Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.784
Revised: November 10, 2012
Accepted: December 15, 2012
Published online: February 7, 2013
Processing time: 113 Days and 16.4 Hours
Mucositis is a known complication following use of chemotherapy, but fatal mucositis is unusual and management of such cases may be challenging. Pathologically there is denudation of mucosa of gastrointestinal tract. Severe cases can develop ileus and even perforation of bowel wall. We report here a case of multiple myeloma who developed World Health Organization grade 4 gut mucositis following the use of high dose melphalan with the expulsion of “intestine-like” material.
- Citation: Sharma SK, Handoo A, Choudhary D, Dhamija G, Gupta N. Severe gastrointestinal mucositis following high dose melphalan therapy for multiple myeloma. World J Gastroenterol 2013; 19(5): 784-785
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/784.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.784
Mucositis is the most common complication of chemotherapeutics agents. Melphalan when used in high doses for treatment for multiple myeloma can lead to severe mucositis[1,2]. Patient can develop gastrointestinal ulcers, vomiting and diarrhea with the use of high dose melphalan as a conditioning regimen for autologous transplant for multiple myeloma[3-5]. Pathologically there is denudation of mucosa of gastrointestinal tract. Severe cases can develop ileus and even perforation of bowel wall. We report here a case of multiple myeloma who developed World Health Organization (WHO) grade 4 gut mucositis following the use of high dose melphalan with the expulsion of “intestine-like” material.
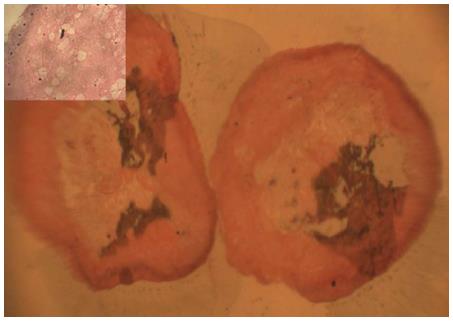
A 62-years-old male was admitted for autologous stem cell transplant for multiple myeloma. He was in complete remission following 4 cycles of bortezomib and dexamethasone. Filgrastim (G-CSF) 10 mg/kg was given for 4 d for peripheral stem cell mobilization followed by melphalan 200 mg/m2 as conditioning regimen. The CD34+ stem cell dose infused was 6 × 106/kg. Cephasol (supersaturated calcium phosphate rinse) was used six times daily to prevent oral mucositis. On day 3 patient developed vomitings and by day 5 he had WHO grade 4 gut mucositis with ileus. Patient also developed fever and was started on empirical antibiotics including meropenem (1 g three times daily, intravenously), metronidazole (500 mg three times daily, intravenously), and teicoplanin (400 mg once daily, intravenously) as per hospital policy, alongwith Ryle’s tube aspiration and parenteral feeding. On day 7 he passed 25 cm × 1 cm pinkish, mucosa coated partly digested material mimicking segment of small intestine (Figure 1). Histopathological section of the segment revealed numerous neutrophils surrounding the partly digested material, without any muscle tissue (Figure 2). Blood culture was negative for bacteria and fungus. Viral serology tests for cytomegalovirus immunoglobulin M (IgM) and herpes simplex virus 1 and 2 IgM were negative. Stool examination did not reveal ova or cysts and was negative for Clostridial difficile toxin. Computed tomography of abdomen showed dilated bowel loops, with caecal wall thickness of 10 mm, there was no evidence of perforation or intussusception. Though he had neutrophil engraftment (absolute neutrophil count more than 0.5 × 109/L) on day 13, platelets remained below 10 × 109/L. Patient succumbed on day 20 because of enterocolitis and sepsis.
Oral and gastrointestinal mucositis due to cancer chemotherapy or radiotherapy continues to be an important clinical problem. Alimentary tract mucositis can involve any part of oral and gastrointestinal mucosa, from the mouth to the anus. The incidence of WHO grade 3 or 4 gastrointestinal mucositis can be as high as 20%-60% in patients undergoing hematopoietic stem cell transplantation, depending on the intensity of the conditioning regimen used[3,4]. In the study by Krishna et al[2], of the 47 patients who developed lower alimentary tract mucositis there was no mortality. Our patient developed severe gastrointestinal toxicity following high dose of melphalan, with excretion of a large mucosa coated segment mimicking part of the small intestine, and succumbed to the complications related to mucositis.
| 1. | Peterson DE, Bensadoun RJ, Roila F. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22 Suppl 6:vi78-vi84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Krishna SG, Zhao W, Grazziutti ML, Sanathkumar N, Barlogie B, Anaissie EJ. Incidence and risk factors for lower alimentary tract mucositis after 1529 courses of chemotherapy in a homogenous population of oncology patients: clinical and research implications. Cancer. 2011;117:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Vera-Llonch M, Oster G, Ford CM, Lu J, Sonis S. Oral mucositis and outcomes of autologous hematopoietic stem-cell transplantation following high-dose melphalan conditioning for multiple myeloma. J Support Oncol. 2007;5:231-235. [PubMed] |
| 4. | Tuncer HH, Rana N, Milani C, Darko A, Al-Homsi SA. Gastrointestinal and hepatic complications of hematopoietic stem cell transplantation. World J Gastroenterol. 2012;18:1851-1860. [PubMed] |
| 5. | Grazziutti ML, Dong L, Miceli MH, Krishna SG, Kiwan E, Syed N, Fassas A, van Rhee F, Klaus H, Barlogie B. Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: incidence, risk factors and a severity predictive model. Bone Marrow Transplant. 2006;38:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
P- Reviewer Grant G S- Editor Gou SX L- Editor A E- Editor Zhang DN