Published online Mar 25, 1996. doi: 10.3748/wjg.v2.i1.27
Revised: January 31, 1996
Accepted: February 10, 1996
Published online: March 25, 1996
AIM: To perform a clinical evaluation of the surgical procedures of extrahepatic bile duct cancer and their influence on prognosis.
METHODS: A total of 55 patients with pathologically and clinically verified extrahepatic bile duct cancer treated in our department between January 1984 and December 1993 were analyzed retrospectively. Clinical courses, with respect to the surgical procedures, investigated by follow-up and the survival period was assessed.
RESULTS: Among the 55 patients, 24 received surgery that involved the upper third of extrahepatic biliary tract, 12 involving the middle third, and 19 involving the lower third. The diagnosis of bile duct cancer was confirmed histopathologically in 42 of the patients, with a clear predominance of adenocarcinoma (97.6%). Eleven (26.2%) of the patients received curative resection, 30 received palliative procedures (i.e. biliary-enteric bypass (n = 14) and external drainage (n = 16)), 6 received permanent percutaneous transhepatic cholangio-drainage (PTCD) alone, and 8 received exploratory laparotomy only or conservative treatment. Forty-eight patients (87.3%) were followed-up. The overall mean survival period was 10.8 ± 9.7 mo (¯x ± s); patients with curative resection had the longest survival period (21.4 ± 16.7 mo, P < 0.01) and highest survival rate (P < 0.05). A significant survival difference was observed for patients with biliary-enteric anastomosis as compared with those who had external drainage, etc. (P < 0.05), but there was no significant difference in survival period between patients who had preoperative PTCD (n = 23) and those who did not (n = 26) (P < 0.05).
CONCLUSION: Curative resection is the treatment of choice for suitable patients with extrahepatic bile duct cancer; biliary-enteric anastomosis is preferable for those with unresectable tumor in order to improve prognosis and quality of life.
- Citation: Fan YZ, Cai TN, Wang BC. Studies on surgical operations and prognosis of extrahepatic bile duct cancer. World J Gastroenterol 1996; 2(1): 27-29
- URL: https://www.wjgnet.com/1007-9327/full/v2/i1/27.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i1.27
Despite the widespread application of new imaging techniques in diagnosis of bile duct cancer, the prognosis remains discouraging[1-3]. As a result of difficult early-detection and the low resectability rate of such tumors, only a few reports are available on the relationship between the surgical procedures and the prognosis of extrahepatic bile duct cancer. This study made an effort to evaluate the influence of various surgical procedures on survival and prognosis of patients with extrahepatic bile duct cancer by retrospective analysis in order to identify the best choice of management of the disease.
A total of 55 consecutive patients with extrahepatic bile duct cancer were surgically treated between January 1984 and December 1993. This group included 30 men and 25 women, with a mean age of 65.0 years (range: 39.2-85.0 years). Taking into account the general condition of the patients and stage of the disease, we grouped the 55 cases according to the subsequent treatment, as follows: (1) curative resection, (2) biliary-enteric anastomosis, (3) operational external drainage, (4) permanent percutaneous transhepatic cholangio-drainage (PTCD), and (5) exploratory laparotomy only or conservative treatment. All patients were followed up routinely.
All the statistical analyses were made by Student's t-test and Student-Newman-Keuls test for comparison between the groups. Survival curves were constructed by the Kaplan-Meier method[4] and were compared by the log-rank test[5]. P < 0.05 was considered to be of statistical significance.
Among the 55 patients, diagnosis of extrahepatic bile duct cancer was confirmed histopathologically in 42, with a clear predominance of adenocarcinoma (97.6%), including 24 infiltrative type, 6 nodular type and 11 papillary type. The other 13 patients were diagnosed clinically by imaging procedures such as ultrasonography, PTC, ERCP or CT. Of these, 24 (43.6%) involved the upper third of extrahepatic biliary tract, 12 (21.8%) the middle third, and 19 (34.6%) the lower third, according to Longmire's classification[6].
In group 1, 11 patients underwent curative resection, including left hemihepatectomy and biliary-enteric anastomosis (n = 2), resection of tumors with biliary-enteric anastomosis (n = 3), and Whipple's procedure (n = 6). The overall curative resection rate was 26.2%, of which the lower third cholangiocarcinoma had the highest resective rate (42.9%) and the upper third tumor had the lowest resective rate (11.8%). In group 2, 30 patients with unresectable tumors underwent palliative procedures (accounting for 54.6% of all patients and with 71.4% receiving surgical exploration), including biliary-enteric bypass (n = 14; i.e. Roux-en-Y cholangiojejunostomy (n = 9), cholangio-duodenostomy (n = 1), choledochojejuno-intubation internal drainage (n = 1), cholecystojejunostomy (n = 2) and cholecystoduodenostomy (n = 1)) and biliary external drainage by operative stent insertion (n = 16; i.e. extrahepatic (n = 6) or intrahepatic biliary T-tube intubation (n = 7), and hepatobiliary U-tube intubation (n = 3)). In group 3, 29 patients underwent PTCD, including preoperative PTCD (n = 23) and permanent PTCD alone (n = 6). In group 4, 1 patient underwent exploratory laparotomy only. In group 5, 7 patients received conservative treatment (Table 1).
| Tumor site | n | Curative resection, n (%) | Palliative procedures, n (%) | Laparotomy | |
| Biliary-enteric bypass | Stent insertion | ||||
| Upper third | 17 | 2 (11.8) | 5 | 10 (88.2) | - |
| Middle third | 11 | 3 (27.3) | 3 | 4 (63.6) | 1 |
| Lower third | 14 | 6 (42.9) | 6 | 2 (57.1) | - |
| Total | 42 | 11 (26.2) | 14 | 16 (71.4) | 1 |
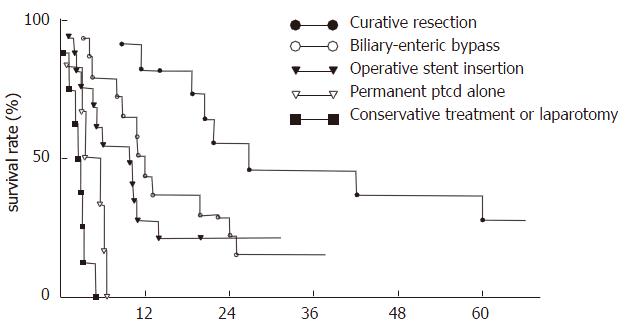
The range of follow-up was 1-62 mo. Seven patients were lost to follow-up. Five patients are still alive and disease-free. Forty-eight (87.3%) patients have data that are evaluable. The overall mean survival period was 10.8 ± 9.7 mo (range: 8 d to 5.2 years). The 1/2-, 1-, 2-, 3- and 5-year survival rates were 68.8%, 39.6%, 20.8%, 6.3% and 2.1%, respectively. Long-term survival following various treatment procedures is shown in Table 2 and Figure 1. Patients with curative resection had the longest mean survival period (21.4 ± 16.7 mo, P < 0.01) as well as the highest rate (P < 0.05). Biliary-enteric anastomosis gave better outcome and longer survival period than operative stent insertion, etc. (P < 0.05). There was no significant difference in the mean survival period and the survival rate between patients with operative stent insertion and permanent PTCD, conservative treatment or exploratory laparotomy only (P > 0.05).
The mean survival period of patients with preoperative PTCD (n = 23) was 12.5 ± 6.6 mo in contrast to those (n = 19) without which had a period of 15.2 ± 13.4 mo (Table 3). There was no significant difference between the two groups (P > 0.05).
| Preoperative PTCD | n | Mean survival period (mean ± SD, mo) |
| Yes | 23 | 12.5 ± 6.6 |
| No | 19 | 15.2 ± 13.4 |
Despite great advances having been made for diagnostic techniques and surgical procedures in hepatobiliary surgery, patients with extrahepatic bile duct cancer, and in particular hepatic hilar cancer, still have a poor prognosis, with less than 5%-30% surviving 5 years. The vast majority of patients died in the first year[1-3]. The cancer is frequently infiltrative and sclerotic in nature, tending to involve the hilum of the liver. As a consequence, resectability rates under 20% are common[1]. A national clinical survey in China determined the overall resectable rate of extrahepatic bile duct cancer to be 11.1%-33.3%, with 10.4% for carcinoma of the upper third of the extrahepatic bile duct[7]. However, it had also been reported that the resectable rate of carcinoma of the upper third might reach 50%[8-10].
Our data showed that the overall resectable rate of extrahepatic bile duct cancer was 26.2%, with 11.8% for carcinoma of the upper third. This finding should be ascribed to the late diagnosis and advanced lesions in our case series; fifty-four (98.2%) of these patients developed icterus at 1-6 mo prior to the operation, and 52.7% had weight loss and 32.7% developed ascites. In addition, lymph node metastasis (42.2%) and hepatic metastasis (25.5%) were present during laparotomy. Therefore, early diagnosis and operation are essential.
Among therapeutic modalities, resection remains the best choice of treatment of these tumors[1-4,8-13]. In this case series, 42 patients received surgical exploratory operation, of whom 11 underwent curative resection and 30 underwent palliative procedures, with 6 receiving permanent PTCD alone and 7 receiving conservative medical treatment. The overall mean survival period of all patients was 10.8 ± 9.7 mo. Curative resection gave the best chance of prolonged survival and the highest survival rate, as shown in Table 2 and Figure 1. The results are consistent with the above-mentioned reports in the literature.
Patients with curative resection had better general condition, as well as being treated at an earlier stage. However, because the lesion has access to the hepatic artery and portal vein, as well as frequent liver invasion, curative resection is not possible and the surgeon is faced with choosing the most appropriate form of palliation[1,14]. Our data indicated that biliary-enteric anastomosis gave longer survival period than operative stent insertion and permanent PTCD, etc. (Table 2). Biliary-enteric anastomosis has a low operative mortality rate, and less post-operative complications, such as cholangitis, which improves quality of life. Besides, there was no significant difference in survival period and survival rate between patients with permanent PTCD alone and conservative medical treatment or exploratory laparotomy only (P > 0.05).
There is considerable debate as to whether preoperative PTCD diminishes postoperative morbidity and reduces the operative mortality rate in patients who submit to surgery for obstructive jaundice. Several early reports showed that decompression and external drainage of the biliary tract can reduce the serum bilirubin level and improve operative morbidity and mortality[15,16]. However, prospective randomized controlled clinical trials from McPherson et al[17], Pitt et al[18] showed negative results.
In the current study, there was no statistically significant difference found for survival period of patients with and without preoperative PTCD (P > 0.05), which is consistent with the above-mentioned reports. Huang did not emphasize on this aspect[8]. The reasons were: (1) PTCD might be complicated with biliary infection resulting in loss of chance for radical operation; (2) septal occlusion may develop and impede successful drainage; (3) a decrease in serum bilirubin will not restore hepatocyte function; and (4) many PTCD-related complications are possible, such as bile leakage, loss of electrolytes, bile peritonitis, intra-abdominal bleeding, pain and catheter dislodgement[8]. Therefore, we consider that PTCD prior to surgery should be performed with caution.
The authors gratefully acknowledge the late Professor Long-Cai Qiang for providing a portion of the valuable materials.
| 1. | Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg. 1988;12:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 239] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Black K, Hanna SS, Langer B, Jirsch DW, Rider WD. Management of carcinoma of the extrahepatic bile ducts. Can J Surg. 1978;21:542-545. [PubMed] |
| 3. | Hadjis NS, Blenkharn JI, Alexander N, Benjamin IS, Blumgart LH. Outcome of radical surgery in hilar cholangiocarcinoma. Surgery. 1990;107:597-604. [PubMed] |
| 4. | Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32610] [Cited by in RCA: 31477] [Article Influence: 462.9] [Reference Citation Analysis (0)] |
| 5. | MANTEL N, HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719-748. [PubMed] |
| 6. | Longmire WP. Tumors of the extrahepatic biliary radicals. Curr Probl Cancer. 1976;1:1-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | China surgical association biliary surgery group. [A national survey of 1098 cases with extrahepatic biliary cancer]. Zhonghua Waike Zazhi. 1990;28:516-21, 572. [PubMed] |
| 8. | Huang ZQ. Diagnosis and treatment of hilar bile duct carcinoma. Putong Linchuang Waike Zazhi. 1991;6:29-34. |
| 9. | Reding R, Buard JL, Lebeau G, Launois B. Surgical management of 552 carcinomas of the extrahepatic bile ducts (gallbladder and periampullary tumors excluded). Results of the French Surgical Association Survey. Ann Surg. 1991;213:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Nagorney DM, Donohue JH, Farnell MB, Schleck CD, Ilstrup DM. Outcomes after curative resections of cholangiocarcinoma. Arch Surg. 1993;128:871-877; discussion 877-879;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 161] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Tompkins RK, Thomas D, Wile A, Longmire WP. Prognostic factors in bile duct carcinoma: analysis of 96 cases. Ann Surg. 1981;194:447-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 170] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Baer HU, Stain SC, Dennison AR, Eggers B, Blumgart LH. Improvements in survival by aggressive resections of hilar cholangiocarcinoma. Ann Surg. 1993;217:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Guthrie CM, Haddock G, De Beaux AC, Garden OJ, Carter DC. Changing trends in the management of extrahepatic cholangiocarcinoma. Br J Surg. 1993;80:1434-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Malangoni MA, McCoy DM, Richardson JD, Flint LM. Effective palliation of malignant biliary duct obstruction. Ann Surg. 1985;201:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Nakayama T, Ikeda A, Okuda K. Percutaneous transhepatic drainage of the biliary tract: technique and results in 104 cases. Gastroenterology. 1978;74:554-559. [PubMed] |
| 16. | Yanagisawa J, Ichimiya H, Kuwano N, Nakayama F. The role of preoperative biliary decompression in the treatment of bile duct cancer. World J Surg. 1988;12:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | McPherson GA, Benjamin IS, Hodgson HJ, Bowley NB, Allison DJ, Blumgart LH. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984;71:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 237] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Pitt HA, Gomes AS, Lois JF, Mann LL, Deutsch LS, Longmire WP. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost. Ann Surg. 1985;201:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 252] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Liu WX