Published online Mar 7, 2013. doi: 10.3748/wjg.v19.i9.1387
Revised: November 7, 2012
Accepted: November 24, 2012
Published online: March 7, 2013
Processing time: 175 Days and 0.7 Hours
AIM: To investigate and clarify, for the first time, the role of inosine triphosphate pyrophosphatase (ITPA) polymorphism in Egyptian chronic hepatitis C virus (HCV) patients.
METHODS: The human genomic DNA of all patients was extracted from peripheral blood cells in order to determine the single nucleotide polymorphism (SNP) of ITPA (rs1127354). SNP genotyping was performed by real time polymerase chain reaction (PCR, ABI TaqMan allelic discrimination kit) for 102 treatment-naive Egyptian patients with chronic HCV. All patients had no evidence of cardiovascular or renal diseases. They received a combination treatment of pegylated interferon α (PEG-IFNα) as a weekly subcutaneous dose plus an oral weight-adjusted dose of ribavirin (RBV). The majority received PEG-IFNα2a (70.6%) while 29.4% received PEG-IFNα2b. The planned duration of treatment was 24-48 wk according to the viral kinetics throughout the course of treatment. Pre-treatment liver biopsy was done for each patient for evaluation of fibrosis stage and liver disease activity. The basal viral load level was detected quantitatively by real time PCR while viral load throughout the treatment course was performed qualitatively by COBAS TaqMan assay.
RESULTS: Ninety-three patients (91.2%) had ITPA SNP CC genotype and 9 (8.8%) had non-CC genotype (CA and AA). The percentage of hemoglobin (Hb) decline was higher for CC patients than for non-CC patients, particularly at weeks 4 and 8 (P = 0.047 and 0.034, respectively). During the first 12 wk of treatment, CC patients had significantly more Hb decline > 3 g/dL than non-CC patients: 64.5% vs 22.2% at weeks 8 and 12, respectively, (P = 0.024 and 0.038). Reduction of the amount of the planned RBV dose was significantly higher for CC patients than non-CC patients during the first 12 wk (18% ± 12.1% vs 8.5% ± 10.2%, P = 0.021). The percentage of CC patients with RBV dose reduction was significantly greater than that of non-CC patients (77.4% vs 44.4%, P = 0.044). Multivariate analysis identified only the percentage of RBV dose as a predictor for Hb decline. Platelet decline was significantly higher in non-CC patients than CC patients at weeks 12, 24 and 48 (P = 0.018, 0.009 and 0.026, respectively).
CONCLUSION: Rs1127354 ITPA polymorphism plays a decisive role in protecting against treatment-induced anemia and the need for RBV dose reduction in Egyptian HCV patients.
- Citation: Ahmed WH, Furusyo N, Zaky S, Sharaf Eldin A, Aboalam H, Ogawa E, Murata M, Hayashi J. Pre-treatment role of inosine triphosphate pyrophosphatase polymorphism for predicting anemia in Egyptian hepatitis C virus patients. World J Gastroenterol 2013; 19(9): 1387-1395
- URL: https://www.wjgnet.com/1007-9327/full/v19/i9/1387.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i9.1387
Hepatitis C virus (HCV) infection is considered one of the most horrifying diseases. It affects approximately 3% of the world’s total population, more than 170 million people worldwide. It can range in severity from a mild illness lasting a few weeks to a serious lifelong disease that may end with cirrhosis and hepatocellular carcinoma[1,2]. The prevalence of HCV infection shows considerable differences between countries; the lowest prevalence rate has been estimated in European countries. Parenteral anti-schistosomal treatment that was used in the 1960s and 1970s played a major role in the spread of HCV throughout Egypt[3,4]. Egypt has been reported as having the highest prevalence rate of HCV worldwide, with HCV antibodies estimated at 10% and 20% of Egyptian adults in urban and rural areas, respectively[5].
The standard treatment for HCV treatment worldwide, including Egypt, is pegylated interferon α (PEG-IFNα), which acts as an antiviral and an immunomodulatory cytokine, and ribavirin (RBV), a guanosine analogue that interrupts the viral RNA metabolism[6,7]. Anemia is a well-known, serious side effect associated with the PEG-IFN plus RBV combination treatment that has been reported to occur in 54% of patients during the course of treatment[8]. It leads to the necessity of dose reduction or premature discontinuation of treatment by 10%-14% of the patients[6]. Anemia is mainly due to a RBV hemolytic effect on red blood cells (RBCs) or by IFN through either its suppressive effect on bone marrow or an autoimmune hemolytic reaction from excessive apoptosis of erythroid cells[9-11]. Pretreatment screening of biomarkers is essential for evaluating both the risks and benefits of the available treatment regimen. In the last few years, some genome wide association studies have discussed the impact of host genetic factors on the treatment of hepatitis C. Some of these studies targeted the single nucleotide polymorphism (SNP) in the inosine triphosphate pyrophosphatase (ITPA) gene of patients mono-infected with HCV[12-14]. Domingo et al[15] reported a strong association between this polymorphism and induction of anemia in HCV/human immunodeficiency virus (HIV) co-infected patients. This gene is located on chromosome 20, and the SNP occurs at nucleotide 94 in exon 2 of the ITPA gene and leads to substitution of proline residue at position 32 to threonine (P32T, 94C→A)[16]. Although HCV is widespread in Egypt, it has not been spotlighted from the standpoint of host genetic studies. Our motivation to carry out this study was to clarify for the first time in Egyptian patients the association between the variants of the ITPA gene (encoding ITPase enzyme) and anemia induced during PEG-IFNα and RBV combination treatment, regardless of the impact on treatment outcome.
The data of this study were collected from 102 Egyptian chronic hepatitis C patients. All were positive for HCV RNA for more than 6 mo and negative for any other viral infection. The pretreatment demographic data are displayed in Table 1. All patients were mono-infected with chronic hepatitis C with no evidence of HIV or hepatitis B infection. All participants were treatment-naive patients with no evidence of the presence of any associated liver diseases other than chronic viral hepatitis C. Patients with cardiovascular problems or renal impairment were not eligible for the study. They received standard doses of the combination treatment of PEG-IFNα and RBV in the Assiut National Center for the Treatment of Chronic Hepatitis under the supervision of the Egyptian Ministry of Health.
In the first week of treatment, all patients were given the fully recommended dosages of RBV. At week 2 and throughout the course of treatment, the dose of RBV was modified according to the hemoglobin (Hb) level. In the current study, we calculated the values of Hb at weeks 2 and 4, then every 4 wk throughout the treatment course. Informed consent was obtained from all participants for the collection and storage of blood samples for ITPA polymorphism testing. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
In relation to the polymorphism of ITPA genotyping, we had some clinical endpoints, including the absolute and percentage decline of Hb during the first 12 wk, RBV dose reduction following anemia, and the percentage of platelet (plt) count change.
The protocol specified a treatment duration of 24-48 wk depending on viral genotype and the response of patients during the treatment course. An additional 24 wk of follow-up was done for evaluation of efficacy. The majority of patients (70.6%) received PEG-IFNα2a (Pegasys) at a dose of 180 μg per week and 29.4% received PEG-IFNα2b (Peginteron) at a dose of 1.5 μg/kg per week, given as a subcutaneous injection. All patients received a 15 mg/kg daily oral dose of RBV (Rebetol).
The study had a planned treatment duration of 48 wk. However, the study was designed with a futility-stopping rule that would halt the trial if there was < 2 log10 HCV RNA decline at week 12 or persistent viremia at week 24. The treatment duration was prolonged to 72 wk only for patients who had detectable HCV RNA at week 12 but undetectable at week 24. All patients were followed for 24 wk after cessation of treatment.
The pre-treatment HCV RNA level was detected by quantitative real time polymerase chain reaction (PCR) technique with a lower limit of detection of 12 IU/mL. Qualitative PCR by COBAS TaqMan assay[17] was used for detection of viral load at weeks 12, 24 and 48. All patients underwent a pretreatment liver biopsy from which the liver disease activity and fibrosis stage were scored by the Metavir scoring system[18]. HCV genotype determination was by sequence determination in the 5’-nonstructual region of the HCV genome followed by phylogenetic analysis[7].
Human genomic DNA was extracted from peripheral blood. Genotyping of the ITPA SNP (rs1127354) was performed using the ABI TaqMan allelic discrimination kit (7500 Real Time PCR System; Applied Biosystems, Carlsbad, CA, United States). Patients were genotyped as CC, CA, or AA at the polymorphic site rs1127354.
The statistical analysis of comparisons of the categorical variables of the groups was performed by two tailed Fischer’s exact probability test calculated on 2 × 2 contingency tables. Data are shown as mean ± SD. Student t test was used to compare the direct continuous variables between groups with a significant P value of less than 0.05. Odds ratios (OR) for the relative risk and 95%CI in the univariate analysis were estimated by binary logistic regression (program written by Pezzullo JC and Sullivan KM, version 05.07.20). The multivariate regression analysis for the identification of the predictors of Hb decline more than 3 g/dL were performed by JMP software (JMP IN, Version 9.0.2, SAS Institute Inc., Cary, NC, United States).
Baseline demographic and biochemistry criteria for all 102 patients are reported in Table 1. Comparison of the ITPA genotyping groups (CC and non-CC) and their relationships to the baseline data are shown in Table 2. Baseline plt count and body mass index were the only significant baseline characters, and both were higher for non-CC than for CC patients (both P < 0.05). Hb data were missing for 6 patients from week 16 till end of treatment. Eleven patients stopped the treatment at week 24 due to continuous positivity of HCV RNA, with 91 receiving the complete course of 48 wk.
| Criteria | Total |
| Number | 102 |
| Age (yr) | 32.5 ± 7.3 |
| Sex (male/female) | 90/12 |
| BMI | 25.6 ± 2.7 |
| Baseline Hb (g/dL) | 14.07 ± 1.1 |
| plt (× 103/μL) | 238.5 ± 62.7 |
| WBCs (× 103/μL) | 6.3 ± 1.4 |
| ALT > ULN, n (%) | 78 (76.5) |
| Creatinine level (mg/dL) | 0.91 ± 0.44 |
| Baseline HCV RNA (log IU/mL) | 5.4 ± 0.7 |
| Metavir fibrosis stage, n (%) | |
| F1 | 69 (67.7) |
| F2 | 30 (29.4) |
| F3 | 3 (2.9) |
| Grade of inflammation, n (%) | |
| A1 | 64 (62.7) |
| A2 | 33 (29.4) |
| A3 | 5 (5.4) |
| PEG IFN, n (%) (dose) | |
| α2a | 72 (70.6) (180 μg/wk) |
| α2b | 30 (29.4) (1.5 μg/kg per week) |
| Ribavirin dose (mg/kg per day) | 12.5 (10-15) |
| HCV genotype (G), n (%) | G4, 62 (60.8); G1, 40 (39.2) |
| Criteria | CC | CA + AA | P value |
| Number, n (%) | 93 (91.2) | 9 (8.8) | |
| Age (yr) | 32.3 ± 7.3 | 34.4 ± 7.7 | 0.457 |
| Sex (male/female) | 82/11 | 8/1 | |
| BMI | 25.4 ± 2.8 | 26.8 ± 1.6 | 0.046 |
| Baseline Hb (g/dL) | 14 ± 1.2 | 14.3 ± 1.03 | 0.441 |
| plt (× 103/μL) | 233.2 ± 62.1 | 292.2 ± 74.3 | 0.046 |
| WBCs (× 103/μL) | 6.2 ± 1.5 | 6.8 ± 1.1 | 0.172 |
| ALT > ULN, n (%) | 71 (76.3) | 7 (77.7) | > 0.99 |
| Creatinine level (mg/dL) | 0.9 ± 0.46 | 0.98 ± 0.22 | 0.349 |
| Baseline HCV RNA (log IU/mL) | 5.46 ± 0.7 | 5.4 ± 0.8 | > 0.99 |
Rs1127354 SNP genotyping of all participants was successful, and the distribution is as follows: 93 (91.2%) patients were identified as CC and 9 as non-CC (8 with the CA and one with the AA genotype) (8.8%). The minor allele frequency of rs1127354 was 0.04, which means the chance of (A) allele occurring was 4%; the distribution here is consistent with Hardy Weinberg Equilibrium (P = 0.108).
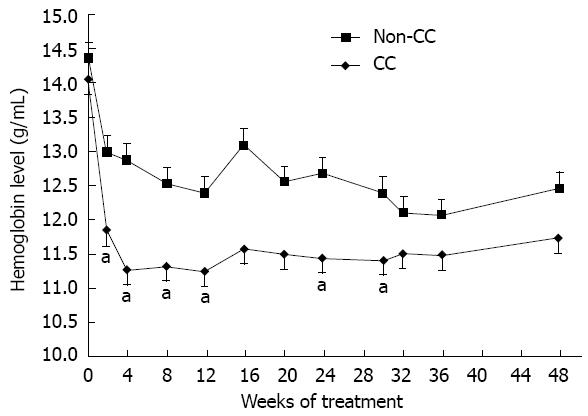
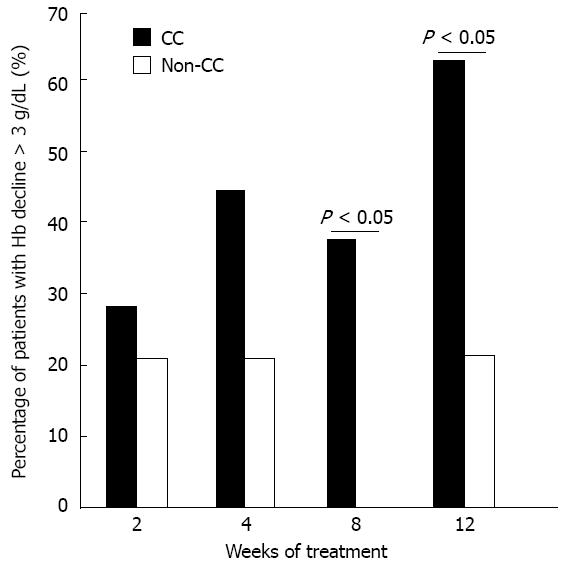
In the present study, anemia was defined as an Hb level decreased by > 3 g/dL and severe anemia was reported when Hb level declined to less than 10 g/dL. Figure 1 shows the difference in Hb level between the CC and non-CC groups throughout the treatment course. It was significantly different at weeks 2, 4, 8, 12, 24 and 30 (P = 0.019, 0.016, 0.015, 0.013, 0.036 and 0.047, respectively). Hb decline > 3 g/dL during the first 12 wk was faster and more vigorous in the anemia-susceptible group CC than in the protective non-CC group at weeks 4 and 8 (P = 0.047 and 0.034, respectively). By week 16, Hb had settled in the CC group with no further decrease, in contrast to the non-CC genotype group that showed a sudden increase in Hb followed by a continuous slow decrease until the end of treatment (Figure 1). The Hb decline > 3 g/dL was significantly higher among CC patients than non-CC patients at week 8 [36 of 93 (38.7%) vs none of 9 (0%), P = 0.024] and week 12 [60 of 93 (64.5%) vs 2 of 9 (22.2%), P = 0.038] (Figure 2). This indicated a protective advantage for patients with the minor allele A of rs1127354 SNP of ITPA gene against early onset anemia during combination treatment for Egyptian patients with chronic hepatitis C. During the first 12 wk, severe anemia with an Hb level less than 10 g/dL was identified in 29% of the CC patients compared with 11.1% of the non-CC patients (P = 0.438). Interestingly, the only patient in this study harboring a homozygous variant for the minor allele (AA) did not suffer anemia at any time during the treatment. In an attempt to identify factors predictive of the incidence of Hb decline more than 3 g/dL during the first 12 wk, we performed both univariate and multivariate regression analyses (Table 3). The univariate logistic regression analysis detected four factors as independent variables: ITPA genotype, percentage of received RBV dose and the baseline Hb and plt levels. On the other hand, the percentage of the received RBV dose (≥ 80% of total dose), was the only significant independent variable in the multivariate analysis.
| Variable | Univariate | Multivariate | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Sex (female) | 0.49 (0.1-2.4) | 0.386 | 4.7 (0.9-26.4) | 0.123 |
| Age (≥ 40 yr) | 0.66 (0.24-1.8) | 0.421 | 1.02 (0.9-1.1) | 0.462 |
| BMI ( ≤ 24) | 2.03 (0.82-5.01) | 0.124 | 1.2 (1-1.5) | 0.068 |
| Baseline Hb (< 15) | 0.14 (0.04-0.49) | 0.002 | 0.8 (0.5-1.2) | 0.610 |
| Baseline plt (< 200) | 0.26 (0.1-0.65) | 0.004 | 0.9 (0.9-1) | 0.073 |
| ITPA genotype (CC) | 6.36 (1.24-32.41) | 0.025 | 0.4 (0.04-4.08) | 0.671 |
| RBV dose ≥ 80% | 0.08 (0.01-0.3) | 0.0009 | 0.87 (0.82-0.92) | 0.00002 |
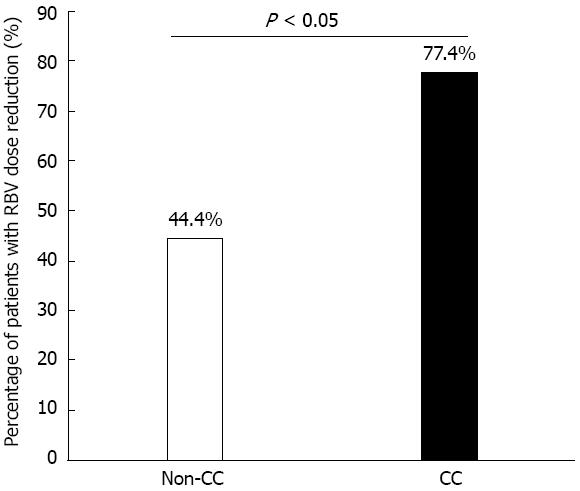
The dose of RBV was modified according to the presence of treatment side effects. During the first 12 wk, the total number of patients needing an RBV dose reduction was 76 of 102 (74.5%). The percentage of RBV dose reduction was higher for the CC than for the non-CC patients (18% ± 12.1% vs 8.5% ± 10.2% of the recommended dose, P = 0.021). The percentage of CC patients requiring an RBV dose reduction was greater than that of non-CC patients (77.4% vs 44.4%, respectively, P = 0.044) (Figure 3). Calculation of the time-point of reduction showed a significant difference between CC and non-CC patients at week 4, at which time none of the non-CC patients required an RBV dose reduction compared to 35 CC patients having a high reduction of RBV (P = 0.025). Although the reduction of RBV started earlier for patients with the CC than with the non-CC genotype (6.1 ± 5.3 wk vs 10 ± 8.5 wk, respectively), this did not reach statistical significance (P = 0.325). After the first 12 wk, 11 CC patients stopped the treatment due to non-responsiveness, whereas all non-CC patients completed the full course of treatment. Table 4 shows a comparison of the average RBV dose during the first 12 wk with the following 36 wk of treatment course between CC and non-CC patients. A significant difference was found (P = 0.035 and 0.029, respectively).
| During first 12 wk | 12-48 wk of treatment | |||||
| Total | CC | CA + AA | Total | CC | CA + AA | |
| No. of patients | 102 | 93 | 9 | 91 | 82 | 9 |
| RBV dose (mg/wk), mean ± SD | 947 ± 151.4 | 936 ± 148.3 | 1059 ± 144.1 | 943 ± 153.2 | 930.5 ± 149.6 | 1059 ± 144.1 |
| P value | 0.035 | 0.029 | ||||
Almost all of the patients in this study had an RBV dose reduction in the first 12 wk of treatment, with 200 mg the initial reduction when Hb declined by more than 3 g/dL. Patients with severe anemia had a higher reduction when their Hb reached less than 10 g/dL. Usually, discontinuation of treatment is considered when the Hb declines to less than 8.5 g/dL; however, in this study, none of the patients had treatment discontinued due to this cause. During the first 12 wk, 74.2% of the CC patients (69 of 93) received 80% or more of the initially recommended dosage of RBV, whereas all non-CC patients received more than 80% of the dosage (P = 0.112).
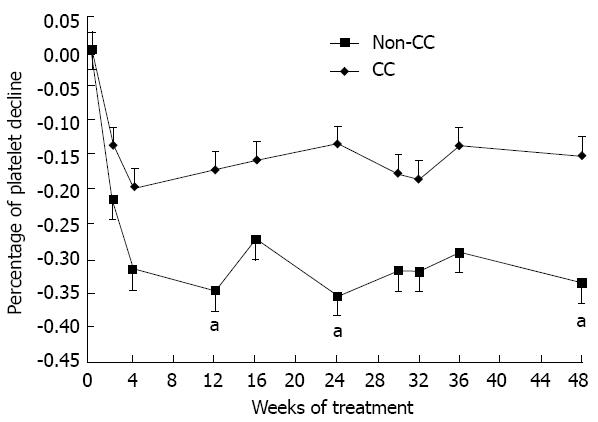
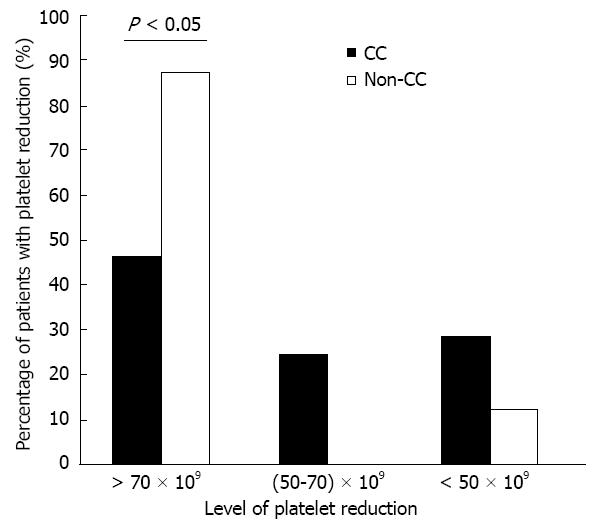
Calculation of plt count change throughout the 48 wk of treatment showed a difference in the percentage of plt reduction between the CC and non-CC patients (Figure 4). Significant differences were found at weeks 12, 24 and 48 (P = 0.018, 0.009 and 0.026, respectively). Although CC patients had a lower baseline median plt count than non-CC patients (233.2 vs 292.2, respectively), they had a lower percentage of plt reduction during the early weeks of treatment (17.4% vs 28.5%, respectively). Figure 5 shows the difference between ITPA genotypes according to three levels of maximum plt reduction at week 4 (plt reduction more than 70 × 109/L, (50-70) × 109/L, and less than 50 × 109/L). The percentage of patients was significantly different among those with plt reduction more than 70 × 109/L: 87.5% of non-CC vs 46.4% of CC (P = 0.029). This result indicates that the anemia-susceptible patients of group CC are less likely to develop a higher degree of plt reduction than non-CC patients. Notably, the percentage of the relative reactive increase in the plt count during weeks 1-4 was higher for CC patients than for non-CC patients but did not reach statistical significance (34.4% vs 11%, respectively, P = 0.264). However, the reactive plt increase of the CC and non-CC patients did not correlate with either an Hb reduction > 3 g/dL or with the baseline plt count of weeks 1-4.
No significant difference in the white blood cells was found between the CC and non-CC patients during the early weeks of treatment (P > 0.05) (Figure 6). Also, there was no significant association between ITPA genotype and early virological response at week 12.
To our knowledge, this is the first study to assess the impact of the rs1127354 ITPA genotype on the anemia of Egyptian patients infected with chronic hepatitis C, regardless of the outcome of treatment. Marsh et al[19] and Cao et al[20] reported the distribution of ITPA genotypes in multiple populations, which reached the highest rate among Asians (11%-19%) and lowest in Central/South Americans (1%-2%), while in Caucasian and African populations the distributions were constant (5%-7%). In our study, the minor allele distribution of ITPA polymorphism was 4%, which is almost comparable with previous reports. Previous studies have demonstrated the protective benefit of the minor allele A of the rs1127354 ITPA SNP against RBV-induced anemia during the first 12 wk of combination treatment[14,15,21-23]. Similarly, Hb decline > 3 g/dL was detected in about 64.5% of the anemia-susceptible group CC, which means that about 35.5% did not develop anemia.
The difference of Hb decline between the CC and non-CC groups throughout the treatment is comparable with previous studies[14,15,21,24]. Hb decline in the first 4 wk has been reported to be an independent predictor of development of anemia at some time in the course of treatment[25-29]. This indicates the value of early monitoring of Hb and the consequence of RBV dose adjustment to obviate further hazards of anemia. Anemia is a major cause of RBV dose reduction and premature withdrawal from treatment by 10%-14% of HCV-infected patients in the first 12 wk[6]. RBV dose reduction was obviously more necessary for patients in the CC group than for those in the protective non-CC group in the early weeks of treatment, which may explain the relative stability of the Hb level by week 16 in the CC group in comparison with the non-CC group. Since the mean age of all participants in this study was 35.5 years (range 21-50), their mean baseline Hb was 14 ± 1.1 g/dL and almost of them showed mild liver disease, thus there was no need for pretreatment reduction of the RBV dose. There are two explanations for the lack of statistical significance for the ITPA genotypes in multivariate analysis. First, the RBV dose in the univariate analysis showed higher significance than the ITPA genotype (0.0009 vs 0.025, respectively). Second, the colinearity between RBV dose reduction and hemoglobin decline makes the variable of RBV dose reduction strong enough to prevent the impact of ITPA genotypes in the multivariate analysis. This interpretation was previously confirmed by Domingo et al[15]. In previous studies[12-14,21-23], the ITPA genotypes were statistically significant in their multivariate analysis; this may be due to the different ethnic cohorts and the greater number of patients included in their studies. Accumulation of the RBV metabolite (triphosphorylated RBV) in RBCs causes a relative deficiency of ATP, and hence antioxidative damage of the cells with erythrophagocytosis[30]. Additionally, the phosphorylation of RBV is reversible in nucleated cells, and the half-life of RBV elimination from RBCs has been reported to be greater than from plasma, 40 d vs only 24 h respectively[2,11], which in turn enhances the destructive effect on RBCs. Recently, it has been reported that the accumulated ITP in RBCs starts to substitute guanosine triphosphate, which has already been depleted by RBV, for biosynthesis of ATP[31]. According to the classification of predicted ITPase deficiency by Thompson et al[14], we can classify our results of rs1127354 ITPA genotypes into _ , ++, +++ (CC, CA, and AA, respectively). The wild type CC usually shows no deficiency (-) with 100% ITPase activity, while the heterozygous genotype CA was predicted to have 25% of ITPase activity. The mutant homozygous AA genotype represents 0% activity (the highest level of deficiency +++). This deficiency in turn leads to accumulation of ITP inside RBCs, instead of RBV triphosphate[32].
There is a robust point in this study; we demonstrated the association between ITPA polymorphism (rs1127354) and RBV-induced anemia without resorting to checking the other SNP, rs7270101, of the same gene (a splice altering single-nucleotide polymorphism in intron 2). This was previously confirmed by Suzuki et al[33]. In our study, the difference in plt change of CC and non-CC genotype patients supported, to some extent, the previous results of Tanaka et al[34]; however, it was different than those reported by Thompson et al[14]. Tanaka et al[34] identified a significant difference in their population at a maximum plt reduction of < 30 × 109/L, but in the present study a significant difference was determined at plt count reduction of > 70 × 109/L. In the current study, we did not find a direct association between the ITPA genotype and plt count change. On the other hand, the association is thought to be indirect through the role of endogenous erythropoietin (EPO), which may increase to confront the reduction of Hb during treatment[25,34-36]. The later study showed sequence homology between EPO and thrombopoietin[36], which may lead to similarity in their action. Actually, the effect of EPO on the plt count is controversial. In the current study, EPO was not prescribed for any patient. Although EPO prescription is not stated in the established protocol of the Egyptian Ministry of Health for the treatment of hepatitis C in Egypt, some cases may require the addition of EPO in other studies.
There are some limitations in the present study: (1) the small number of female participants did not enable us to evaluate possible associations with gender; (2) the impact of the ITPA polymorphism on the treatment outcome has not been assessed. This may be attributed to the lower distribution rate of the ITPA protective minor allele among populations, which reflects its lack of significance to change the treatment outcome[12,14]; and (3) the unavailability of data to evaluate the endogenous serum level of EPO to determine if there was any association between it and the reactive increase in the plt count among the two ITPA groups.
Further research is needed to cover the following points: First, efficacy of the clinical use of this approach and the elucidation of its cost effectiveness. This may enable the physician to take precautions before starting therapy of those patients who are likely to develop anemia during therapy (ITPA CC genotype). These precautions may include pretreatment initial doses of EPO, initial reduction of RBV doses or even postponement of combined treatment in susceptible patients with no or mild liver disease. Second, verification is needed of the correlation between ITPA genotypes and RBV-induced hemolytic anemia by use of the new Specifically Targeted Antiviral Therapy for hepatitis C among Egyptian HCV patients. Third, the impact of ITPA polymorphism should be determined in targeted cohorts of Egyptian HCV patients, including old age populations and those suffering from advanced liver disease or chronic kidney disease. Finally, the association between ITPA variants and the plasma concentration of RBV in Egyptian patients should be investigated.
In summary, the minor allele in ITPA rs1127354 variants (CA/AA) plays a decisive role in protection against treatment-induced anemia and RBV dose reduction of Egyptian HCV patients. Additionally, pretreatment clinical decisions regarding RBV dose adjustment can be bolstered by identifying such polymorphisms.
We would like to thank all of the doctors of the Assiut Center for the Treatment of Chronic Hepatitis, Egypt for sample gathering. We thank Mr. Hisham Galal, the Technician in the Department of Microbiology and Immunology, Egypt, for sample analysis. We appreciate the effort of Mr. Yoshitaka Etoh for his lab work on ITPA SNP genotyping.
Hepatitis C infection is a widespread disease caused by hepatitis C virus (HCV). It infects the liver, mainly causing acute disease developing to chronic if left without treatment. Its current standard therapy is a combination therapy of pegylated interferon injection plus oral ribavirin drug. The treatment is effective in more than half of patients but has moderate to severe side effects. Anemia is a common side effect which may be a leading cause of decreasing the doses of therapy or even premature withdrawal of the treatment. Herein, the authors reported a correlation between host genetic polymorphisms and pre-treatment prediction of anemia in Egyptian HCV patients.
Due to the multiple side effects associated with the therapy of hepatitis C disease, many researchers are focusing on studying the pretreatment predictors of these side effects. This will help in obviating the dose reduction and the premature withdrawal of therapy. Although Egypt has the highest rate of HCV infection, no research has been done on its HCV patients in that field. Therefore, the authors of this study targeted this cohort.
The authors reported, for the first time, the implication of inosine triphosphate pyrophosphatase (ITPA) single nucleotide polymorphism (SNP) genotypes in predicting the incidence of anemia in Egyptian HCV patients during the combination therapy. The mutant genotype of this polymorphism has a crucial role in protection against treatment-induced anemia and ribavirin (RBV) dose reduction in Egyptian HCV patients. Further studies are needed to elucidate the cost effectiveness of this approach.
ITPA genotyping is a promising pretreatment predictor of treatment-induced anemia before starting the combination therapy of HCV disease. This will enhance the pretreatment decision of early adjustment of RBV dose in patients with the unfavorable genotype.
SNP is the most common type of genetic variation of the human DNA. There are millions of human SNPs. They occur when a single nucleotide in a genome differs among members of the same species. This difference is due to substitution, insertion or deletion of one nucleotide within the genetic structural unit of human DNA. Many studies have found a robust correlation between SNPs and different diseases.
The present study provides interesting results and novelty to genetic factors related to ribavirin-induced anemia during HCV therapy in an Egyptian HCV cohort. The manuscript is well written and the conclusion drawn in the data is appropriate.
| 1. | World Health Organization. Hepatitis C Fact Sheet (Accessed February 8, 2012). 2011; Available from: http: //www.who.int/mediacentre/factsheets/fs164/en/. |
| 2. | Hayashi J, Furusyo N, Ariyama I, Sawayama Y, Etoh Y, Kashiwagi S. A relationship between the evolution of hepatitis C virus variants, liver damage, and hepatocellular carcinoma in patients with hepatitis C viremia. J Infect Dis. 2000;181:1523-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Dartmouth Medical Center. Hepatitis C: An Epidemic for Anyone. Available from: http: //www.epidemic.org/theFacts/theEpidemic. World Prevalence Last Updated 2010. |
| 4. | Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Aly Ohn ES, Anwar W. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887-891. [PubMed] |
| 5. | Mostafa A, Taylor SM, el-Daly M, el-Hoseiny M, Bakr I, Arafa N, Thiers V, Rimlinger F, Abdel-Hamid M, Fontanet A. Is the hepatitis C virus epidemic over in Egypt? Incidence and risk factors of new hepatitis C virus infections. Liver Int. 2010;30:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [PubMed] |
| 7. | Furusyo N, Katoh M, Tanabe Y, Kajiwara E, Maruyama T, Shimono J, Sakai H, Nakamuta M, Nomura H, Masumoto A. Interferon alpha plus ribavirin combination treatment of Japanese chronic hepatitis C patients with HCV genotype 2: a project of the Kyushu University Liver Disease Study Group. World J Gastroenterol. 2006;12:784-790. [PubMed] |
| 8. | Krishnan SM, Dixit NM. Ribavirin-induced anemia in hepatitis C virus patients undergoing combination therapy. PLoS Comput Biol. 2011;7:e1001072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Kowdley KV. Hematologic side effects of interferon and ribavirin therapy. J Clin Gastroenterol. 2005;39:S3-S8. [PubMed] |
| 10. | Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850. [PubMed] |
| 11. | De Franceschi L, Fattovich G, Turrini F, Ayi K, Brugnara C, Manzato F, Noventa F, Stanzial AM, Solero P, Corrocher R. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology. 2000;31:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 331] [Article Influence: 12.7] [Reference Citation Analysis (8)] |
| 12. | Fellay J, Thompson AJ, Ge D, Gumbs CE, Urban TJ, Shianna KV, Little LD, Qiu P, Bertelsen AH, Watson M. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature. 2010;464:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 373] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 13. | Ochi H, Maekawa T, Abe H, Hayashida Y, Nakano R, Kubo M, Tsunoda T, Hayes CN, Kumada H, Nakamura Y. ITPA polymorphism affects ribavirin-induced anemia and outcomes of therapy--a genome-wide study of Japanese HCV virus patients. Gastroenterology. 2010;139:1190-1197. [PubMed] |
| 14. | Thompson AJ, Fellay J, Patel K, Tillmann HL, Naggie S, Ge D, Urban TJ, Shianna KV, Muir AJ, Fried MW. Variants in the ITPA gene protect against ribavirin-induced hemolytic anemia and decrease the need for ribavirin dose reduction. Gastroenterology. 2010;139:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | Domingo P, Guardiola JM, Salazar J, Torres F, Mateo MG, Pacho C, Del Mar Gutierrez M, Lamarca K, Fontanet A, Martin J. Association of ITPA gene polymorphisms and the risk of ribavirin-induced anemia in HIV/hepatitis C virus (HCV)-coinfected patients receiving HCV combination therapy. Antimicrob Agents Chemother. 2012;56:2987-2993. [PubMed] |
| 16. | Arenas M, Duley J, Sumi S, Sanderson J, Marinaki A. The ITPA c.94C& gt; A and g.IVS2+21A& gt; C sequence variants contribute to missplicing of the ITPA gene. Biochim Biophys Acta. 2007;1772:96-102. [PubMed] |
| 17. | Ogawa E, Furusyo N, Toyoda K, Taniai H, Otaguro S, Kainuma M, Murata M, Sawayama Y, Hayashi J. Excellent superiority and specificity of COBAS TaqMan HCV assay in an early viral kinetic change during pegylated interferon alpha-2b plus ribavirin treatment. BMC Gastroenterol. 2010;10:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3128] [Article Influence: 104.3] [Reference Citation Analysis (1)] |
| 19. | Marsh S, King CR, Ahluwalia R, McLeod HL. Distribution of ITPA P32T alleles in multiple world populations. J Hum Genet. 2004;49:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Cao H, Hegele RA. DNA polymorphisms in ITPA including basis of inosine triphosphatase deficiency. J Hum Genet. 2002;47:620-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Thompson AJ, Santoro R, Piazzolla V, Clark PJ, Naggie S, Tillmann HL, Patel K, Muir AJ, Shianna KV, Mottola L. Inosine triphosphatase genetic variants are protective against anemia during antiviral therapy for HCV2/3 but do not decrease dose reductions of RBV or increase SVR. Hepatology. 2011;53:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Azakami T, Hayes CN, Sezaki H, Kobayashi M, Akuta N, Suzuki F, Kumada H, Abe H, Miki D, Tsuge M. Common genetic polymorphism of ITPA gene affects ribavirin-induced anemia and effect of peg-interferon plus ribavirin therapy. J Med Virol. 2011;83:1048-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Sakamoto N, Tanaka Y, Nakagawa M, Yatsuhashi H, Nishiguchi S, Enomoto N, Azuma S, Nishimura-Sakurai Y, Kakinuma S, Nishida N. ITPA gene variant protects against anemia induced by pegylated interferon-α and ribavirin therapy for Japanese patients with chronic hepatitis C. Hepatol Res. 2010;40:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Bodenheimer HC, Lindsay KL, Davis GL, Lewis JH, Thung SN, Seeff LB. Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: a multicenter trial. Hepatology. 1997;26:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 264] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Kobayashi T, Hige S, Terashita K, Nakai M, Horimoto H, Sho T, Nakanishi M, Ogawa K, Chuma M, Sakamoto N. Anemia and thrombocytosis induced by ribavirin monotherapy in patients with chronic hepatitis C. J Gastroenterol. 2012;47:1228-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Hiramatsu N, Kurashige N, Oze T, Takehara T, Tamura S, Kasahara A, Oshita M, Katayama K, Yoshihara H, Imai Y. Early decline of hemoglobin can predict progression of hemolytic anemia during pegylated interferon and ribavirin combination therapy in patients with chronic hepatitis C. Hepatol Res. 2008;38:52-59. [PubMed] |
| 27. | Sievert W, Dore GJ, McCaughan GW, Yoshihara M, Crawford DH, Cheng W, Weltman M, Rawlinson W, Rizkalla B, Depamphilis JK. Virological response is associated with decline in hemoglobin concentration during pegylated interferon and ribavirin therapy in hepatitis C virus genotype 1. Hepatology. 2011;53:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Oze T, Hiramatsu N, Kurashige N, Tsuda N, Yakushijin T, Kanto T, Takehara T, Kasahara A, Kato M, Yoshihara H. Early decline of hemoglobin correlates with progression of ribavirin-induced hemolytic anemia during interferon plus ribavirin combination therapy in patients with chronic hepatitis C. J Gastroenterol. 2006;41:862-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Reau N, Hadziyannis SJ, Messinger D, Fried MW, Jensen DM. Early predictors of anemia in patients with hepatitis C genotype 1 treated with peginterferon alfa-2a (40KD) plus ribavirin. Am J Gastroenterol. 2008;103:1981-1988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Homma M, Hosono H, Hasegawa Y, Kohda Y. Morphological transformation and phosphatidylserine exposure in erythrocytes treated with ribavirin. Biol Pharm Bull. 2009;32:1940-1942. [PubMed] |
| 31. | Hitomi Y, Cirulli ET, Fellay J, McHutchison JG, Thompson AJ, Gumbs CE, Shianna KV, Urban TJ, Goldstein DB. Inosine triphosphate protects against ribavirin-induced adenosine triphosphate loss by adenylosuccinate synthase function. Gastroenterology. 2011;140:1314-1321. [PubMed] |
| 32. | Shipkova M, Lorenz K, Oellerich M, Wieland E, von Ahsen N. Measurement of erythrocyte inosine triphosphate pyrophosphohydrolase (ITPA) activity by HPLC and correlation of ITPA genotype-phenotype in a Caucasian population. Clin Chem. 2006;52:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Suzuki F, Suzuki Y, Akuta N, Sezaki H, Hirakawa M, Kawamura Y, Hosaka T, Kobayashi M, Saito S, Arase Y. Influence of ITPA polymorphisms on decreases of hemoglobin during treatment with pegylated interferon, ribavirin, and telaprevir. Hepatology. 2011;53:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Tanaka Y, Kurosaki M, Nishida N, Sugiyama M, Matsuura K, Sakamoto N, Enomoto N, Yatsuhashi H, Nishiguchi S, Hino K. Genome-wide association study identified ITPA/DDRGK1 variants reflecting thrombocytopenia in pegylated interferon and ribavirin therapy for chronic hepatitis C. Hum Mol Genet. 2011;20:3507-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 35. | Tseng KC, Chen LH, Chen CY, Chang TT, Chou AL, Wu IC, Cheng PN. Low dose erythropoietin-beta improves anemia and maintains ribavirin dose in chronic hepatitis C patients receiving combination therapy with ribavirin plus pegylated interferon Alfa-2b. Hepatol Res. 2009;39:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Bilic E, Bilic E. Amino acid sequence homology of thrombopoietin and erythropoietin may explain thrombocytosis in children with iron deficiency anemia. J Pediatr Hematol Oncol. 2003;25:675-676. [PubMed] |
P- Reviewers Fusco DN, Steinmann E S- Editor Song XX L- Editor Logan S E- Editor Li JY