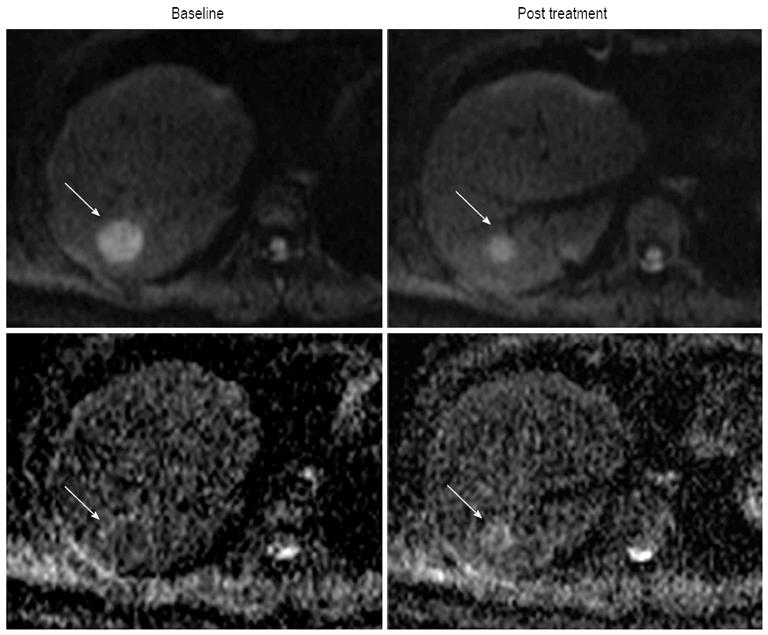
Molecular diffusion, or Brownian motion, was first formally described by Einstein[48] in 1905. Various tissue types have unique diffusion characteristics, as measured by the apparent diffusion coefficient (ADC), which can be calculated by the diffusion-weighted imaging (DWI) measurements acquired with a different gradient duration and amplitude (b-values). The movement of water molecules in biological tissues within the body is typically limited by interactions with cell membranes macromolecules, and fibers in tissue compartments. Therefore, DWI has been suggested as a tool to distinguish different tissue compartments and detect changes in cellular tissue structures and viability, which could be used to monitor the response to treatment. DWI has been discussed as cancer biomarker in a consensus meeting and a publication on consensus and recommendations for DWI as a cancer biomarker has been published recently highlighting the potential of this promising technique in cancer patients[49]. In lung cancer, a previous study reported that ADC values differ based on histological type, which suggested a possible correlation between ADC values and tumor characteristics, such as histology, response to therapies and prognosis[50]. Monitoring effectiveness of treatment is often challenging, especially following liver directed therapy. In HCC, the usefulness of DWI in the evaluation of therapeutic efficacy after targeted therapy or TACE has already been reported in several studies[51-55]. Some of those studies reported that the ADC value in HCC showed significant increases after TACE[51-53]. Yuan et al[55] reported that high baseline ADC value of HCC could predict poor response to TACE and that responding lesions had a significant increase in %ADC values than nonresponding during TACE. They demonstrated that an alteration %ADC value ≥ 16.21% could be used to identify HCC to early response to chemoembolization. In HCC treated with an antiangiogenic agent (sorafenib), Schraml et al[54] reported that early decrease in ADC of tumor after therapy was followed by an increase (Figure 4). However, there are some limitations regarding ADC values reproducibility, which depend on magnetic field strength, technical factors (e.g., b-value selection) and on the ROI localization on ADC maps[56,57]. In addition, particularly in abdomen, DWI still represents a technical challenge because of the strong influence of motion caused by breathing and vascular pulsation, resulting in image artifacts that may lead to inaccurate ADC measurements[58]. Nevertheless, DWI is one of the promising techniques for the noninvasive assessment of tumor burden. Future studies are necessary to correlate the time course of ADC changes with HCC therapy response, and additional technical developments are necessary to improve DWI quality and spatial resolution.
PET is a quantitative imaging modality using various tracers such as 18F-fluorodeoxyglucose (18F-FDG)[59-63], 11C-acetate (11C-Act)[64-67], 11C- or 18F-F-choline (11C-Cho, 18F-F-Cho)[68] and 18F-fluorothymidine (18F-FLT)[69] to assess metabolism, lipogenesis, cellular membrane metabolism and proliferation respectively.
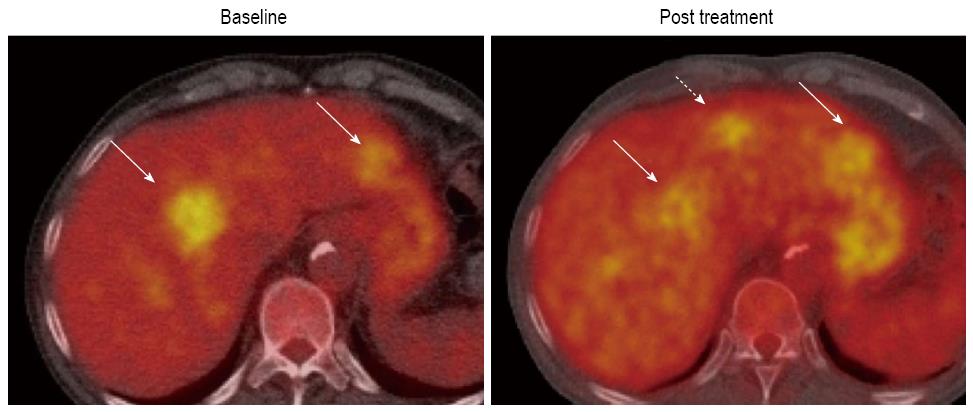
18F-FDG, which can be used for assessing glucose metabolism of tumors, is the most widely available clinical PET tracer (Figure 5). Generally, malignant tumors show increased 18F-FDG uptake due to the increased number of glucose transporters and the increased hexokinase activity. Nevertheless, FDG-PET shows poor sensitivity for the detection of HCC with reports ranging from 50% to 55%[70-74]. In spite of the poor sensitivity of 18F-FDG PET in HCC, Song et al[75] reported that the increase of 18F-FDG uptake in HCC was significantly associated with tumor burdens such as size and number of tumors, and they concluded that 18F-FDG PET could provide effective information on the prognosis of the treatment response. In addition, it has been demonstrated that 18F-FDG uptake after TACE might be a favorable marker to assess tumor viability after TACE[66-76]. Similar findings have been reported in detecting local tumor progression following radiofrequency ablation of HCC[77].
Despite the rapid integration of PET with 18F-FDG into clinical practice, there has been relatively little systematic integration of PET into clinical trials of new cancer treatments. Given the clinical importance and quantitative nature of PET, it is important to have methods to allow inclusion of PET response criteria into clinical trials. Therefore, the European Organization for Research and Treatment of Cancer (EORTC) has defined response assessment criteria for PET in 1999[78]. Although some use the EORTC criteria, methods for PET performance and interpretation are typically highly variable across studies and typically only exploratory. Therefore, in 2009, Wahl et al[79] described the Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) 1.0 to standardize therapy-monitoring method with PET. They classified responses by use of percentage changes in SUVs in the “hottest” lesions per scan. The basics of PERCIST 1.0 are shown in Table 2, where they are contrasted with the EORTC criteria. It is clear that further efforts are needed to validate usefulness of SUV as a sensitive biomarker to assess tumor burden, response and clinical outcome. At present, PET still plays a small role in imaging assessment of HCC tumor burden, compared with other modalities, but tumor-specific tracers may be the key in future.
| EORTC | PERCIST | |
| CMR | Complete resolution of 18F-FDG uptake within the tumor volume so that it is indistinguishable from surrounding normal tissue | Complete resolution of 18F-FDG uptake within measurable target lesion so that the liver activity was less than the mean and indistinguishable from surrounding background blood-pool levels plus disappearance of all other lesions to background blood pool levels and appearance of no new 18F-FDG-avid lesions |
| PMR | Minimum 15%-25% reduction in tumor 18F-FDG SUV after 1 chemotherapy cycle and > 25% reduction after ≥ 1 treatment cycle; reduction in extent of tumor 18F-FDG uptake not required | ≥ 30% relative and ≥ 0.8 SUL unit absolute reduction in target measurable tumor 18F-FDG SUL peak and no increase > 30% in SUL or size (per RECIST) of target or nontarget lesions or appearance of new lesions; reduction in extent of tumor 18F-FDG uptake not required ≥ 30% decrease in the sum of the longest diameters |
| SMD | < 25% increase or < 15% decrease in tumor 18F-FDG SUV and no visible increase in extent of 18F-FDG tumor uptake (> 20% in the longest dimension) | Not CMR, PMR, nor PMD |
| PMD | > 25% increase in 18F-FDG tumor SUV within the tumor region defined on the baseline examination or visible increase in the extent of 18F-FDG tumor uptake (> 20% in the longest dimension) or appearance of new 18F-FDG uptake in metastatic lesions | > 30% increase in 18F-FDG SUL peak, with > 0.8 SUL unit increase in tumor SUV peak from baseline scan in pattern typical of tumor and not of infection/treatment effect or visible increase in extent of 18F-FDG tumor uptake (75% in total lesion glycolysis volume with no decline in SUL) or new 18F-FDG-avid lesions typical of cancer and not related to treatment effect or infection |
Accurate tumor burden assessment is a critical component of patient management and the investigation of new therapies. Morphological tumor burden analysis has been served as golden standard. However, with the increasing clinical use of novel biologic targeted agents or LRTs, morphological analysis confronted limitations, and new methods to assess tumor burden were desired. Advances in software and hardware of imaging technique enable us to assess tumor function such as viability, vascular physiology, or metabolism. Since targeted therapy or LRTs can induce biological changes much earlier than morphological changes, these functional tumor burden analyses are very promising. However, some of them have not gone thorough all steps for standardization and validation. Nevertheless, these new techniques and criteria will play an important role in the cancer management, and provide each patient more tailored therapy.
| 1. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [PubMed] |
| 2. | Nishino M, Jackman DM, Hatabu H, Yeap BY, Cioffredi LA, Yap JT, Jänne PA, Johnson BE, Van den Abbeele AD. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol. 2010;195:W221-W228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 22770] [Article Influence: 1339.4] [Reference Citation Analysis (1)] |
| 4. | Taylor M, Rössler J, Geoerger B, Vassal G, Farace F. New anti-angiogenic strategies in pediatric solid malignancies: agents and biomarkers of a near future. Expert Opin Investig Drugs. 2010;19:859-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Murakami T, Imai Y, Okada M, Hyodo T, Lee WJ, Kim MJ, Kim T, Choi BI. Ultrasonography, computed tomography and magnetic resonance imaging of hepatocellular carcinoma: toward improved treatment decisions. Oncology. 2011;81 Suppl 1:86-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging. 2013;12:530-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Shields AF. Positron emission tomography measurement of tumor metabolism and growth: its expanding role in oncology. Mol Imaging Biol. 2006;8:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. [PubMed] |
| 9. | Sohaib SA, Turner B, Hanson JA, Farquharson M, Oliver RT, Reznek RH. CT assessment of tumour response to treatment: comparison of linear, cross-sectional and volumetric measures of tumour size. Br J Radiol. 2000;73:1178-1184. [PubMed] |
| 10. | Welsh JL, Bodeker K, Fallon E, Bhatia SK, Buatti JM, Cullen JJ. Comparison of response evaluation criteria in solid tumors with volumetric measurements for estimation of tumor burden in pancreatic adenocarcinoma and hepatocellular carcinoma. Am J Surg. 2012;204:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10523] [Article Influence: 584.6] [Reference Citation Analysis (9)] |
| 12. | Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, Meyer T. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 283] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 13. | Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, Reig M, Bianchi L, Llovet JM, Bruix J. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 340] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 14. | Sahani DV, Jiang T, Hayano K, Duda DG, Catalano OA, Ancukiewicz M, Jain RK, Zhu AX. Magnetic resonance imaging biomarkers in hepatocellular carcinoma: association with response and circulating biomarkers after sunitinib therapy. J Hematol Oncol. 2013;6:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Zhu AX, Holalkere NS, Muzikansky A, Horgan K, Sahani DV. Early antiangiogenic activity of bevacizumab evaluated by computed tomography perfusion scan in patients with advanced hepatocellular carcinoma. Oncologist. 2008;13:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Jiang T, Kambadakone A, Kulkarni NM, Zhu AX, Sahani DV. Monitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST). Invest Radiol. 2012;47:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027-3035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 18. | Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293-4300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 914] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 19. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 20. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3431] [Article Influence: 214.4] [Reference Citation Analysis (43)] |
| 21. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1332] [Article Influence: 74.0] [Reference Citation Analysis (1)] |
| 22. | Keppke AL, Salem R, Reddy D, Huang J, Jin J, Larson AC, Miller FH. Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. AJR Am J Roentgenol. 2007;188:768-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Riaz A, Miller FH, Kulik LM, Nikolaidis P, Yaghmai V, Lewandowski RJ, Mulcahy MF, Ryu RK, Sato KT, Gupta R. Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. JAMA. 2010;303:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 24. | Prajapati HJ, Spivey JR, Hanish SI, El-Rayes BF, Kauh JS, Chen Z, Kim HS. mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE). Ann Oncol. 2013;24:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2654] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 26. | Park JW, Finn RS, Kim JS, Karwal M, Li RK, Ismail F, Thomas M, Harris R, Baudelet C, Walters I. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2011;17:1973-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Edeline J, Boucher E, Rolland Y, Vauléon E, Pracht M, Perrin C, Le Roux C, Raoul JL. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 28. | Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1137] [Article Influence: 59.8] [Reference Citation Analysis (1)] |
| 29. | Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, Macapinlac HA, Podoloff DA. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004;183:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 349] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 30. | Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Charnsangavej C. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 415] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 31. | Faivre S, Zappa M, Vilgrain V, Boucher E, Douillard JY, Lim HY, Kim JS, Im SA, Kang YK, Bouattour M. Changes in tumor density in patients with advanced hepatocellular carcinoma treated with sunitinib. Clin Cancer Res. 2011;17:4504-4512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Miles KA. Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol. 2003;76 Spec No 1:S36-S42. [PubMed] |
| 33. | Miles KA, Hayball M, Dixon AK. Colour perfusion imaging: a new application of computed tomography. Lancet. 1991;337:643-645. [PubMed] |
| 34. | Kambadakone AR, Sahani DV. Body perfusion CT: technique, clinical applications, and advances. Radiol Clin North Am. 2009;47:161-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Morgan B, Thomas AL, Drevs J, Hennig J, Buchert M, Jivan A, Horsfield MA, Mross K, Ball HA, Lee L. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol. 2003;21:3955-3964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 496] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 36. | van Laarhoven HW, Rijpkema M, Punt CJ, Ruers TJ, Hendriks JC, Barentsz JO, Heerschap A. Method for quantitation of dynamic MRI contrast agent uptake in colorectal liver metastases. J Magn Reson Imaging. 2003;18:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Jiang T, Zhu AX, Sahani DV. Established and novel imaging biomarkers for assessing response to therapy in hepatocellular carcinoma. J Hepatol. 2013;58:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Goh V, Padhani AR. Imaging tumor angiogenesis: functional assessment using MDCT or MRI? Abdom Imaging. 2006;31:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1454] [Article Influence: 66.1] [Reference Citation Analysis (2)] |
| 40. | Lind JS, Meijerink MR, Dingemans AM, van Kuijk C, Ollers MC, de Ruysscher D, Postmus PE, Smit EF. Dynamic contrast-enhanced CT in patients treated with sorafenib and erlotinib for non-small cell lung cancer: a new method of monitoring treatment? Eur Radiol. 2010;20:2890-2898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Hahn OM, Yang C, Medved M, Karczmar G, Kistner E, Karrison T, Manchen E, Mitchell M, Ratain MJ, Stadler WM. Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol. 2008;26:4572-4578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 42. | Flaherty KT, Rosen MA, Heitjan DF, Gallagher ML, Schwartz B, Schnall MD, O’Dwyer PJ. Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther. 2008;7:496-501. [PubMed] |
| 43. | Hsu CY, Shen YC, Yu CW, Hsu C, Hu FC, Hsu CH, Chen BB, Wei SY, Cheng AL, Shih TT. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J Hepatol. 2011;55:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Goh V, Halligan S, Hugill JA, Gartner L, Bartram CI. Quantitative colorectal cancer perfusion measurement using dynamic contrast-enhanced multidetector-row computed tomography: effect of acquisition time and implications for protocols. J Comput Assist Tomogr. 2005;29:59-63. [PubMed] |
| 45. | Ng CS, Chandler AG, Wei W, Herron DH, Anderson EF, Kurzrock R, Charnsangavej C. Reproducibility of CT perfusion parameters in liver tumors and normal liver. Radiology. 2011;260:762-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Lavini C, Verhoeff JJ. Reproducibility of the gadolinium concentration measurements and of the fitting parameters of the vascular input function in the superior sagittal sinus in a patient population. Magn Reson Imaging. 2010;28:1420-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Vlieger EJ, Lavini C, Majoie CB, den Heeten GJ. Reproducibility of functional MR imaging results using two different MR systems. AJNR Am J Neuroradiol. 2003;24:652-657. [PubMed] |
| 48. | Einstein A. Investigations on the Theory of the Brownian Movement. New York, NY: Dover Publications 1956; 17. |
| 49. | Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102-125. [PubMed] |
| 50. | Matoba M, Tonami H, Kondou T, Yokota H, Higashi K, Toga H, Sakuma T. Lung carcinoma: diffusion-weighted mr imaging--preliminary evaluation with apparent diffusion coefficient. Radiology. 2007;243:570-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 51. | Kamel IR, Bluemke DA, Ramsey D, Abusedera M, Torbenson M, Eng J, Szarf G, Geschwind JF. Role of diffusion-weighted imaging in estimating tumor necrosis after chemoembolization of hepatocellular carcinoma. AJR Am J Roentgenol. 2003;181:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Goshima S, Kanematsu M, Kondo H, Yokoyama R, Tsuge Y, Shiratori Y, Onozuka M, Moriyama N. Evaluating local hepatocellular carcinoma recurrence post-transcatheter arterial chemoembolization: is diffusion-weighted MRI reliable as an indicator? J Magn Reson Imaging. 2008;27:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Chen CY, Li CW, Kuo YT, Jaw TS, Wu DK, Jao JC, Hsu JS, Liu GC. Early response of hepatocellular carcinoma to transcatheter arterial chemoembolization: choline levels and MR diffusion constants--initial experience. Radiology. 2006;239:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 54. | Schraml C, Schwenzer NF, Martirosian P, Bitzer M, Lauer U, Claussen CD, Horger M. Diffusion-weighted MRI of advanced hepatocellular carcinoma during sorafenib treatment: initial results. AJR Am J Roentgenol. 2009;193:W301-W307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Yuan Z, Ye XD, Dong S, Xu LC, Xu XY, Liu SY, Xiao XS. Role of magnetic resonance diffusion-weighted imaging in evaluating response after chemoembolization of hepatocellular carcinoma. Eur J Radiol. 2010;75:e9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Dale BM, Braithwaite AC, Boll DT, Merkle EM. Field strength and diffusion encoding technique affect the apparent diffusion coefficient measurements in diffusion-weighted imaging of the abdomen. Invest Radiol. 2010;45:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 57. | Ogura A, Hayakawa K, Miyati T, Maeda F. Imaging parameter effects in apparent diffusion coefficient determination of magnetic resonance imaging. Eur J Radiol. 2011;77:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Marcus CD, Ladam-Marcus V, Cucu C, Bouché O, Lucas L, Hoeffel C. Imaging techniques to evaluate the response to treatment in oncology: current standards and perspectives. Crit Rev Oncol Hematol. 2009;72:217-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Talbot JN, Gutman F, Fartoux L, Grange JD, Ganne N, Kerrou K, Grahek D, Montravers F, Poupon R, Rosmorduc O. PET/CT in patients with hepatocellular carcinoma using [(18)F]fluorocholine: preliminary comparison with [(18)F]FDG PET/CT. Eur J Nucl Med Mol Imaging. 2006;33:1285-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Talbot JN, Fartoux L, Balogova S, Nataf V, Kerrou K, Gutman F, Huchet V, Ancel D, Grange JD, Rosmorduc O. Detection of hepatocellular carcinoma with PET/CT: a prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. J Nucl Med. 2010;51:1699-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 61. | Yamamoto Y, Nishiyama Y, Kameyama R, Okano K, Kashiwagi H, Deguchi A, Kaji M, Ohkawa M. Detection of hepatocellular carcinoma using 11C-choline PET: comparison with 18F-FDG PET. J Nucl Med. 2008;49:1245-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 62. | Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, Lee WJ, Kim CM, Nam BH. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008;49:1912-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 63. | Ho CL, Chen S, Yeung DW, Cheng TK. Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma. J Nucl Med. 2007;48:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 64. | Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213-221. [PubMed] |
| 65. | Hwang KH, Choi DJ, Lee SY, Lee MK, Choe W. Evaluation of patients with hepatocellular carcinomas using [(11)C]acetate and [(18)F]FDG PET/CT: A preliminary study. Appl Radiat Isot. 2009;67:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Salem N, Kuang Y, Corn D, Erokwu B, Kolthammer JA, Tian H, Wu C, Wang F, Wang Y, Lee Z. [(Methyl)1-(11)c]-acetate metabolism in hepatocellular carcinoma. Mol Imaging Biol. 2011;13:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Yun M, Bang SH, Kim JW, Park JY, Kim KS, Lee JD. The importance of acetyl coenzyme A synthetase for 11C-acetate uptake and cell survival in hepatocellular carcinoma. J Nucl Med. 2009;50:1222-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 68. | Salem N, Kuang Y, Wang F, Maclennan GT, Lee Z. PET imaging of hepatocellular carcinoma with 2-deoxy-2[18F]fluoro-D-glucose, 6-deoxy-6[18F] fluoro-D-glucose, [1-11C]-acetate and [N-methyl-11C]-choline. Q J Nucl Med Mol Imaging. 2009;53:144-156. [PubMed] |
| 69. | Eckel F, Herrmann K, Schmidt S, Hillerer C, Wieder HA, Krause BJ, Schuster T, Langer R, Wester HJ, Schmid RM. Imaging of proliferation in hepatocellular carcinoma with the in vivo marker 18F-fluorothymidine. J Nucl Med. 2009;50:1441-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Takayasu K, Arii S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, Sato M, Yamanaka N, Shimamura Y, Ohto M. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 71. | Guan YS, Sun L, Zhou XP, Li X, Zheng XH. Hepatocellular carcinoma treated with interventional procedures: CT and MRI follow-up. World J Gastroenterol. 2004;10:3543-3548. [PubMed] |
| 72. | Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991;32:623-48; discussion 649-50. [PubMed] |
| 73. | Okazumi S, Isono K, Enomoto K, Kikuchi T, Ozaki M, Yamamoto H, Hayashi H, Asano T, Ryu M. Evaluation of liver tumors using fluorine-18-fluorodeoxyglucose PET: characterization of tumor and assessment of effect of treatment. J Nucl Med. 1992;33:333-339. [PubMed] |
| 74. | Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, Collins BT, Di Bisceglie AM. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792-797. [PubMed] |
| 75. | Song MJ, Bae SH, Yoo IeR, Park CH, Jang JW, Chun HJ, Choi BG, Lee HG, Choi JY, Yoon SK. Predictive value of ¹⁸F-fluorodeoxyglucose PET/CT for transarterial chemolipiodolization of hepatocellular carcinoma. World J Gastroenterol. 2012;18:3215-3222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 76. | Torizuka T, Tamaki N, Inokuma T, Magata Y, Yonekura Y, Tanaka A, Yamaoka Y, Yamamoto K, Konishi J. Value of fluorine-18-FDG-PET to monitor hepatocellular carcinoma after interventional therapy. J Nucl Med. 1994;35:1965-1969. [PubMed] |
| 77. | Kuehl H, Stattaus J, Hertel S, Hunold P, Kaiser G, Bockisch A, Forsting M. Mid-term outcome of positron emission tomography/computed tomography-assisted radiofrequency ablation in primary and secondary liver tumours--a single-centre experience. Clin Oncol (R Coll Radiol). 2008;20:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773-1782. [PubMed] |
| 79. | Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S-150S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2916] [Cited by in RCA: 2895] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
P- Reviewers: Karatag O, Sinakos E S- Editor: Gou SX L- Editor: A E- Editor: Wang CH