Published online Mar 25, 1996. doi: 10.3748/wjg.v2.i1.44
Revised: October 11, 1995
Accepted: December 16, 1995
Published online: March 25, 1996
AIM: To investigate the relationship between spleen deficiency substance, spleen deficiency and gastric cancer.
METHODS: We adopted the IBAS 2000 image analysis system and the 501B SEM with 9100/60 energy chromatic dispersing X-ray analysis instrument, along with histologic chemistry and radioimmunoassay to investigate the ultramicrostructure, intestinal metaplasia subtypes, cAMP, DNA, trace element series and their oxides of patients with gastric cancer.
RESULTS: The incidence rates of gastric cancer, incomplete colonic intestinal metaplasia (IM) and background lesion (P < 0.05 0.001). Additionally, the patients with incomplete IM and those with small bowel IM have lower levels of cAMP, Zn, Cu, ZnO and CuO and higher levels of p53 gene expression in gastric mucosa than patients with complete IM and those with colonic IM (P < 0.05-0.001). However, the levels of p53 DNA, cAMP, Zn, Cu, ZnO and CuO in gastric mucosa of incomplete colonic IM tissue are not remarkably different from those in gastric cancer tissue.
CONCLUSION: Gastric diseases in patients with spleen Qi deficiency or Qi stagnation have the tendency of turning into cancer. There is a close relationship between the incidence of incomplete colonic IM and gastric cancer.
- Citation: Guang-Yao Y, Fen-Xue H, Fen YY. Clinical and experimental study on gastric mucosal pathology, gene expression, cAMP and trace elements of pixu patients. World J Gastroenterol 1996; 2(1): 44-50
- URL: https://www.wjgnet.com/1007-9327/full/v2/i1/44.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i1.44
The incidence of gastric carcinoma is highest among all malignant tumors. Early gastric cancer has no distinct symptoms; when discovered, it usually has developed to intermediate and late gastric carcinoma, both of which represent serious threats to a patient’s life. Observations in clinical practice and pathologic tissue examination have suggested that “spleen deficiency” of traditional Chinese medicine and gastric mucosa intestinal metaplasia have a very close relationship with gastric carcinoma[1]. This study sought to determine the underlying mechanism of evolution for these three diseases, from both quantitative and qualitative aspects, and to provide experimental evidence to help promote earlier diagnosis of gastric cancer and guide clinical prevention and treatment approaches. Comprehensive statistical analysis of the data helps to provide insight into this important topic.
Sixty-seven patients who underwent fiber-optic gastroscopy, histopathologic examination and/or partial stomach resection and were diagnosed with gastric cancer or benign gastric diseases (including gastric ulcer and duodenal bulb ulcer) were divided into two groups according to spleen Qi deficiency or Qi stagnation (excess).
Fifty of the total 67 patients in the study had intestinal metaplasia. Among the 31 cases of spleen Qi deficiency (22 males, 9 females; average age: 45 years, age range: 22-74 years) there were 11 cases of gastric ulcer, 12 cases of duodenal bulb ulcer, and 8 cases of gastric cancer. Seventeen of the patients in this group had intestinal metaplasia. Among the 36 cases of spleen Qi stagnation (22 males, 11 females; average age: 52 years, age range: 29-68 years) there were 10 cases of gastric ulcer, 6 cases of duodenal bulb ulcer, and 20 cases of gastric cancer. Thirty-three of the patients in this group had intestinal metaplasia.
The gastric mucosa specimens taken from each patient were divided into non-focal and focal disease according to pathological examination; the levels of bioactive substances in these two tissue types were measured to determine the change in mucosa patterns. The ultramicroscopic structure of the gastric mucosa, plus the levels of trace elements and their oxides within, were evaluated by scanning electron microscope and ESI, as previously described[2,3]. The levels of cAMP and cGMP were assayed from 20 mg moist gastric mucosa tissue, with the former assayed by a radio-immune competitive protein binding method and the latter by a radio-immune diaphragm separation method. The weight unit was pmol/20 mg. To assay DNA expression in the gastric mucosa specimens, we first prepared a mucosal smear, stained it with Feulgen, and then made microscopic examination using an IBAS 2000 image analysis system equipped with a TV camera and processing computer. The integrated optical density (IOD) values of the assayed cellular karyons were calculated digitally and compared to the level of DNA. When more than 100 karyons from each patient were randomly assayed, the results were printed out in the form of a histogram.
To assay the body’s cellular immune function, we performed the tritium thymidine lymphocyte conversion test, with units of measurement at cpm/0.2 mL in whole blood. Results from histochemical staining allowed for the gastric mucosa specimens to be classified for presence of intestinal metaplasia. Staining with ABpH/PAS was performed as well. If only acid mucin was found in the epithelium the tissue was classified as having complete intestinal metaplasia. If there was a neutral mucin finding, the tissue was classified as incomplete intestinal metaplasia. By staining with HiD/AB pH 2.5, if there was sulfur mucin found in the epithelium, the tissue was classified as colonic intestinal metaplasia, but if there was no sulfur mucin found then the tissue was classified as small intestinal metaplasia. By staining with HiD/AB pH 2.5/PAS, intestinal metaplasia was classified into the following 4 types: complete, incomplete, colonic and small.
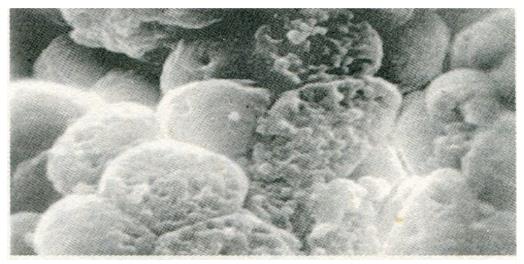
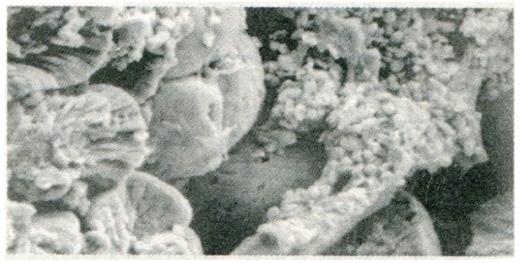
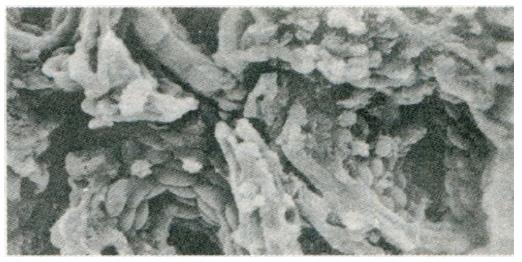
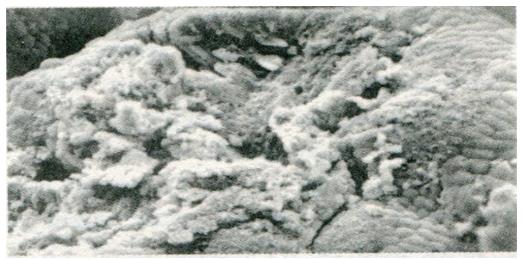
Change in gastric mucosa structural patterns is one of the features of local structural state alteration related to gastric disease with spleen deficiency. Scanning electron microscopic observation of duodenal bulb ulcer, gastric ulcer, and gastric cancer, we found that in addition to the typical characteristic lesions there existed different degrees of chronic inflammation (Figure 1), focal intestinal metaplasia (Figure 2), focal atrophic gastritis (Figure 3) and micro-ulcerations (Figure 4) in both the non-focal areas of the body of the stomach and the gastric antrum mucosa. This phenomenon represented the “background lesion”, and there were distinct differences in the incidence rates of such corresponding to the different spleen deficiency types (P < 0.05; see Table 1). Among the 18 cases of duodenal bulb ulcer, there were 7 of complete and 2 of incomplete small intestinal metaplasia and 2 of complete colonic intestinal metaplasia. Among the 21 cases of gastric ulcer, there were 3 of complete and 5 of incomplete small intestinal metaplasia, as well as 3 of complete and 4 of incomplete colonic intestinal metaplasia. Among the 28 cases of gastric cancer, there were 3 of incomplete small intestinal metaplasia, 5 of complete and 15 of incomplete colonic intestinal metaplasia. There were distinct differences in the incidence rates of intestinal metaplasia and gastric cancer between the two spleen deficiency types (P < 0.001; see Table 2).
| Pathologic changes in non-focal gastric mucosa | Deficiency of spleen energy (n= 31) | Spleen deficiency with energy stagnation (n= 36) | ||
| Gastric antrum cases (%) | Body of stomach cases | Gastric antrum cases | Body of stomach cases | |
| Focal chronic superficial inflammatory lesion | 31 (100.0) | 31 (100.0) | 36 (100.0) | 36 (100.0) |
| Focal chronic atrophic inflammatory lesion | 19 (61.3) | 12 (38.7) | 32 (88.9) | 29 (80.6) |
| Focal intestinal metaplasia | 12 (38.8) | 13 (41.9) | 25 (69.4) | 30 (83.3) |
| Micro-ulcer | 18 (58.1) | 9 (29.0) | 17 (47.2) | 28 (77.8) |
| Gastric cancer | Intestinal metaplasia | Small intestinal metaplasia | Colonic intestinal metaplasia | |||
| Complete | Incomplete | Complete | Incomplete | |||
| Deficiency of spleen energy (n = 31) | 8 (25.81) | 15 (48.39) | 6 (19.35) | 5 (16.13) | 2 (6.45) | 2 (6.15) |
| Spleen deficiency with energy stagnation (n = 36) | 20 (55.56) | 33 (91.66) | 4 (11.11) | 5 (13.89) | 7 (19.44) | 17 (47.22) |
ESI was used to assay the trace element series and their oxides in normal and focal tissues in the gastric mucosa of 41 patients with gastric disease and spleen Qi deficiency or stagnation. For each specimen, ESI automatically detected elements above atomic number 12, in the range of 0.1-0.001 mm2. The computer calculated the weight percentage of each element in the element series, which was expressed as comparative weight unit (WT%). The corresponding oxide and its WT% were also assayed synchronically at the same point. We set 15 points respectively for the normal and focal tissues, which were repeatedly and synchronically assayed 15 times, and obtained an average value of WT%. The average value of WT% of the sum of the normal and focal tissues was designated as the comprehensive value.
Twenty elements, including Na, Mg, Al, Si, P, S, K, Ca, Fe, Cu, Zn, Ti, Ni, Cr, etc., and their corresponding oxides were assayed in the gastric mucosa of 41 patients. The WT% change of Zn, Cu, ZnO and CuO were the most distinct. In normal tissue, the WT% of Zn was 3.31 ± 1.26, of Cu was 4.64 ± 1.94, of ZnO was 1.48 ± 0.71, and of CuO was 2.39 ± 0.98. In focal tissue, the WT% of Zn was 1.44 ± 0.85, of Cu was 2.42 ± 0.92, of ZnO was 0.73 ± 0.41, and of CuO was 1.35 ± 0.48. All of these differential values showed statistical significance (P < 0.001). In the normal gastric mucosa of 15 cases of benign gastric disease due to deficiency of spleen energy, Zn was 4.39 ± 0.33, Cu was 6.28 ± 0.54, ZnO was 2.11 ± 0.13, and CuO was 3.28 ± 0.25; in the focal tissue, Zn was 1.99 ± 0.01, Cu was 3.05 ± 0.12, ZnO was 1.04 ± 0.08, and CuO was 1.73 ± 0.26. There were distinct differences between the two tissues (P < 0.001). In the normal gastric mucosa of 15 cases of benign gastric disease due to spleen deficiency with energy stagnation, Zn was 3.23 ± 0.37, Cu was 4.84 ± 0.89, ZnO was 1.51 ± 0.38, and CuO was 2.34 ± 0.35; in the focal tissue, Zn was 1.57 ± 0.07, Cu was 2.24 ± 0.23, ZnO was 0.84 ± 0.14, and CuO was 1.48 ± 0.10. Again, there were distinct differences between the two tissues (P < 0.001). In the normal gastric mucosa of 10 cases of gastric cancer due to spleen deficiency with energy stagnation, Zn was 1.97 ± 0.41, Cu was 2.77 ± 0.95, ZnO was 0.44 ± 0.04, and CuO was 1.10 ± 0.39; in focal tissues, Zn was 0.50 ± 0.22, Cu was 1.63 ± 0.85, ZnO was 0.09 ± 0.03, and CuO was 0.72 ± 0.31. There were distinct differences between the two tissues as well (P < 0.001). There were also distinct differences between the spleen Qi types (P < 0.01-0.001). The comprehensive value of WT% was also remarkably different between the spleen Qi types, between the disease types, and between the intestinal metaplasia subtypes (see Tables 3 and 4).
| Intestinal metaplasia | cAMP | DNA | Zn | ZnO | Cu | CuO |
| 1 Non-intestinal metaplasia | 15.63 ± 1.06 (n = 15) | 10.95 ± 1.32 (n = 9) | 3.27 ± 0.61 (n = 14) | 1.62 ± 0.64 (n = 14) | 4.66 ± 0.62 (n = 14) | 2.67 ± 0.53 (n = 14) |
| 2 Small intestinal metaplasia | 9.55 ± 1.62 (n = 20) | 15.62 ± 5.04 (n = 13) | 2.44 ± 0.44 (n = 11) | 0.71 ± 0.10 (n = 11) | 3.51 ± 0.66 (n = 11) | 1.62 ± 0.32 (n = 11) |
| P value | < 0.001 | < 0.01 | < 0.001 | < 0.001 | < 0.01 | < 0.001 |
| 3 Colonic intestinal metaplasia | 4.35 ± 0.93 (n = 22) | 29.99 ± 12.41 (n = 17) | 1.51 ± 0.51 (n = 16) | 0.31 ± 0.09 (n = 16) | 2.48 ± 0.68 (n = 16) | 1.07 ± 0.09 (n = 16) |
| NO 1:3 P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.01 | < 0.001 |
| NO 2:3 P | < 0.001 | < 0.00 | < 0.001 | < 0.0 | < 0.001 | < 0.001 |
| Complete | Incomplete | P value | ||
| Colonic intestinal metaplasia | cAMP | 5.96 ± 0.42 (n = 9) | 2.74 ± 0.64 (n = 13) | < 0.001 |
| Small intestinal metaplasia | cAMP | 12.31 ± 1.51 (n = 10) | 6.78 ± 1.80 (n = 10) | |
| P value | < 0.001 | < 0.001 | ||
| Colonic intestinal metaplasia | DNA | 19.53 ± 9.55 (n = 6) | 35.71 ± 9.79 (n = 117) | < 0.01 |
| Small intestinal metaplasia | DNA | 11.06 ± 0.99 (n = 9) | 20.17 ± 4.78 (n = 4) | |
| P value | < 0.01 | < 0.05 | < 0.01 | |
| Colonic intestinal metaplasia | Zn | 1.77 ± 0.48 (n = 6) | 1.25 ± 0.32 (n = 10) | < 0.05 |
| Small intestinal metaplasia | Zn | 2.82 ± 0.50 (n = 6) | 2.06 ± 0.41 (n = 5) | < 0.05 |
| P value | < 0.01 | < 0.01 | ||
| Colonic intestinal metaplasia | ZnO | 0.57 ± 0.10 (n = 6) | 0.25 ± 0.08 (n = 10) | < 0.001 |
| Small intestinal metaplasia | ZnO | 1.01 ± 0.20 (n = 6) | 0.41 ± 0.16 (n = 5) | < 0.001 |
| P value | < 0.001 | < 0.05 | ||
| Colonic intestinal metaplasia | Cu | 2.78 ± 0.70 (n = 6) | 2.11 ± 0.40 (n = 10) | < 0.05 |
| Small intestinal metaplasia | Cu | 3.86 ± 0.51 (n = 6) | 3.11 ± 0.50 (n = 5) | < 0.05 |
| P value | < 0.05 | < 0.01 | ||
| Colonic intestinal metaplasia | CuO | 1.56 ± 0.13 (n = 6) | 0.58 ± 0.05 (n = 10) | < 0.001 |
| Small intestinal metaplasia | CuO | 2.41 ± 0.43 (n = 6) | 0.83 ± 0.21 (n = 5) | < 0.001 |
| P value | < 0.001 | < 0.001 |
The level of gastric mucosa cGMP showed no distinct quantitative change. In the normal tissue of 28 cases of deficiency of spleen energy, the level of gastric mucosa cAMP was 14.86 ± 1.97, and in focal tissue was 6.10 ± 1.13; there was a distinct difference between the two tissues (P < 0.001). In the normal tissues of 29 cases of spleen deficiency with energy stagnation, the cGMP level was 8.87 ± 1.73, and in the focal tissue was 2.89 ± 1.11; there was a distinct difference between the two tissues (P < 0.001). There were also distinct differences between the spleen Qi types (P < 0.05-0.001). The comprehensive quantity of gastric mucosa cAMP was also remarkably different between the spleen deficiency types, between the disease types, and between the intestinal metaplasia subtypes (see Tables 3-5).
| Deficiency of spleen energy | Spleen deficiency with energy stagnation | P value | ||
| Gastric cancer | cAMP | 8.34 ± 1.23 (n = 8) | 4.15 ± 1.36 (n = 14) | < 0.001 |
| Benign gastric disease | cAMP | 12.62 ± 1.87 (n = 20) | 7.61 ± 1.84 (n = 15) | < 0.001 |
| P value | < 0.001 | < 0.001 | ||
| Gastric cancer | DNA | 26.54 ± 3.94 (n = 4) | 36.11 ± 9.75 (n = 11) | < 0.05 |
| Benign gastric disease | DNA | 10.72 ± 0.92 (n = 15) | 13.84 ± 3.52 (n = 9) | < 0.05 |
| P value | < 0.05 | < 0.01 | ||
| Gastric cancer | Zn | 1.60 ± 0.01 (n = 1) | 1.24 ± 0.23 (n = 10) | < 0. 001 |
| Benign gastric disease | Zn | 3.19 ± 0.17 (n = 15) | 2.40 ± 0.83 (n = 15) | < 0.001 |
| P value | < 0.01 | < 0.001 | ||
| Gastric cancer | ZnO | 0.71 ± 0.02 (n = 1) | 0.27 ± 0.17 (n = 10) | < 0.001 |
| Benign gastric disease | ZnO | 1.57 ± 0.11 (n = 15) | 1.18 ± 0.33 (n = 15) | < 0.001 |
| P value | < 0.001 | < 0.001 | ||
| Gastric cancer | Cu | 2.68 ± 0.03 (n = 1) | 2.20 ± 0.57 (n = 10) | < 0.05 |
| Benign gastric disease | Cu | 4.66 ± 0.33 (n = 15) | 3.63 ± 1.21 (n = 15) | < 0.01 |
| P value | < 0.001 | < 0.001 | ||
| Gastric cancer | CuO | 1.36 ± 0.03 (n = 1) | 0.91 ± 0.19 (n = 10) | < 0.001 |
| Benign gastric disease | CuO | 2.51 ± 0.26 (n = 15) | 1.91 ± 0.43 (n = 15) | < 0.001 |
| P value | < 0.001 | < 0.001 | ||
In 24 cases of benign gastric disease, the level of DNA was 11.89 ± 2.73, while in 15 cases of gastric cancer it was 33.55 ± 9.58; there were distinct differences between these two diseases (P < 0.001). In 19 cases of deficiency of spleen energy, the level was 14.04 ± 6.75, and in 20 cases of spleen deficiency with energy stagnation it was 26.09 ± 13.44; there were distinct differences between the two spleen deficiency types (P < 0.01). DNA was remarkably different between the spleen deficiency types, between the disease types, and between the intestinal metaplasia subtypes (see Tables 3-5).
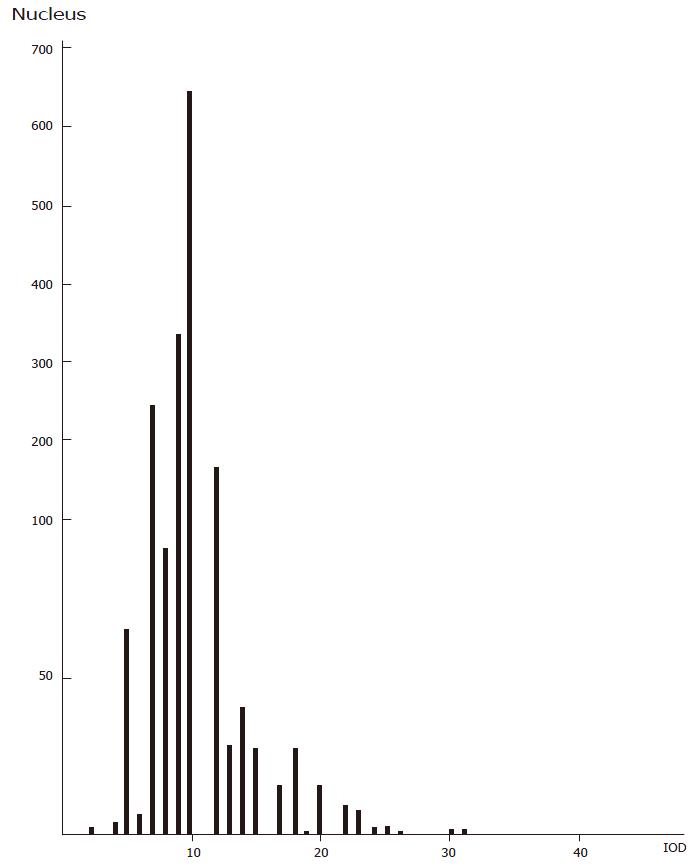
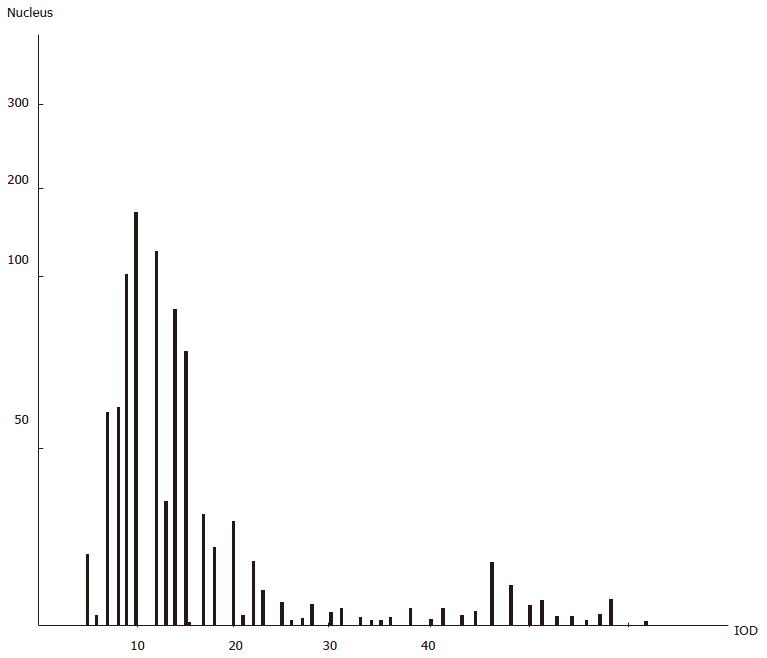
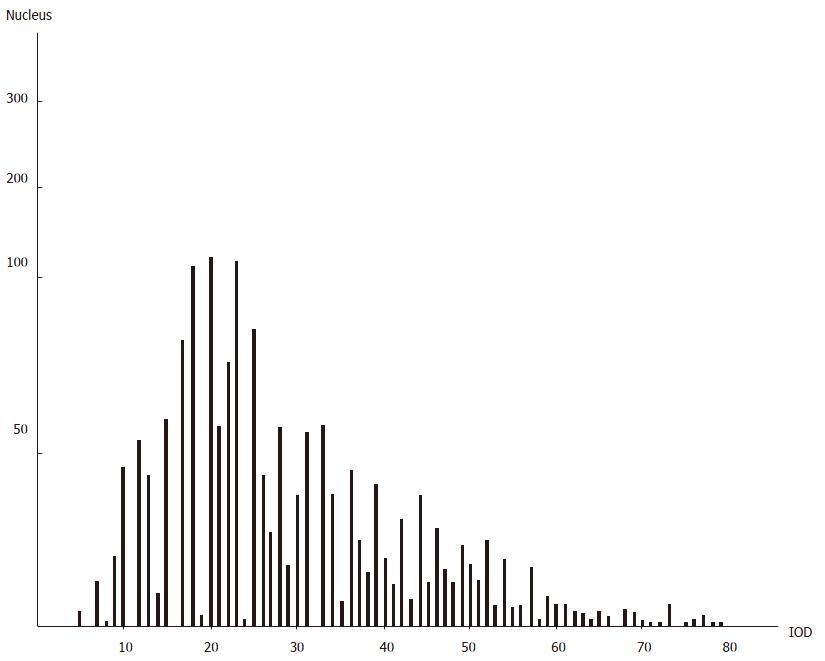
The DNA histogram of benign disease due to spleen deficiency with energy stagnation was similar to that of gastric cancer and different from that of benign gastric disease due to deficiency of spleen energy (see Figure 5, Figure 5, 7).
In 35 cases of benign gastric disease, the 3H-TdR LCT was 21404 ± 15962, and in 16 cases of gastric cancer it was 9946 ± 10498. In 27 cases of deficiency of spleen energy, it was 28578 ± 15101, and in 24 cases of spleen deficiency with energy stagnation it was 7093 ± 6887. In 73 cases of healthy people, it was 38083 ± 18296. The differences between each group were significant (P < 0.05-0.001).
Collectively, the above data show that the levels of cAMP, Zn, Cu, ZnO and CuO in gastric mucosa from patients with gastric disease due to spleen deficiency decreased in the order from deficiency of spleen energy to spleen deficiency with energy stagnation, from normal to focal tissue, from complete to incomplete intestinal metaplasia, and from small to colonic intestinal metaplasia. Meanwhile, DNA increased in the above order (P < 0.05-0.001). The incidence rates of “background lesion” in non-focal gastric mucosa and incomplete colonic intestinal metaplasia increased from deficiency of spleen energy to spleen deficiency with energy stagnation, and from benign gastric disease to gastric cancer (P < 0.05-0.001). The incidence rates of incomplete colonic intestinal metaplasia, gastric cancer and “background lesion” of gastric disease due to spleen deficiency with energy stagnation were all higher than those in patients with deficiency of spleen energy (P < 0.05-0.001). The levels of cAMP, DNA, Zn, Cu, ZnO and CuO in the incomplete colonic intestinal metaplasia tissue of gastric mucosa showed no distinct differences from those detected in gastric cancer tissues (see Tables 3-5).
The cellular immune function of patients suffering from gastric cancer and spleen deficiency with energy stagnation was also poor.
The quantitative change of cAMP, Zn, Cu, ZnO and CuO in gastric mucosa is the material foundation for spleen deficiency types and cellular cancerization, as well as for the physiopathologic and histopathologic reaction of gastric disease due to spleen deficiency. The body’s cellular immune function of gastric disease due to spleen deficiency, the quantitative changes of cAMP, Zn, Cu, ZnO and CuO in gastric mucosa tissue, and the concomitant changes between “background lesion” in non-focal gastric mucosa and intestinal metaplasia subtypes can be considered the body’s systemic, local and phasic dynamic resistance of physiology against pathology the occurs during the disease process, which manifests as the synchronic changes in degrees and levels of the body’s metabolic state, structural state and functioning state.
When the levels of Zn and Cu decline in the organism, the synthesis and activities of various Zn- and Cu-dependent enzymes will be inhibited and the metabolism of sugar, lipids, proteins, nucleic acids and vitamins will be disturbed subsequently. Indeed, when the activity of adenylate cyclase does not coordinate with that of cAMP diesterase, the synthetic metabolism of cAMP is lower and the level of cAMP in tissue becomes decreased; moreover, the translation and transcription of genes related to the metabolism of nucleic acids through phosphorylase kinase and protein kinase is disturbed, which in turn causes mismatch of DNA base pairing, disrupts cellular heredity, and influences cellular division and differentiation, thereby creating damage in the epithelial cells of gastric mucosa. In addition, this mechanism may promote ulcer formation (due to atrophy of the glandular body) and increased regeneration of the epithelial cells, the latter of which presents increased opportunities for gene mutation and may result in intestinal metaplasia and anaplasia, even cancerization.
In this study, the ratio of the speed of multiplication of cellular division was inverse to cAMP density in cells and direct to DNA density. The quantitative changes of cAMP and DNA were particularly distinct in incomplete colonic intestinal metaplasia cells and cancer cells. The 3H-TdR LCT decreased when the metabolic paths of lymphocytes were influenced, and transcription was inhibited by the quantitative changes of Zn and cAMP.
Functional state changed abnormally due to the changes of metabolic state and structural state, and symptoms of spleen deficiency occurred in conjunction. As the disease developed, the degree of synchronic change and level of the body’s systemic, local and phasic metabolic state, structural state and functioning state differed; the evolution and transformation of symptoms differed, as well. If the state developed to kidney-related changes in degrees, deficiency of spleen energy evolved to positive splenonephric deficiency. If it developed to the degree and level of energy stagnation hypostasis, it evolved to spleen deficiency with energy stagnation. If it developed to the physiologic state, the condition was moving towards recovery.
Symptoms have relation to the manifested disease, and its extent. Thus, symptoms may be useful for comprehensively diagnosing the degrees and levels of the three disease characteristics (entirety, locality and phasity) and the three states by using a quantitative change range based upon multilevel correlative indexes. Each ZANG and FU (vivscera) stated by the traditional Chinese Visceral Manifestation Theory is actually a systematic level that is based on “comprehensive function” associated with some anatomical structure. When various kinds of diseases occur, as repercussions of the body in its entirety adjusting to an individual heredity diathesis, the disease’s impact on each “comprehensive functional unit” (with viscera as its systematic level) results in the clinical phenomena of “different diseases with the same symptoms”, “the same disease with different symptoms” and “the evolution of symptoms” as the degrees and levels that are different for entire, local and phase reactions.
The histogram of DNA in gastric disease tissue due to spleen deficiency with energy stagnation was similar to that of gastric cancer; the incidence rate of incomplete colonic intestinal metaplasia in the gastric mucosa of this spleen deficiency type was high. As for the incomplete colonic intestinal metaplasia, the DNA content was found to be abnormally increased. Sulfuric acid mucin was detected in many samples and the detective rate of CEA was also found to be increased with regard to changes in cellular heredity substances, mucosal histochemistry findings and antigenicity[4]. The levels of cAMP, Zn, Cu, ZnO and CuO in the tissues of these cases were more or less the same as those in gastric cancer tissue, indicating that this kind of intestinal metaplasia is undifferentiated and possibly prone towards progressing to cancer (so it is possibly an indicator of impending cancerization). In clinical practice, more attention should be paid to the cancerization of gastric disease due to spleen deficiency with energy stagnation; the intestinal metaplasia subtypes, cAMP, DNA, Zn, Cu and CEA should also be monitored over time in the gastric mucosa of patients with gastric disease due to spleen deficiency with energy stagnation, as the findings may be helpful towards making an early diagnosis of gastric cancer.
Duodenal bulb ulcer due to spleen deficiency, and gastric ulcer focality in gastric tissue of gastric cancer are special lesions of the disease, and often exist as “background lesions” of different degrees in the non-focal gastric mucosa and may be a common feature of phase structural state of the symptom-disease combination. Although partial surgical excision of the stomach can remove these special lesions, the procedure may not entirely remove the “background lesion”. The residual “background lesion” may then become the original pathologic foundation for residual gastric disease, while the flow of gall and duodenal juices related to the operation mode may also inducing residual gastric disease. Therefore, we recommend avoiding operative treatment for benign gastric disease that has not cancerized or has no expected cancerization trend, especially in cases where there is no serious complication or risk of such. The operation method should be selected carefully according to the comprehensive treatment principles, and postoperative treatment measures should be designed according to an integrative approach for traditional Chinese medicine and Western medicine in order to best prevent and cure residual gastric disease.
| 1. | Yin GY. The relation of plasma cAMP and the chance of gastric disease due to spleen deficiency. Zhongguo Zhongxiyi Jiehe Zazhi. 1983;3:104-105. |
| 2. | Yin GY, C Y. Observation on ultrastructure of gastric mucosa. Zhonghua Waike Zazhi. 1988;16:419-421. |
| 3. | Yin GY, Wang DH. Clinical significance of the measurement of gastric mucosa trace elements and their oxides in patients with spleen deficiency and gastric cancer. Zhongguo Zhongxiyi Jiehe Zazhi. 1989;9:724-725. |
| 4. | Fang DC, Liu EF. Immunohistochemical study of certain different subtypes of intestinal metaplasia. Zhonghua Xiaohua Zazhi. 1988;8:144-146. |
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Liu WX