INTRODUCTION
The performance of hepatic surgery without a parenchyma-sparing strategy carries significant risks for patient survival because of the not negligible occurrence of postoperative liver failure, which is definitely related to the amount of the sacrificed parenchyma[1,2]. Indeed, major or extended hepatic resections are independent negative prognostic factors with regard to short- and long-term outcomes[2-7]. The key factor of modern hepatic surgery is the use of the intraoperative ultrasound (IOUS) not only to stage the disease, but more importantly to guide resection, with the specific aim of maximizing parenchyma-sparing, removing only the tumoral tissue[8]. Whether in patients with hepatocellular carcinoma (HCC) or in patients with colorectal liver metastasis (CLM), IOUS allows the performance of the so-called “conservative but radical surgery”[9], which is the pivotal factor to offer a chance of cure to an increasing proportion of patients, who until few years ago were considered only for palliative care. Indeed, in cases of HCC with cirrhosis the underlying liver function is generally marginal, and the prognosis of the patient might be more related to the residual liver function rather than to the presence of HCC. In such patients, the hepatectomy should always be tailored on the basis of both tumoral features and functional liver reserve. Similarly, in cases of CLM the rationale of the surgical approach described here is based on the need to minimize the rate of major or extended resections with the aim of reducing operative risk, and at the same time preserving the liver parenchyma, which could be the site for future hepatic recurrence potentially re-treated with curative intent.
OPERATIVE TECHNIQUE
The J-shaped laparotomy is the preferred incision for liver surgery, and the access into the right thoracic cavity following the 9th intercostal space is carried out to control the hepatocaval confluence. In particular, the thoracoabdominal approach is selected in obese patients, in patients with a deep chest, and during complex reoperations. Thus, the liver is partially mobilized by dividing the round and the falciform ligaments. Sometimes the coronary and triangular ligaments are also divided early to obtain enough space for IOUS. This should in fact be performed before complete mobilization of the liver to avoid any artifact made by the surgical maneuvers.
INTRAOPERATIVE ULTRASOUND
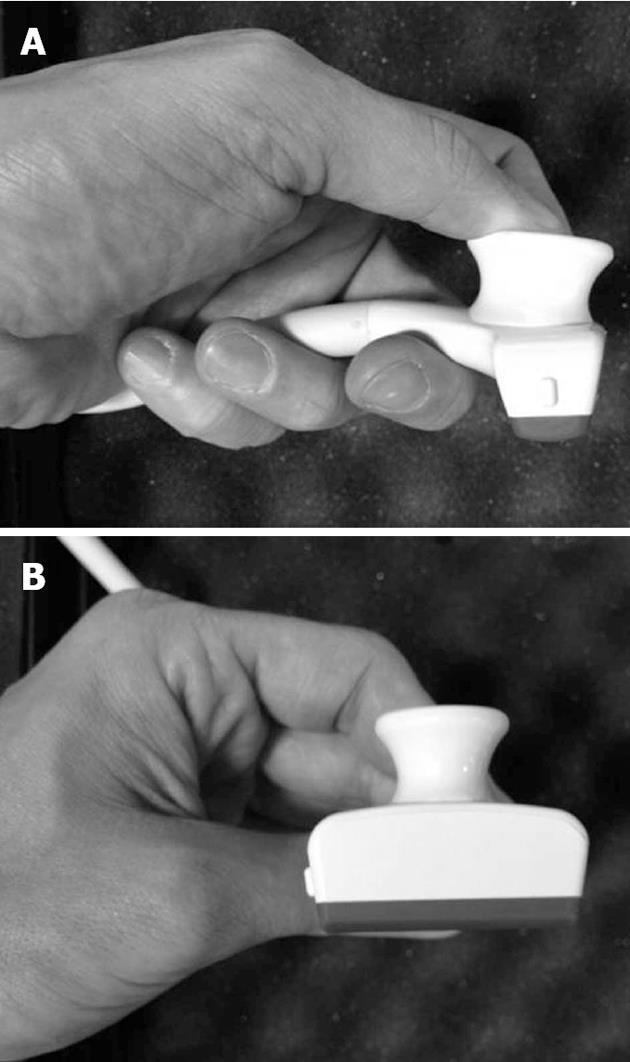
IOUS is the procedure of choice to stage disease in patients with liver tumors. It should be fully performed by the surgeon in charge for the operation rather than by the assistants, radiologists or technicians. This is because the information gathered during the exploration requires interpretation to have most impact on the surgical strategy. Thus, IOUS is mainly performed to plan the surgical strategy rather than to locate the lesions. Generally, high frequency probes (7.5-10 MHz) are recommended for IOUS, since they allow for a higher spatial resolution than those working at lower frequencies (3.5-5 MHz). However, those latter probes are very useful for the initial exploration providing a better panoramic view. Lower frequency probes are also useful for allowing contrast-enhanced IOUS. Different shapes of probes are available for intraoperative use: the linear T-shaped, the inter-digital, and micro-convex probes. The best probe is the one that ensures the optimal compromise between the volume of the probe itself, which should be minimal, the scanning windows, which should be the largest, and the stability once in contact with the liver surface. In this sense, a new micro-linear probe with trapezoid scanning windows probably represents the best compromise among all the aforementioned requirements; this probe is furthermore designed to meet the requirements for those surgical maneuvers discussed here (Figure 1). Of note, the performance of IOUS may take time, and it requires experience to be effective and beneficial[10].
Figure 1 New probe for intraoperative ultrasound.
This probe has a trapezoid scanning area, and an ergonomic shape, which help during intraoperative ultrasound-guided maneuvers. A: Lateral view; B: Front view.
RESECTION GUIDANCE
Apart from staging, IOUS is essential to guide resection. It is almost impossible to correctly define the hepatic segmental boundaries without IOUS, nor the boundaries of the tumor itself because of the existing wide variations in anatomy. The main advantage of IOUS-guided resection is modification of the traditional approach to liver tissue dissection, which involves dissection in vertical planes to avoid tumor exposure on the cut surface. With IOUS, the relationship between the dissection plane and the tumor edges can be followed in real time, and the direction of the dissection plane can be modified when needed. Versatile dissection planes around the tumors can avoid tumor exposure while sparing important vascular structures, thus sparing vital liver parenchyma. This approach has been recently redefined by the authors as the “radical but conservative approach”, and should be applied in liver surgery to maximize the results[9]. Also, in patients in whom major resections should be required, IOUS allows better design of the dissection plane, leading to conservative surgery even in patients with complex tumoral presentations[11]. Specific, and original IOUS techniques have already been developed to help the surgeon during the operation[12-15]. The following paragraphs will focus on two crucial techniques for defining the area of resection using IOUS findings.
PLANNING OF THE SURGICAL STRATEGY
The information achieved from the preoperative imaging workup, which has an essential role in staging intra- and extra-hepatic disease, should be used to plan the surgical strategy. However, the surgical strategy should be intraoperatively defined only after IOUS exploration. The impact of IOUS on the operative decision-making, when compared with that of preoperative imaging techniques, is reported to be around 4%-7%[16,17]. These relatively low rates may be explained because of the different surgical policies applied by the different centers as well as the different tumor types considered. Indeed, IOUS, when used in a systematic and extensive way to map the tumor nodules, allows a 3-dimensional reconstruction of the relationships between the tumor and the main intrahepatic vascular structures [glissonian pedicles and hepatic veins (HVs)], which is pivotal in planning the individualized surgical strategy for each patient. Indeed, some experienced authors reported better results in terms of IOUS accuracy[18-21]. Some important tumor-vessel relationship rules have been developed by the authors, both for HCC and for CLM, with the aim of providing an intraoperative guide to individualize the surgical strategy and minimize parenchyma sacrifice.
Tumor in contact with a glissonian pedicle
The glissonian pedicle may be spared when in contact with an encapsulated HCC or with a CLM once the integrity of the vessel wall is confirmed at IOUS, without any sign of distal bile duct dilation. For CLM, the contact should extend for less than one-third of the pedicle circumference. In the presence of bile duct dilation, tumor thrombus, or invasion of the vessel wall, the pedicle must be divided[9].
Tumor in contact with a HV
The HV may be spared when in contact with an encapsulated HCC or with CLM once the integrity of the vessel wall is confirmed at IOUS. For CLM, the contact should extent for less than two-thirds of the vessel circumference. Thus, in the presence of a tumor thrombus, invasion of the vessel wall, and wider contact the HV must be divided[15]. However, as described below, the extension of the hepatectomy to the portion of the liver theoretically drained by the resected HV is not systematically performed, but only when accessory HVs and/or communicating veins are missing or when inversion of the portal flow is demonstrated by IOUS[22].
INFLOW MODULATION
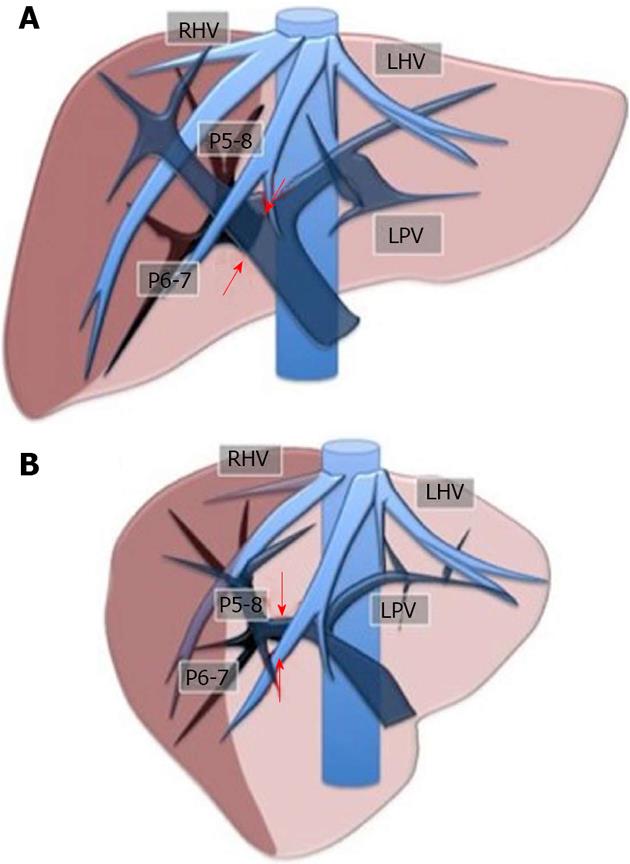
Initially used for tumors located in the left hemiliver[14], the inflow modulation technique has more recently been successfully extended to any liver segment[23], including segment 8, and even to sectional portions of the liver[24]. Once the feeding portal branch is identified at IOUS, it can be compressed using the IOUS probe by one side of the liver, and by the finger in the opposite side with the aim to induce a transient ischemia of the portion of the liver distal to the compression site. This portion can then be marked with the electrocautery, and when released, resection can be performed. This technique is simple, fast, non-invasive, and reversible. Also, the possibility of modifying the site of the compression, and then the corresponding resection volume allows tailoring of the resection according to tumor features, and more importantly to the status of the background liver. This is of paramount importance in patients with HCC and cirrhosis, in which the functional liver reserve may be marginal. Such a technique allows for precise anatomical resection of a subsegment, segment or section of the liver. As is well-established, anatomical resection of HCC is recommended to offer a higher chance of cure[25-29]. For segments such as segment 1 and 4 superior, for which direct compression of the feeding portal branch may not be feasible, the compression of the adjacent segmental branches allows definition of their segmental margins. Indeed, our technique can be used in a counter-compression perspective similar to the counter-staining technique reported by Takayama et al[30]. Figure 2 illustrates the layout of the liver with the compression technique applied to delineate the right posterior section, while Figures 3 and 4 show an actual case.
Figure 2 Layout of the liver for the inflow modulation.
Ischemic demarcation of the right posterior sector by intraoperative ultrasound-guided finger compression at its origin of the right portal bifurcation. A: Front view; B: Lateral view. RHV: Right hepatic vein; LHV: Left hepatic vein; LPV: Left portal vein; P5-8: Right anterior portal braches; P6-7: Right posterior portal branches. The arrows indicate the point for the compression.
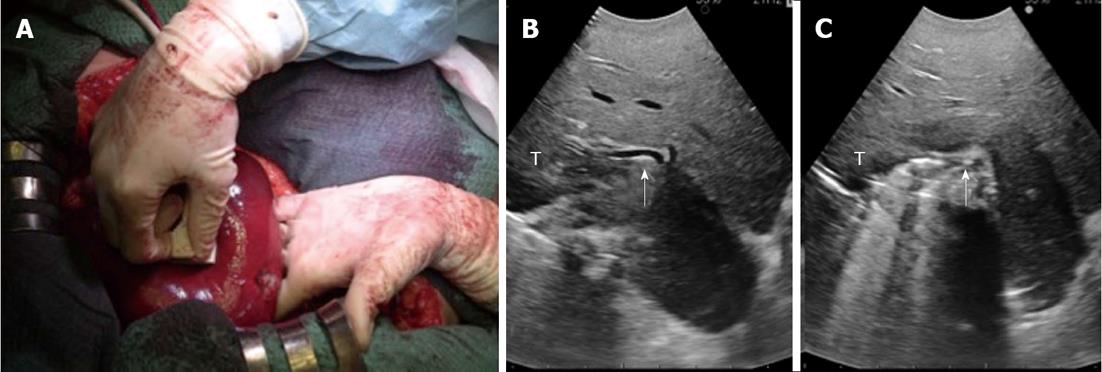
Figure 3 A case of intraoperative ultrasound-guided finger compression of segment 6.
A: The portal pedicle for segment 6 is compressed by the probe in the right hand and by the finger in the left hand; B: Intraoperative ultrasound (IOUS) focused on the portal pedicle (arrow) for segment 6 before the compression; C: IOUS focused on the portal pedicle (arrow) for segment 6 during the compression. T: Tumor.
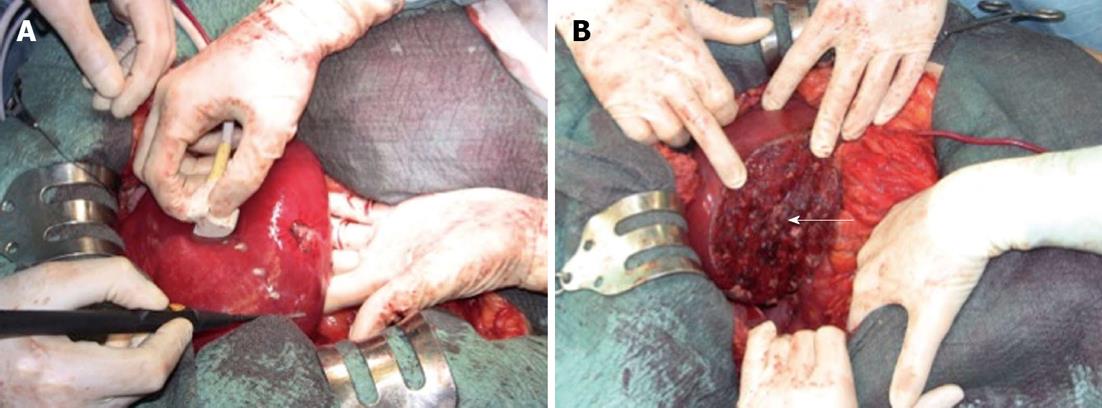
Figure 4 Demarcation of the compressed area by electrocautery.
A: The operative field before the resection; B: The operative field at the end of the resection. The arrow indicates the stump of the portal pedicle for segment 6.
OUTFLOW MODULATION
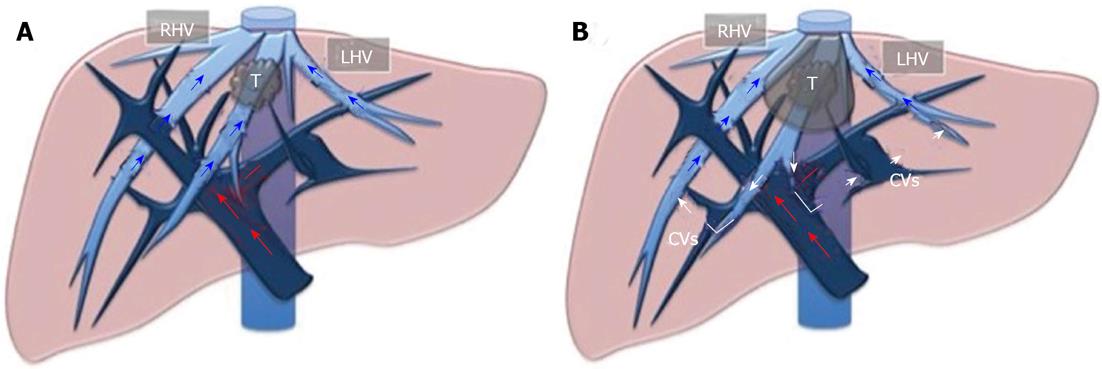
The area of resection may be tailored not only using US-guided finger compression of the portal branch as described above, but also using IOUS outflow modulation. Indeed, we have already showed how to minimize the sacrifice of liver parenchyma even in those patients with a tumor at the hepatocaval confluence, for which a standard major or extended hepatectomy should be indicated based on traditional criteria. In addition, we have introduced some new operations, such as mini-mesohepatectomy, and systematic extended right posterior sectionectomy[22-31], which simultaneously limit the need for formal major resection, and improve the chances of resection for those patients with complex tumoral presentation. The definition of the resection area using the outflow control is based on the extensive use of IOUS flow analyses, with the aim of checking the outflow modifications once the HV that should be resected is clamped. For this purpose rather than the direct closure of the vein by a vessel loop, the US-guided fingertip compression at the caval confluence might be adequate[32]. Certainly, the HV may already be closed by the tumor. At that case, the search is focused on at least one of the following criteria: reversal of flow direction in the peripheral portion of the compressed HV, which suggests drainage through the collateral circulation in adjacent HVs or inferior vena cava (IVC); direct detection of collaterals between the compressed HV and adjacent HV or IVC; or persistence of hepatopetal flow in the portal branches corresponding to the area drained by the compressed HV. In particular, in the case of hepatofugal flow direction in the portal branches, the resection should not be minimal but extended to the parenchyma fed by those portal branches. The presence of hepatofugal flow in the portal branches is a clear signal of insufficient drainage of the corresponding HV. Once at least one of the aforementioned criteria has been satisfied, full mobilization of the right and left hemiliver is performed, preserving most of the posterior short HVs to minimize the risk of congestion of the residual liver. Thus, the area of resection may be marked on the liver surface using electrocautery and IOUS to define the caudal, medial and lateral limits of the parenchyma to be removed, while the surgeon’s left fingertip is visualized in the most cranial portion, and it is used to mark the dissection area. Parenchyma transection is then carried out with the surgeon’s left hand behind the right hemiliver with the aim of guiding resection by the right hand in real time. Figure 5 illustrates the layout of the liver with the outflow modulation technique, while Figure 6 shows an actual case.
Figure 5 Layout of the liver for outflow modulation.
A: A tumor in contact with the middle hepatic vein at the caval confluence; B: Once that vein is infiltrated and/or compressed, some collateral veins (CVs) shunting the flow from the middle hepatic vein territory to right hepatic vein (RHV) and/or left hepatic vein (LHV) territories can be detected. T: Tumor.
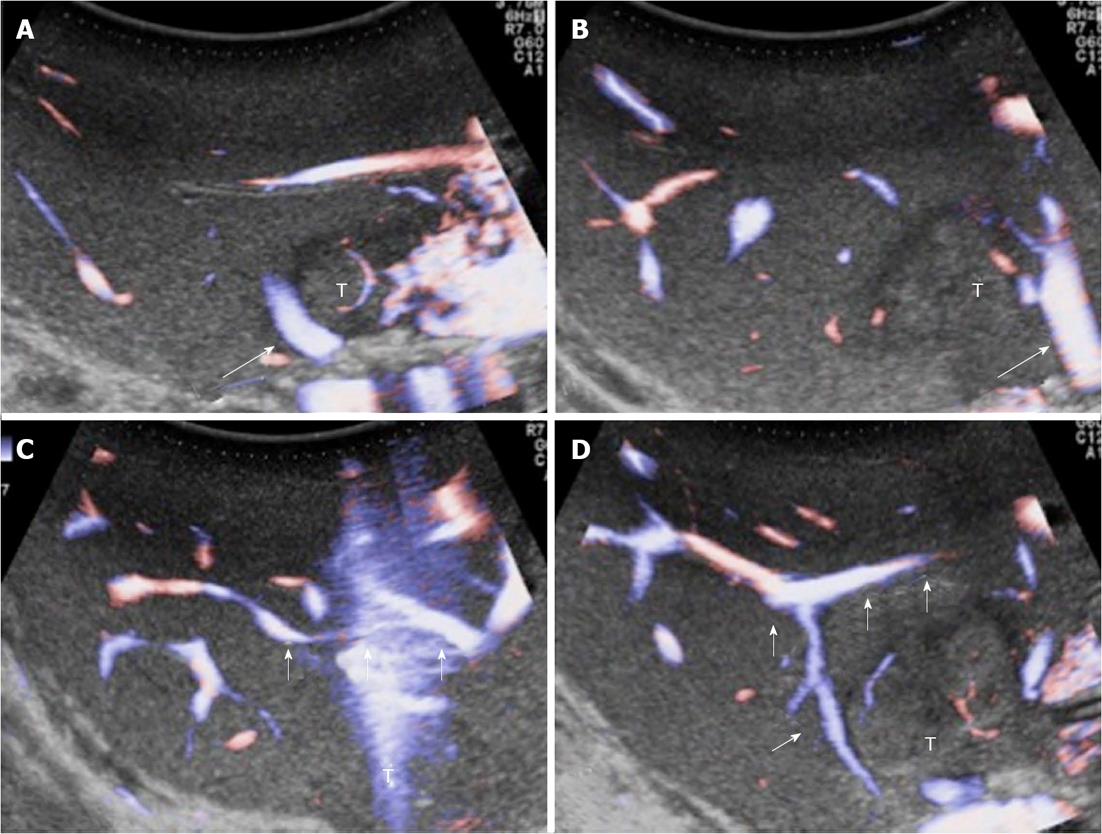
Figure 6 Intraoperative ultrasound study of communicating veins.
A: A tumour located between the middle hepatic vein (MHV) (arrow) and the left hepatic vein (LHV) at their confluence into the inferior vena cava; B: The arrow indicates the LHV; C, D: Evidence of communicating veins (arrows) between the LHV and the MHV. T: Tumor.
PROBLEM OF THE SURGICAL MARGIN
One of the main criticisms of this surgical approach is the problem of the surgical margin. Both for HCC and CLM the detachment of the tumor from a spared vessel may mean zero millimeters surgical margin, which traditionally is classified as R1 resection by the pathologist. Indeed, exposure of the tumor on the dissection plane is sometimes required to spare intrahepatic major vascular structures, which is the mainstay of our surgical policy. However, the effect of surgical margin status on survival of patients with HCC and CLM has been studied, but controversy still remains among surgeons. There is still debate about the real impact of the extent of the surgical margin once tumoral tissue is removed from the cut surface. For HCC, some authors reported that a margin smaller than 1 cm and even 2 cm plays a negative role in terms of long-term survival, while others authors found that a 0 mm margin is acceptable[33-37]. Also for CLM, there is no definitive agreement on the surgical margin[38,39]. It is well known that a positive margin is associated with increased risk of recurrence, but its width does not affect survival[40,41]. Moreover, it has been shown that patients with complex tumoral presentation treated with R1 resection may have the same long-term survival of patients treated with R0 resection if aggressively treated with modern chemotherapy and repeated surgery[42]. Therefore, an anticipated minimal negative surgical margin should not be used as exclusion criterion for resection of HCC or CLM. The keystone is the performance of IOUS to guide the resection with the aim of achieving complete tumor clearance to minimize the risk of non-curative surgery.
In conclusions, IOUS is the best method for staging a liver tumor, and it is certainly the best method for the surgeon to understand in real-time the liver anatomy, and the relationships between tumors and intrahepatic vessels, thus allowing effective surgical operations. IOUS guidance allows for expanding indications offering the chance of cure to a greater proportion of patients, who would otherwise be excluded from the surgical program or submitted to more traditional but more risky operations. A precise tailoring of the area of hepatic resection using inflow and outflow modulation should be part of the surgical armamentarium of the modern liver surgeon.
P- Reviewer Ooi LL S- Editor Gou SX L- Editor Cant MR E- Editor Xiong L