Published online Mar 7, 2013. doi: 10.3748/wjg.v19.i9.1438
Revised: December 26, 2012
Accepted: January 5, 2013
Published online: March 7, 2013
Processing time: 170 Days and 1.2 Hours
AIM: To evaluate the prognostic significance of CD24 expression in patients undergoing adjuvant chemoradiotherapy for extrahepatic bile duct (EHBD) cancer.
METHODS: Eighty-four patients with EHBD cancer who underwent curative resection followed by adjuvant chemoradiotherapy were enrolled in this study. Postoperative radiotherapy was delivered to the tumor bed and regional lymph nodes up to a median of 40 Gy (range: 40-56 Gy). All patients also received fluoropyrimidine chemotherapy for radiosensitization during radiotherapy. CD24 expression was assessed with immunohistochemical staining on tissue microarray. Clinicopathologic factors as well as CD24 expression were evaluated in multivariate analysis for clinical outcomes including loco-regional recurrence, distant metastasis-free and overall survival.
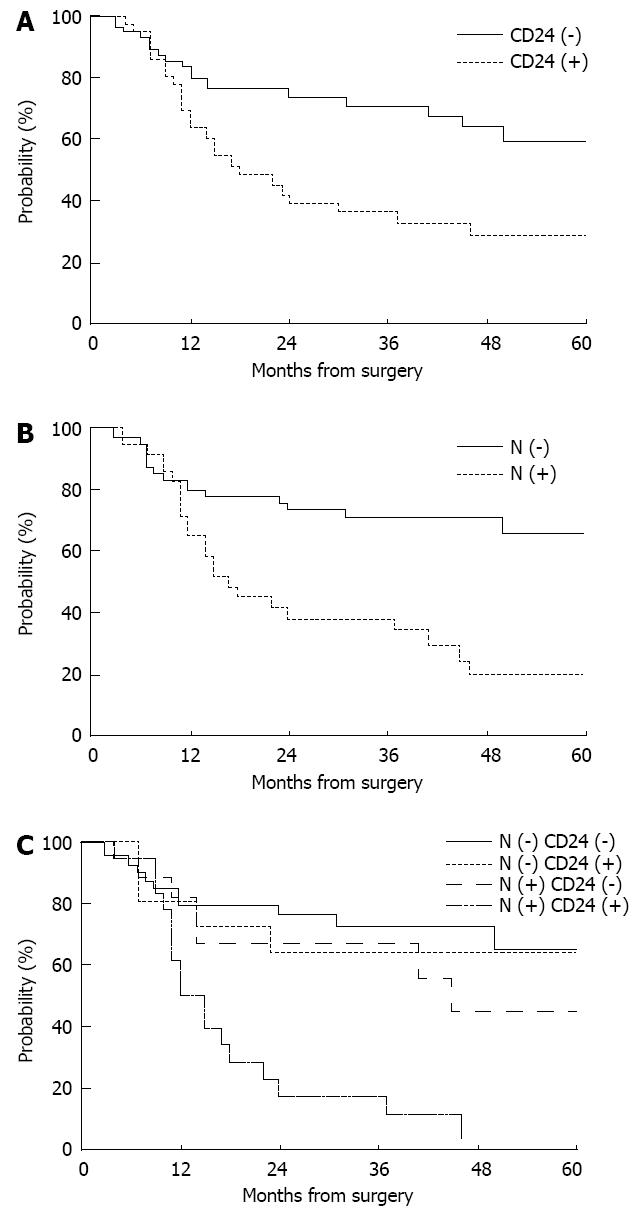
RESULTS: CD24 was expressed in 36 patients (42.9%). CD24 expression was associated with distant metastasis, but not with loco-regional recurrence nor with overall survival. The 5-year distant metastasis-free survival rates were 55.1% and 29.0% in patients with negative and positive expression, respectively (P = 0.0100). On multivariate analysis incorporating N stage, histologic differentiation and CD24 expression, N stage was the only significant factor predicting distant metastasis-free survival (P = 0.0089), while CD24 expression had borderline significance (P = 0.0733). In subgroup analysis, CD24 expression was significantly associated with 5-year distant metastasis-free survival in node-positive patients (38.4% with negative expression vs 0% with positive expression, P = 0.0110), but not in node-negative patients (62.0% with negative expression vs 64.0% with positive expression, P = 0.8599).
CONCLUSION: CD24 expression was a significant predictor of distant metastasis for patients undergoing curative resection followed by adjuvant chemoradiotherapy especially for node-positive EHBD cancer.
- Citation: Kim K, Min HS, Chie EK, Jang JY, Kim SW, Han SW, Oh DY, Im SA, Kim TY, Bang YJ, Jang JJ, Ha SW. CD24 expression predicts distant metastasis in extrahepatic bile duct cancer. World J Gastroenterol 2013; 19(9): 1438-1443
- URL: https://www.wjgnet.com/1007-9327/full/v19/i9/1438.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i9.1438
Extrahepatic bile duct (EHBD) cancer is a rare malignancy with a poor prognosis[1]. Surgical resection is considered as the only curative modality in the management of EHBD cancer, but a significant number of patients have loco-regional recurrences after curative resection alone[2-4]. Therefore, adjuvant radiotherapy with or without chemotherapy has been used for these patients, resulting in the improved loco-regional control and survival[5-9]. As a result of the increased loco-regional control, however, the distant metastasis rate has increased[5,6,10]. In our previous report, only 11.6% of patients had isolated loco-regional recurrences, whereas 44.2% of patients had distant metastases with or without loco-regional failure[10]. Likewise, further technical advances in the delivery of radiotherapy may further increase the proportion of distant metastasis[11].
Recently, metastasis-associated protein CD24 has been reported to be associated with shorter survival in various malignancies[12-20]. As for bile duct cancer, Agrawal et al[21] noted that CD24 expression was predictive of poor survival and possibly poor response to chemotherapy or radiotherapy. However, the patient number was small and the treatment details were heterogeneous.
In this study, we evaluated CD24 expression as a potential prognostic factor for predicting distant metastasis in patients undergoing curative surgery followed by adjuvant chemoradiotherapy for EHBD cancer.
Between January 2000 and August 2006, 108 patients underwent adjuvant radiotherapy after curative resection for EHBD cancer. Of these, 6 patients who did not receive concomitant chemotherapy and 18 patients whose paraffin-embedded tissue was unavailable were excluded from the analysis. Therefore, 84 patients were the subjects of this study. This study was approved by Institutional Review Board, and all patients gave informed consent prior to treatment.
All patients underwent adjuvant chemoradiotherapy. In 69 patients, a total dose of 40 Gy was delivered using 2 Gy/fraction, 5 d/wk with 2 wk of planned rest after 20 Gy. Concomitant 5-fluorouracil (5-FU, 500 mg/m2 per day iv bolus) was administered for the first 3 d of each 2 wk course of radiotherapy. Fifteen patients received a continuous course of radiotherapy, and the total dose ranged from 50 to 56 Gy in conventional fractionation. Of 15 patients, 13 patients received concomitant 5-FU (500 mg/m2 per day iv bolus for 3 d) on weeks 1 and 5 of radiotherapy. Capecitabine was prescribed for the 2 remaining patients during radiotherapy.
Fluoropyrimidine-based maintenance chemotherapy was administered to 68 patients after the completion of concurrent chemoradiotherapy. The scheduled duration of maintenance chemotherapy was 6-12 mo.
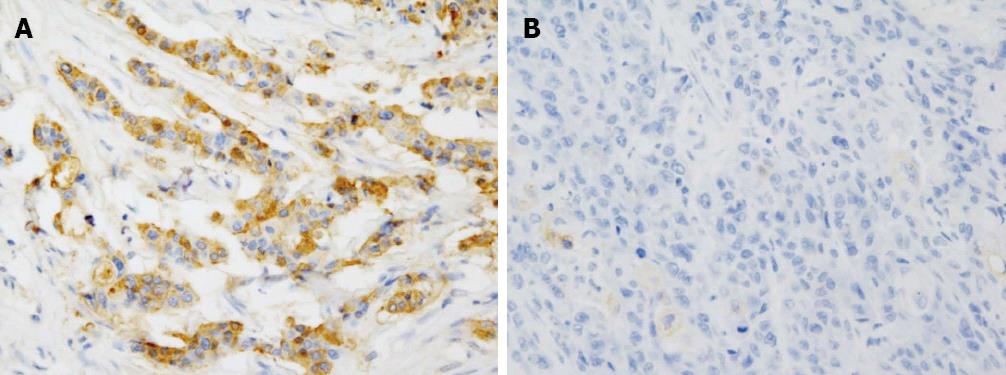
All 84 cases diagnosed as adenocarcinoma of EHBD were retrieved from the archives in Seoul National University Hospital, which contained enough paraffin-embedded tissue for the study. All the hematoxylin and eosin-stained slides were reviewed and confirmed as adenocarcinoma. Representative paraffin blocks were selected and the tissue microarray of 4 mm core was produced. Immunohistochemical staining was done on all 84 cases using antibody to CD24 (clone SN3b, Thermo Scientific, Fremont, CA, United States; 1:200) automatically, according to the manufacturer’s protocol based on the conventional streptavidin-biotin-peroxidase method. For statistical analysis, CD24 expression was scored in 4 tiers: 0, no staining; 1, staining in less than 20% of the cells; 2, staining in 20%-50% of the cells; and 3, staining in more than 50% of the cells.
Survival was calculated from the date of surgical resection. Statistical analysis was performed using SPSS software (release 12.0.1. SPSS Inc. Chicago, IL, United States). Differences in categorical variables between the parameters were compared with the standard χ2 test or Fisher’s exact test. The actuarial survival rates were calculated using the Kaplan-Meier method, and statistical significance between the actuarial survival rates was evaluated by the log-rank test. The Cox proportional hazard model was used for multivariate analysis.
There were 62 males and 22 females. The median age of all patients was 62 years (range: 36-86 years). Sixty-two patients had no residuum (R0), whereas 22 patients had microscopic residual disease (R1). As for the location of the tumor, 59 patients had hilar or proximal tumors, and 22 patients had intrapancreatic (distal) tumors. Three patients had tumors extending from the proximal to the distal EHBD. Stage was determined according to the American Joint Committee on Cancer staging system, 6th edition[22]. For T classification, 3 patients had T1, 30 patients had T2, 40 patients had T3, and 11 patients had T4 disease. Thirty-three patients had lymph node involvement, whereas 48 patients did not. Lymph node dissection was not performed in 3 patients. The degree of histologic differentiation was as follows; well differentiated in 15, moderately differentiated in 59, and poorly differentiated in 8 patients. Histologic differentiation information was missing for 2 patients.
CD24 was expressed in 36 patients (42.9%) and not in 48 patients (score = 0, 57.1%) (Figure 1). The percentage of positive cells was variable and the staining intensity was more than intermediate in most cases. Twenty-nine cases expressed CD24 in less than 20% of the tumor cells (score = 1, 80.6%), 6 cases expressed in 20%-50% of the tumor cells (score = 2, 16.7%) and only one case was diffusely positive for CD24 (score = 3, 2.8%).
Patients with CD24 expression were more likely to have node-positive tumors (P = 0.0360), and less likely to have well differentiated tumors (P = 0.0405). Also, patients with CD24 expression tended to have proximal tumors, but the correlation was statistically marginal (P = 0.0770, Table 1). As for a treatment-related factor, there was no association between CD24 expression and the use of maintenance chemotherapy.
| Variables | Patients | P value | |
| CD24 (-) | CD24 (+) | ||
| (n = 48) | (n = 36) | ||
| Age | |||
| ≤ 60 yr | 21 | 16 | 0.949 |
| > 60 yr | 27 | 20 | |
| Residual disease | |||
| R0 | 37 | 25 | 0.431 |
| R1 | 11 | 11 | |
| Tumor location1 | |||
| Proximal | 30 | 29 | 0.077 |
| Distal | 16 | 6 | |
| T stage | |||
| T1-2 | 19 | 14 | 0.949 |
| T3-4 | 29 | 22 | |
| N stage2 | |||
| N0 | 33 | 15 | 0.036 |
| N1 | 15 | 18 | |
| Histologic differentiation3 | |||
| W/D | 13 | 2 | 0.041 |
| M/D | 30 | 29 | |
| P/D | 4 | 4 | |
| Use of maintenance chemotherapy | |||
| No | 9 | 7 | 0.936 |
| Yes | 39 | 29 | |
CD24 expression had a significant impact on the 5-year distant metastasis-free survival (29.0% with positive expression vs 55.1% with negative expression, P = 0.0100, Figure 2A), but not on loco-regional recurrence-free or overall survival. N stage and histologic differentiation were also significantly correlated with distant metastasis-free survival (P = 0.0003 and 0.0394, respectively), while tumor location had marginal significance (P = 0.0623, Table 2). On multivariate analysis incorporating N stage, histologic differentiation, and CD24 expression, N stage was the only significant prognostic factor predicting distant metastasis-free survival (P = 0.0089, Figure 2B), while CD24 expression had borderline significance (P = 0.0733). When the use of maintenance chemotherapy was added in this model, the statistical significance of N stage and CD24 expression was similarly maintained (P = 0.0100 and 0.0683, respectively).
| Variables | n | 5-yr LRRFS | P value | 5-yr DMFS | P value | 5-yr OS | P value |
| Age | |||||||
| ≤ 60 yr | 37 | 62.1% | 0.3615 | 53.6% | 0.6837 | 35.1% | 0.3495 |
| > 60 yr | 47 | 63.0% | 37.9% | 51.4% | |||
| Residual disease | |||||||
| R0 | 62 | 76.3% | 0.0356 | 49.6% | 0.7748 | 53.1% | 0.2421 |
| R1 | 22 | 33.4% | 25.6% | 27.3% | |||
| Tumor location1 | |||||||
| Proximal | 59 | 54.1% | 0.1236 | 34.8% | 0.0623 | 38.7% | 0.0288 |
| Distal | 22 | 79.9% | 67.0% | 59.7% | |||
| T stage | |||||||
| T1-2 | 33 | 59.2% | 0.6311 | 48.3% | 0.4501 | 49.4% | 0.9502 |
| T3-4 | 51 | 64.3% | 40.4% | 42.2% | |||
| N stage2 | |||||||
| N0 | 48 | 67.5% | 0.7606 | 64.2% | 0.0003 | 51.7% | 0.0514 |
| N1 | 33 | 54.4% | 15.8% | 30.8% | |||
| Histologic differentiation3 | |||||||
| W/D | 15 | 69.8% | 0.2003 | 66.0% | 0.0394 | 35.0% | 0.3538 |
| M/D | 59 | 60.7% | 41.4% | 50.2% | |||
| P/D | 8 | 36.5% | 0% | 25.0% | |||
| Use of maintenance chemotherapy | |||||||
| No | 16 | 59.5% | 0.5444 | 35.2% | 0.1976 | 33.7% | 0.1435 |
| Yes | 68 | 62.7% | 45.2% | 47.3% | |||
| CD24 expression | |||||||
| Negative | 48 | 68.9% | 0.3519 | 55.1% | 0.0100 | 50.3% | 0.1873 |
| Positive | 36 | 49.9% | 29.0% | 37.2% | |||
In the subgroup analysis according to nodal involvement, CD24 expression was significantly associated with 5-year distant metastasis-free survival in node-positive patients (38.4% with negative expression vs 0% with positive expression, P = 0.0110), but not in node-negative patients (62.0% with negative expression vs 64.0% with positive expression, P = 0.8599, Figure 2C).
As for loco-regional relapse-free survival, residual disease status was the only significant prognostic factor on univariate analysis (P = 0.0356). Tumor location (P = 0.0288) and N stage (P = 0.0514) were correlated with overall survival on univariate analysis.
This study indicates that CD24 expression is associated with poorer distant metastasis-free survival in patients undergoing curative surgery followed by adjuvant chemoradiotherapy for node-positive EHBD cancer.
CD24 is reported to be expressed in various malignancies including tumors of the ovary, lung, breast, etc. Because CD24 is known to be a ligand for P-selectin, which is expressed on platelets and endothelial cells, CD24-positive tumor cells can attach more easily to activated platelets and endothelial cells, and are involved in cell adhesion and metastatic tumor spread. The prognostic value of CD24 has also been evaluated in the aforementioned tumors, and CD24 expression was demonstrated to be associated with poor survival[12-20].
As for cholangiocarcinoma, Riener et al[23] reported that CD24 expression was observed in 21% of intrahepatic cholangiocarcinoma, 58% of extrahepatic cholangiocarcinoma, and 42% of gallbladder carcinoma. Regarding the prognostic value of CD24, Agrawal et al[21] evaluated CD24 expression in 22 patients with cholangiocarcinoma by immunohistochemical staining, but information on the tumor, such as location, was not described in detail. CD24 was expressed in 81.8% of patients, and the median survival times of patients with low and high CD24 expression were 36 and 8 mo, respectively (P = 0.02). They also tried to correlate CD24 expression with the response to chemotherapy or radiotherapy. Better survival was observed in patients with low CD24 expression in the subgroup analyses, which included patients treated with either chemotherapy or radiotherapy. However, the patient number was small, and the treatment details were heterogeneous. Moreover, the patterns of failure were not given, and therefore the prognostic value of CD24 as a metastasis-associated protein could not be fully evaluated. Su et al[24] also reported that CD24 was expressed in 36 of 70 patients (51%) with resected intrahepatic cholangiocarcinoma, and that the median survival times of patients with CD24 positive and negative tumors were 8.1 and 17.2 mo, respectively (P = 0.028). However, the association between CD24 expression and distant metastasis was also not reported in their study.
In the current study, CD24 expression was observed in 42.9% of patients, and the 5-year distant metastasis-free survival rate was significantly inferior in patients with CD24 positive tumors. CD24 expression was correlated with nodal involvement and histologic differentiation, all of which are known to be predictive of distant metastasis[10,25]. On multivariate analysis incorporating these risk factors as well as CD24 expression, however, CD24 expression still showed a borderline significance. Keeratichamroen et al[26] observed similar findings in 34 patients with resected cholangiocarcinoma, that is, less nodal involvement and more well differentiated tumors in patients with low CD24 expression. Multivariate analysis in the aforementioned study showed that CD24 expression was the only independent risk factor for survival. However, details on adjuvant treatment were unavailable. In the present study, CD24 expression was not associated with maintenance chemotherapy, and moreover, the statistical significance of CD24 expression remained even after the use of maintenance chemotherapy was added in the multivariate analysis. Therefore, the correlation between CD24 expression and distant metastasis was not confounded by maintenance chemotherapy.
Several strengths of our study are the relatively large sample size including only EHBD cancer patients (n = 84), the relatively homogeneous treatment (curative resection followed by adjuvant chemoradiotherapy), and the relevant endpoint (distant metastasis). As previously mentioned, the reported proportion of distant metastasis was increased in resected EHBD cancer as the result of increased loco-regional control with the use of adjuvant chemoradiotherapy[5,6,10]. In addition to nodal involvement, CD24 expression can be used as a relevant predictor of distant metastasis in these populations. A distinct finding of our study is that the prognostic significance of CD24 was limited to those patients with nodal involvement. There was no difference in 5-year distant metastasis-free survival between CD24 positive and negative expression in node-negative patients. Therefore, those patients with higher risk of distant metastasis, that is, nodal involvement and CD24 expression, should be considered as potential candidates for more intensive systemic therapy.
However, due to the retrospective nature of our study, conclusions drawn from this study are limited and need further validation through another patient cohort, and possibly, through a prospective trial. In addition, it is unknown why the prognostic significance of CD24 was limited to those patients with nodal involvement. Further studies are needed to confirm and elucidate this observation.
In conclusion, CD24 expression was a relevant predictor of distant metastasis in patients undergoing curative resection followed by adjuvant chemoradiotherapy for node-positive EHBD cancer. CD24 expression may be used as an additional index for selecting patients with higher risk of distant metastasis in future trials.
Extrahepatic bile duct (EHBD) cancer is a rare malignancy with a poor prognosis. The major pattern of failure after surgical resection has been shifted from loco-regional recurrence to distant metastasis as a result of increased loco-regional control with the use of adjuvant chemoradiotherapy. Given these observations, a prognostic factor predicting distant metastasis needs to be evaluated to select patients who need more intensive systemic therapy.
There are a number of studies demonstrating that CD24 was associated with shorter survival in various malignancies, but only a few for EHBD cancer. In the current study, the prognostic significance of CD24 expression in EHBD cancer patients who underwent adjuvant chemoradiotherapy after curative resection was evaluated.
There were a few reports on the prognostic value of CD24 in patients with cholangiocarcinoma. However, patient numbers were small and tumor location and treatment details were heterogeneous. Moreover, the patterns of failure were not given, and therefore the prognostic value of CD24 as a metastasis-associated protein could not be fully evaluated. Several strengths of the present study are the relatively large sample size including only EHBD cancer patients, relatively homogeneous treatment, and a relevant endpoint, that is, distant metastasis. The authors found that CD24 expression was associated with poorer distant metastasis-free survival in node-positive EHBD cancer patients who underwent adjuvant chemoradiotherapy after curative resection.
From these results, CD24 expression along with nodal involvement can be used as a relevant predictor of distant metastasis and as a potential indicator for more intensive systemic therapy.
CD24, metastasis-associated protein, is reported to be expressed in various malignancies including tumors of ovary, lung and breast. Because CD24 is known to be a ligand for P-selectin, which is expressed on platelets and endothelial cells, CD24-positive tumor cells can attach more easily to activated platelets and endothelial cells and are involved in cell adhesion and metastatic tumor spread.
The topic and the results are interesting, well presented and of great clinical importance. The number of involved patients is also notable considering the observed type of cancer. The main conclusion of the study, namely that the timorous CD24 expression is a useful predictor of distant metastasis in patients undergoing curative resection followed by adjuvant chemoradiotherapy, especially for node-positive cases, is important both clinically and therapeutically, as CD24 immunohistochemistry (IHC) may provide a basis for patient selection for perioperative bile duct cancer treatment. The methodology is clear, tissue microarray analysis scores are relevant, and the IHC figures are representative.
| 1. | Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303-1314. [PubMed] |
| 2. | Jarnagin WR, Ruo L, Little SA, Klimstra D, D’Angelica M, DeMatteo RP, Wagman R, Blumgart LH, Fong Y. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689-1700. [PubMed] |
| 3. | Kim JK, Ha HK, Han DJ, Auh YH. CT analysis of postoperative tumor recurrence patterns in periampullary cancer. Abdom Imaging. 2003;28:384-391. [PubMed] |
| 4. | de Castro SM, Kuhlmann KF, van Heek NT, Busch OR, Offerhaus GJ, van Gulik TM, Obertop H, Gouma DJ. Recurrent disease after microscopically radical (R0) resection of periampullary adenocarcinoma in patients without adjuvant therapy. J Gastrointest Surg. 2004;8:775-784; discussion 784. [PubMed] |
| 5. | Borghero Y, Crane CH, Szklaruk J, Oyarzo M, Curley S, Pisters PW, Evans D, Abdalla EK, Thomas MB, Das P. Extrahepatic bile duct adenocarcinoma: patients at high-risk for local recurrence treated with surgery and adjuvant chemoradiation have an equivalent overall survival to patients with standard-risk treated with surgery alone. Ann Surg Oncol. 2008;15:3147-3156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Gwak HK, Kim WC, Kim HJ, Park JH. Extrahepatic bile duct cancers: surgery alone versus surgery plus postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 2010;78:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Kim TH, Han SS, Park SJ, Lee WJ, Woo SM, Moon SH, Yoo T, Kim SS, Kim SH, Hong EK. Role of adjuvant chemoradiotherapy for resected extrahepatic biliary tract cancer. Int J Radiat Oncol Biol Phys. 2011;81:e853-e859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Bonet Beltrán M, Allal AS, Gich I, Solé JM, Carrió I. Is adjuvant radiotherapy needed after curative resection of extrahepatic biliary tract cancers? A systematic review with a meta-analysis of observational studies. Cancer Treat Rev. 2012;38:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Shinohara ET, Mitra N, Guo M, Metz JM. Radiotherapy is associated with improved survival in adjuvant and palliative treatment of extrahepatic cholangiocarcinomas. Int J Radiat Oncol Biol Phys. 2009;74:1191-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Kim K, Chie EK, Jang JY, Kim SW, Han SW, Oh DY, Im SA, Kim TY, Bang YJ, Ha SW. Adjuvant chemoradiotherapy after curative resection for extrahepatic bile duct cancer: a long-term single center experience. Am J Clin Oncol. 2012;35:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Zamboglou C, Messmer MB, Becker G, Momm F. Stereotactic radiotherapy in the liver hilum. Basis for future studies. Strahlenther Onkol. 2012;188:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Kristiansen G, Sammar M, Altevogt P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol. 2004;35:255-262. [PubMed] |
| 13. | Kristiansen G, Denkert C, Schlüns K, Dahl E, Pilarsky C, Hauptmann S. CD24 is expressed in ovarian cancer and is a new independent prognostic marker of patient survival. Am J Pathol. 2002;161:1215-1221. [PubMed] |
| 14. | Kristiansen G, Winzer KJ, Mayordomo E, Bellach J, Schlüns K, Denkert C, Dahl E, Pilarsky C, Altevogt P, Guski H. CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res. 2003;9:4906-4913. [PubMed] |
| 15. | Kristiansen G, Schlüns K, Yongwei Y, Denkert C, Dietel M, Petersen I. CD24 is an independent prognostic marker of survival in nonsmall cell lung cancer patients. Br J Cancer. 2003;88:231-236. [PubMed] |
| 16. | Darwish NS, Kim MA, Chang MS, Lee HS, Lee BL, Kim YI, Kim WH. Prognostic significance of CD24 expression in gastric carcinoma. Cancer Res Treat. 2004;36:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Weichert W, Denkert C, Burkhardt M, Gansukh T, Bellach J, Altevogt P, Dietel M, Kristiansen G. Cytoplasmic CD24 expression in colorectal cancer independently correlates with shortened patient survival. Clin Cancer Res. 2005;11:6574-6581. [PubMed] |
| 18. | Kwon GY, Ha H, Ahn G, Park SY, Huh SJ, Park W. Role of CD24 protein in predicting metastatic potential of uterine cervical squamous cell carcinoma in patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:1150-1156. [PubMed] |
| 19. | Yang XR, Xu Y, Yu B, Zhou J, Li JC, Qiu SJ, Shi YH, Wang XY, Dai Z, Shi GM. CD24 is a novel predictor for poor prognosis of hepatocellular carcinoma after surgery. Clin Cancer Res. 2009;15:5518-5527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Sano A, Kato H, Sakurai S, Sakai M, Tanaka N, Inose T, Saito K, Sohda M, Nakajima M, Nakajima T. CD24 expression is a novel prognostic factor in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16:506-514. [PubMed] [DOI] [Full Text] |
| 21. | Agrawal S, Kuvshinoff BW, Khoury T, Yu J, Javle MM, LeVea C, Groth J, Coignet LJ, Gibbs JF. CD24 expression is an independent prognostic marker in cholangiocarcinoma. J Gastrointest Surg. 2007;11:445-451. [PubMed] |
| 22. | Agrawal S; AJCC. AJCC Cancer staging handbook. 6th ed. New York: Springer-Verlag 2002; . |
| 23. | Riener MO, Vogetseder A, Pestalozzi BC, Clavien PA, Probst-Hensch N, Kristiansen G, Jochum W. Cell adhesion molecules P-cadherin and CD24 are markers for carcinoma and dysplasia in the biliary tract. Hum Pathol. 2010;41:1558-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Su MC, Hsu C, Kao HL, Jeng YM. CD24 expression is a prognostic factor in intrahepatic cholangiocarcinoma. Cancer Lett. 2006;235:34-39. [PubMed] |
| 25. | Kobayashi A, Miwa S, Nakata T, Miyagawa S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg. 2010;97:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Keeratichamroen S, Leelawat K, Thongtawee T, Narong S, Aegem U, Tujinda S, Praditphol N, Tohtong R. Expression of CD24 in cholangiocarcinoma cells is associated with disease progression and reduced patient survival. Int J Oncol. 2011;39:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
P- Reviewers Sipos F, Oh HC S- Editor Gou SX L- Editor Cant MR E- Editor Li JY