Published online Sep 15, 1996. doi: 10.3748/wjg.v2.i3.176
Revised: July 25, 1996
Accepted: August 14, 1996
Published online: September 15, 1996
AIM: To investigate the relationship between liver functional impairment and sodium and water retention.
METHODS: An animal model of acute liver damage model was established by administering carbon tetrachloride (CCl4) to male Sprague-Dawley rats. Twenty-four and 48 h after CCl4 administration, the excretion of acute sodium and water load was measured. In controls, the excretion of acute sodium and water load was measured 24 h after administration of normal saline. In addition, the concentration of plasma caffeine was analyzed using high pressure liquid chromatography (HPLC). The half-life of plasma caffeine (Caf t1/2) served as a quantitative index of hepatic function. Plasma alanine aminotransferase (ALT) was measured using the Reitman method. Hepatic tissue sections from the same site were used for water content measurement and pathological observation. The serum and urinary sodium levels were measured with flame photometry.
RESULTS: Twenty-four hours after CCl4 administration, plasma ALT level (n = 6, 37.5 ± 12.6 → 189.4 ± 34.4 U, P < 0.01) and water content of hepatic tissue (n = 6, 70.0% ± 0.11% → 73.0% ± 1.0%, P < 0.01) were significantly increased, and Caf t1/2 was prolonged significantly (94.9 ± 18.9 → 326.4 ± 85.8 min, P < 0.01) compared to saline treated control. Renal function, as assessed by excretion of acute salt and water load, was significantly decreased (n = 6, Na+: 92.4% ± 14.1% → 50.1% ± 13.1%, P < 0.01; H2O: 86.3% ± 14.3% → 42.1% ± 8.8%, P < 0.01). The above indices had recovered somewhat 48 h later but were still markedly different from those of control. In addition, the relationships between Caf t1/2 and ALT (r = 0.752, P < 0.01) and between Caf t1/2 and excretory rate of sodium (r = 0.634, P < 0.05) and water remained significant (r = 0.612, P < 0.01) at 48 h.
CONCLUSION: Caf t1/2 is a good index to assess the degree of hepatic damage. Hepatic dysfunction may contribute to impairments in renal excretion following acute sodium and water load.
- Citation: Liu HQ, Ren CY, Jia LS, Yao XX, Ren XL. Effects of acute hepatic damage on natriuresis and water excretion after acute normal saline loading in rats. World J Gastroenterol 1996; 2(3): 176-178
- URL: https://www.wjgnet.com/1007-9327/full/v2/i3/176.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i3.176
Most data to date have shown that there is a significant relationship between hepatic functional damage and sodium retention, but it remains unclear whether acute liver dysfunction results in sodium and water retention. To understand further the relationship between liver function per se and sodium retention, we investigated renal excretion following acute salt and water load in a rat model of acute hepatic damage.
Eighteen male Sprague Dawley rats weighing 270-320 g were divided into three groups: control (n = 6), experimental group 1 (n = 6), and experimental group 2 (n = 6). Reduced salt chow (12.74 mmol/kg) and re-distilled water were available ad libitum. Three days later, carbon tetrachloride (CCl4) (0.02 mL/100 g body wt) was administered intraperitoneally to experimental groups, and normal saline (0.02 mL/100 g body wt) was administered to the control group. Acute normal saline loading was conducted in experimental groups 2 and 3 24 and 48 h after CCl4 administration, respectively, and 24 h after saline administration in the control.
Twelve hours after removing water and food, the animals received under urethane anesthesia normal saline (4 mL/100 g body wt) through the femoral vein. Urine was collected for 4 h and stored in an Eppendorf tube at -20 °C until analysis. One percent caffeine, 0.1 mL (1 mg/rat), was then administered to the femoral vein for 3 h. Four milliliters of blood were taken from the inferior vena cava and anti-agglutinated with heparin. The plasma was separated to measure caffeine, ALT, and sodium. At this time, liver tissues were taken for pathologic examination and water content calculation.
The plasma caffeine concentration was analyzed with high pressure liquid chromatography (HPLC). Plasma ALT was examined with Reitman method, and serum and urinary sodium levels were measured with flame photometry.
The Caf t1/2 was calculated using the following formula:
Caf t1/2 = ln2 × (t / ln (D / (avd × body wt) ln Ccaft)
where D = the dosage of caffeine (1 mg in this experiment); t = time from caffeine injection to blood collection (180 min); Ccaft = caffeine concentration when blood was taken (mg/L); adv = apparent distribution volume (0.64 mL/kg). Data for each parameter were compared between two groups with a t test and among the three groups with an F test. Regression analysis was used to correlate Caf t1/2 and water content in hepatic tissue, sodium excretory rate, water excretory rate, and plasma ALT. p values less than 0.05 were considered to be statistically significant.
In the control group, the hepatic cells were arranged regularly, and the plates radiated from the central vein to the portal canals. Twenty four hours after CCl4 administration, the hepatic tissue exhibited extensive necrosis, hepatocyte swelling, and vacuolization under light microscopy. In the 48 h group, hepatocyte necrosis, swelling, and vacuolization were still clearly seen but to a lesser extent than the 24 h group. Consistent with the histological changes in the hepatic tissue, water content was significantly higher in both experimental groups than the control group.
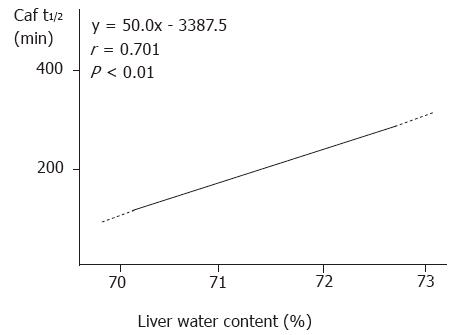
Following structural damage to the liver tissue, the capability of the liver to metabolize caffeine was impaired. The Caf t1/2 was prolonged significantly in the 24 h and 48 h groups compared to that in the control group (Table 1). Caf t1/2 and water content in hepatic tissue were significantly correlated (r = 0.701, P < 0.01, Figure 1).
Consistent with our findings on water content in hepatic tissue and Caf t1/2 , plasma levels of ALT rose significantly in both experimental groups. There was a significant positive correlation between ALT levels and Caf t1/2 (r = 0.753, P < 0.01).
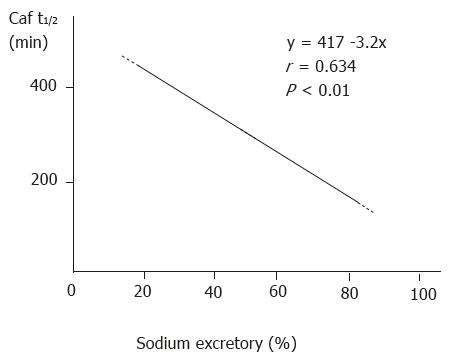
Following the impairment in liver function, the capability of the kidney to excrete acute water and sodium load declined significantly. Although this decline recovered somewhat in the 48 h group, it remained significantly different from that of the control (Table 2). The relationship between Caf t1/2 and renal excretory rate of sodium (Figure 2) and water was significantly correlated (r = -0.612, P < 0.01). There was no significant difference among the three groups in serum sodium level (F = 2.34, P > 0.05, Table 2).
Recently, different models of hepatic damage[1-3] have demonstrated that the decline in liver function is an important cause of salt and water retention. Caffeine is metabolized by hepatic cytochrome P-450, and the ability of the body to clear caffeine reflects metabolic function of the liver. Jost et al[4] showed that the clearance rate of plasma caffeine is in significant agreement with the aminopyrine breath test (r = 0.80, P < 0.01), prothrombin time (r = 0.59, P < 0.01), indo cyanogreen test (r = 0.51, P < 0.01), and galactose clearance rate (r = 0.46, P < 0.01). Therefore, use of Caf t1/2 to quantitate liver function is reliable; HPLC is an efficient and stable means to measure caffeine. Our results found that 24 and 48 h after CCl4 administration, Caf t1/2 was prolonged significantly. Changes in ALT levels and hepatic tissue water content paralleled Caf t1/2 . There was a significant relationship between Caf t1/2 and ALT and between Caf t1/2 and water content of hepatic tissue. These results illustrate that the decrease in liver function is linked to the degree of hepatic tissue damage. Thus, Caf t1/2 may be considered a sensitive index that reflects the degree of acute hepatic tissue damage.
Importantly, following pathological damage of the liver, the plasma Caf t1/2 was prolonged significantly and the ability of the kidney to excrete acute water and sodium load was declined significantly. Caf t1/2 and renal excretory rate of sodium and water were negatively correlated. These results are consistent with the report by Wong et al[5] and identity hepatic tissue damage and liver function decline as important contributors to salt and water retention.
| 1. | Liu HQ, Liang KH, Tang WX, Zhang WY. Renal nerves effect on early sodium retention in bile duct ligated diseases in rats. Zhonghua Xiaohua Zazhi. 1995;15:15-17. |
| 2. | Liu HQ, Liang KH, Tang WX, Zhang WY. Impairment of hepatic function, increment of plasma atrial natriuretic peptide and sodium retention in experimental cirrhosis. Tongji Yike Daxue Xuebao. 1993;23:169-171. |
| 3. | Liu HQ, Jia LS, Liang KH, Tang WX, Zhang WY. The changes of RAAS and sodium metabolism in the process of hepatic functional decline induced with 70% hepatectomy in rats. Zhongguo Bingli Shengli Zazhi. 1996;12:309-302. |
| 4. | Jost G, Wahlländer A, von Mandach U, Preisig R. Overnight salivary caffeine clearance: a liver function test suitable for routine use. Hepatology. 1987;7:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Wong F, Massie D, Hsu P, Dudley F. Renal response to a saline load in well-compensated alcoholic cirrhosis. Hepatology. 1994;20:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Original title:
S- Editor: Yang RC L- Editor: Filipodia E- Editor: Li RF