Published online Dec 15, 1996. doi: 10.3748/wjg.v2.i4.192
Revised: August 15, 1996
Accepted: October 10, 1996
Published online: December 15, 1996
AIM: To investigate the protective effect of various doses of selenium (Se) on cadmium (Cd)-induced hepatic cell damage in pregnant rats.
METHODS: The changes in the microstructure and ultrastructure of hepatic cells were observed by microscopy and electron microscopy.
RESULTS: Groups of 16 μg/mL and 8 μg/mL Se had no remarkable protective effects on Cd-induced hepatic cell damage in pregnant rats, while the microstructure and ultrastructure of hepatic cells in the 4 μg/mL Se group were nearly normal.
CONCLUSION: The results show that the antagonism of Se on Cd-induced hepatic cell damage in pregnant rats is related to the dose of Se given. The addictive action of Se and Cd toxicity was found in group B, but group D showed significant protection against Cd-induced damage of hepatic cells.
- Citation: Han GA, Jiang HM, Zhen XM, Zhou HJ, Xu CZ, Tian JX. Protective effect of various doses of selenium on cadmium-induced hepatic cell damage in pregnant rats. World J Gastroenterol 1996; 2(4): 192-193
- URL: https://www.wjgnet.com/1007-9327/full/v2/i4/192.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i4.192
Selenium (Se) is able to inhibit the toxicity of cadmium (Cd). For instance, it is able to antagonize Cd-induced malformation and absorption[1], but this is related to the way, the dose and the time that Se is given. The safety range of Se is very narrow. To investigate the protective action of various doses of Se taken orally on the day of gestation on Cd-induced hepatic cell damage in pregnant rats, and to select the effective and safe dose of Se, we performed this study.
Sixty Wistar rats weighing 220-250 g were provided by the Experimental Animal Center of Shandong Medical University with a sex ratio of two females to one male. Na2SeO3 (AR grade) was used, and the rest materials were in the same way as described in the preceding paper.
Groups B, C and D were given various doses of Se. The rats in these groups were given water containing 16 μg/mL, 8 μg/mL and 4 μg/mL Se, respectively. The rest was dealt with in the same way as that in the preceding paper.
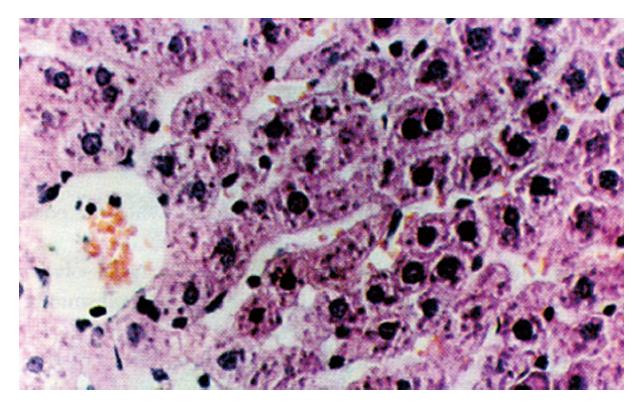
The results of groups A and E were the same as those in the preceding paper. Group B revealed edema of hepatic cells, sporadic acidophilic degeneration, fragmented hepatic cells and bridging necrosis. The infiltration of lymphocytes was found in the necrotic area, which caused the disturbance in the hepatic lobular structure. In group C, hepatic cells showed edema and most of the lobular necrosis foci were visible in the mid-lobular area with infiltration of lymphocytes. In group D, hepatic cells in the mid-lobular area showed slight edema and necrosis of hepatic cells was rarely visible (Figure 1).
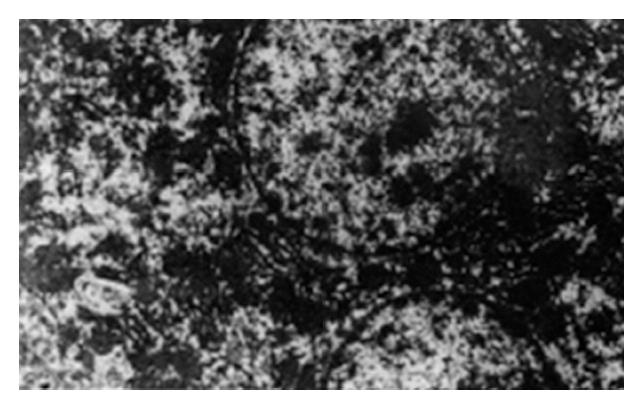
In group B, the hepatic cells were dissolved with a lot of vacuoles, the karyotheca of the hepatic cell was not clear, mitochondria swelled greatly, cristae vanished and endoplasmic reticulum expanded seriously; in group C, the karyotheca of hepatic cell was irregular, few vacuoles were visible in the cytoplasm, parts of mitochondria degenerated and endoplasmic reticulum expanded slightly; in group D, the karyotheca of the hepatic cells was clear and regular, double nuclei were visible, the composition of subcellular structures in the cytoplasm was basically normal, most of the membrane and cristae of mitochondria were clear and the endoplasmic reticulum was normal (Figure 2).
In the organism, Se has antagonism against the toxicity of Cd, mercury (Hg) and arsenic (As). Gunn et al found that among the few known substances having antagonistic action on Cd-induced testicle damage, such as Zn, cobalt (Co), BAL and cysteine, Se has the most effective protective action. Its effect is 100 times more than that of Zn[2,3]. The mitochondrium is a sensitive and most easily damaged cell organ and it can exhibit the severity of cell damage. The order of mitochondrial damage severity for all groups is: B > A > C > D. Group B exhibited an addictive effect of Se and Cd toxicity, while group D showed basically normal microstructure and ultrastructure of liver cells, which suggests that a low dose of Se had remarkable protective action on Cd-induced damage of hepatic cells. However, since the safety range of Se is rather narrow, it is extremely necessary to make further selection of a highly effective, low toxic and safe dose of Se. The antagonistic mechanism of Se on Cd-induced liver damage is as follows. First, Se can cause Cd to be redistributed in tissues and cells[1-3]. Taking Se 4 μg/mL orally could greatly lower Cd contents in the liver, kidney and blood of the pregnant rats by 50.3%, 53% and 78.03%, respectively, in comparison with the Cd-toxication group[1]. Second, taking Se orally at a low dose on the day of pregnancy has made Se in the rat body remain in normal equilibrium. At first, SeO3 is metabolized into selenide; when Cd comes into the body, Se in selenide form can combine Cd to form a compound, which decreases the concentration of free Cd and reduces Cd toxictiy[1,2]. Finally, taking Se 4 μg/mL orally can make the activity of GSH-Px enzyme in the liver, kidney and blood significantly higher than that in the Cd-toxic group and control group[1,4,5], and it can also inhibit Cd-induced lipid peroxidation and effectively clean up free radicals.
| 1. | Han GA, Jiang HM, Gao HF, Shi W. Study on antagonistic effect of selenium on cadmium-induced toxicity of embryo and maternal body. Shandong Yixueyuan Xuebao. 1994;32:5-8. |
| 2. | Yang CF. Research advance on interaction between selenium and cadmium. Guowai Yixue Weishengxue Fence. 1992;2:76-79. |
| 3. | Wang K. Trace elements in life science, 1st ed, Beijing: Measurement Publishing House of China, 1991; 214-225. . |
| 4. | Ip C. Interaction of selenium deficiency and fat intake in the regulation of enzymes associated with peroxide metabolism. Biol Trace Elem Res. 1983;5:139-146. [PubMed] |
| 5. | Jiang HM, Han GA, Li BQ, Gao HF, Yu YN, Sun SA. Study on antagonism of zinc and selenium to cadmiun-induced lipid peroxidation in rats. Shandong Yixueyuan Xuebao. 1994;32:125-129. |
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-).
S- Editor: Cao LB L- Editor: Wang TQ E- Editor: Zhang FF