Published online Sep 15, 1996. doi: 10.3748/wjg.v2.i3.131
Revised: August 13, 1996
Accepted: September 2, 1996
Published online: September 15, 1996
AIM: To investigate the structure-activity relationship of pituitary adenylate cyclase activating polypeptide (PACAP) in guinea pig gallbladder using a synthetic PACAP/vasoactive intestinal peptide (VIP) hybrid.
METHODS: We synthesized PACAP-VIP hybrid peptides using the Fmoc strategy and a simultaneous multiple solid-phase peptide synthesizer. The peptides were tested in isolated guinea pig gallbladders using an improved horizontal type organ bath.
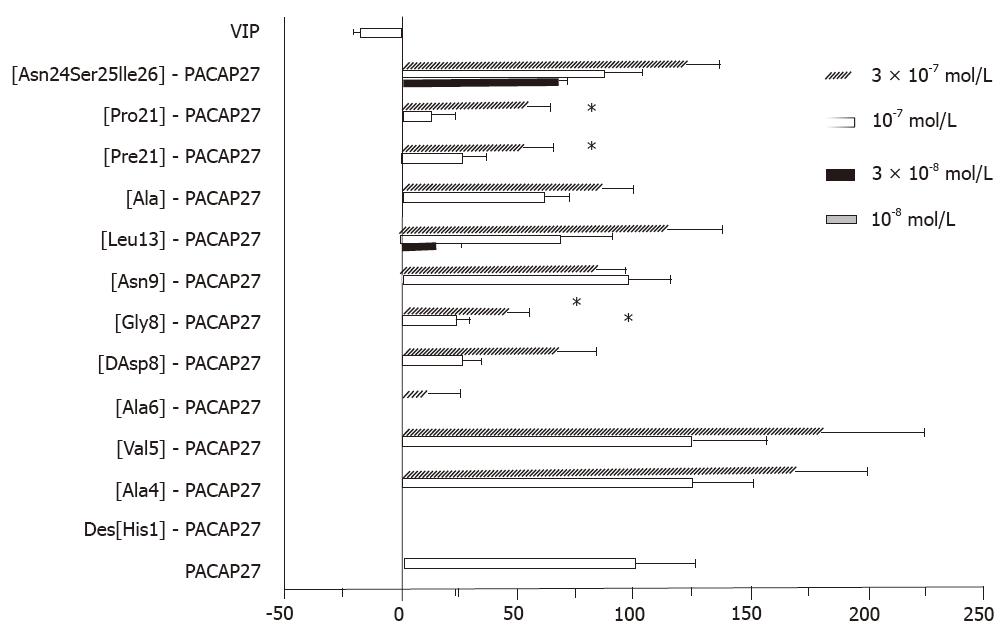
RESULTS: VIP induced relaxation of gallbladder smooth muscle strips, while PACAP27 contracted them. Amino acids at positions 4, 5, 9, and 24-26 were replaced without significant loss of activity. [Leu13]-PACAP27, a substitution in the α-helix domain, also had no significant loss in activity (P < 0.05). It was more potent than [Gly8]- and [DAsp8]-PACAP27 and could substitute peptides at position 21. Des-[His1] and [Ala6]-PACAP27 had no activity at 10-7 mol/L. [Gly8]-, [DAsp8]-, [Phe21]- and [Pro21]-PACAP27 at 10-7 mol/L had about 25% of the activity of PACAP27 at 10-7 mol/L (P < 0.05).
CONCLUSION: The N-terminal disordered region is more important than other regions for determining the physiological activity of PACAP in the guinea pig gallbladder.
- Citation: Wei MX, Naruse S, Nokihara K, Ozaki T, Ando E, Wray V. Structure-activity relationship of pituitary adenylate cyclase activating polypeptide. World J Gastroenterol 1996; 2(3): 131-133
- URL: https://www.wjgnet.com/1007-9327/full/v2/i3/131.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i3.131
Pituitary adenylate cyclase activating polypeptide (PACAP) is a neuropeptide structurally related to vasoactive intestinal polypeptide (VIP)[1]. It exists in the ovine hypothalamus in two molecular forms, PACAP38 and PACAP27. The terminal amino acid 27 residue of PACAP38 (PACAP27) has high sequence homology (68%) with VIP. Many biological actions of PACAP are very similar to those of VIP[1-3]; but we recently found that PACAP contracts the guinea pig gallbladder, while VIP relaxes it[4]. The guinea pig gallbladder is a good animal model system to study the structure-activity relationship of PACAP and VIP. In this investigation, we synthesized PACAP-VIP hybrid peptides using the Fmoc strategy and a simultaneous multiple solid phase peptide synthesizer, and we compared the effects of the peptides on gallbladder smooth muscle in vitro.
Considering the sequence homology of VIP-PACAP, NMR data, and computer-aided molecular graphics[5], positions 4, 5, 6, 8, 9, 10, 21 and 24-26 were selected as mutation points in this study (Figure 1). Peptides were synthesized using the Fmoc strategy and a simultaneous multiple solid phase peptide synthesizer (Model PSSM 8M, Shimadzu Corp.). After simultaneous cleavage with a trifluororacetic acid (TFA) cocktail, the desired crude peptides were easily purified by single step reversed phase-high performance liquid chromatography (RP-HPLC), and then characterized and shown to be homogeneous by HPLC, sequencing, amino acid analysis and liquid secondary ion mass spectrometry (LSIMS).
The peptides were tested in isolated guinea pig gallbladders using an improved horizontal type organ bath. Thirty male Hartley strain guinea pigs (450-580 g) were killed by exanguination. The gallbladders were excised and four 1.2 mm × 10 mm smooth muscle strips were prepared with the aid of a binocular microscope. The strips were suspended in an organ bath containing (4 mL) Krebs-Ringer solution (pH 7.4), maintained at 37 ± 5 °C, aerated with 95% O2 and 5% CO2, and connected to an isotonic displacement transducer. After setting the initial tension at 500 mg, the muscle strips were allowed to equilibrate for 60 min. The length of the strips was recorded using a pen recorder and a computer-controlled data acquisition system. An acetylcholine (Sigma, St. Louis.) control (10-8-10-4 mol/L), synthetic PACAP27, VIP, or the hybrid peptides were added to the organ bath at a concentration of 10-8, 3 × 10-8, 10-7, or 3 × 10-7mol/L. The gallbladder response to the peptides and control solution were standardized to the PACAP27 response. The maximal contraction induced by PACAP27 (10-7 mol/L) was taken as 100% contraction and the resting length as 0%.
The data (Figure 2) were expressed as means ± standard deviation (x-± s). One way factorial analysis of variance (ANOVA) and Fisher’s protected least significant difference (PLSD) multiple comparison test were used to compare the peak responses after adding the peptides. P < 0.05 was taken as the level of significance with n equal to the number of animals.
RESULTSVIP induced relaxation of gallbladder smooth muscle strips, while PACAP27 contracted them. Positions 4, 5, 9, and 24-26 could be replaced without significant loss in activity. [Leu13]-PACAP27, a substitution in the α-helix domain, also had no significant loss in activity (P > 0.05), was more potent than [Gly8]- or [DAsp8]-PACAP27, and could substitute peptides at position 21. Des-[His1]- and [Ala6]-PACAP27 had no activity at 10-7 mol/L. [Gly8]- [DAsp8]-, [Phe21]-, and [Pro21]-PACAP27 at 10-7 mol/L had about 25% of the activity of PACAP27 at 10-7 mol/L (P < 0.05).
The N-terminus of PACAP has a high sequence homology with VIP. At least two types of PACAP binding sites have been demonstrated in rat tissues. One is a type 1 site that is the dominant site in the hypothalamus, and is highly specific for PACAP. The other is a type 2 site that is the dominant site in the lungs and liver, and has almost equal affinities for both PACAP and VIP[6,7]. The action of PACAP on guinea pig gallbladder smooth muscle seemed to be directly via the activation of type 1 PACAP receptors because tetrodotoxin, atropine, and CCK antagonists failed to block it.
In our previous studies, the C-terminus (positions 9-26) of PACAP27, similar to VIP, had a propensity for α-helix formation in a hydrophobic environment, but the N-terminus from positions 1 to 8 did not have a well defined helical or strand structure[5]. PACAP substitutions in this region had a greater loss in activity than was seen with substitutions in other regions. These data suggest that the disordered region from positions 1 to 8 is very important for the recognition of PACAP by its receptor. Position 21 is also important, although it showed no significant loss in activity at a higher dose (3 × 10-7 mol/L).
In conclusion, the N-terminal disordered region is more important than other regions for the physiological activity of PACAP in guinea pig gallbladder.
| 1. | Arimura A. Pituitary adenylate cyclase activating polypeptide (PACAP): discovery and current status of research. Regul Pept. 1992;37:287-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 256] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Naruse S, Suzuki T, Ozaki T. The effect of pituitary adenylate cyclase activating polypeptide (PACAP) on exocrine pancreatic secretion in dogs. Pancreas. 1992;7:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Naruse S, Suzuki T, Ozaki T, Nokihara K. Vasodilator effect of pituitary adenylate cyclase activating polypeptide (PACAP) on femoral blood flow in dogs. Peptides. 1993;14:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Wei MX, Naruse S, Nakamura T, Nokihara K, Ozaki T. The effect of pituitary adenylate cyclase activating polypeptide (PACAP) 38 on gallbladder smooth muscle in vitro. Biomed Res. 1994;15:221-223. |
| 5. | Wray V, Kakoschke C, Nokihara K, Naruse S. Solution structure of pituitary adenylate cyclase activating polypeptide by nuclear magnetic resonance spectroscopy. Biochemistry. 1993;32:5832-5841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Gottschall PE, Tatsuno I, Miyata A, Arimura A. Characterization and distribution of binding sites for the hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide. Endocrinology. 1990;127:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 237] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Shivers BD, Görcs TJ, Gottschall PE, Arimura A. Two high affinity binding sites for pituitary adenylate cyclase-activating polypeptide have different tissue distributions. Endocrinology. 1991;128:3055-3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 240] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
Original title:
S- Editor: Cao LB L- Editor: Filipodia E- Editor: Li RF