Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.761
Revised: November 20, 2012
Accepted: December 25, 2012
Published online: February 7, 2013
Processing time: 184 Days and 18.5 Hours
AIM: To assess the impact of preoperative neoadjuvant bevacizumab (Bev) on the outcome of patients undergoing resection for colorectal liver metastases (CLM).
METHODS: Eligible trials were identified from Medline, Embase, Ovid, and the Cochrane database. The data were analyzed with fixed-effects or random-effects models using Review Manager version 5.0.
RESULTS: Thirteen nonrandomized studies with a total of 1431 participants were suitable for meta-analysis. There was no difference in overall morbidity and severe complications between the Bev + group and Bev - group (43.3% vs 36.8%, P = 0.06; 17.1% vs 11.4%, P = 0.07, respectively). Bev-related complications including wound and thromboembolic/bleeding events were also similar in the Bev + and Bev - groups (14.4% vs 8.1%, P = 0.21; 4.1% vs 3.8%, P = 0.98, respectively). The incidence and severity of sinusoidal dilation were lower in patients treated with Bev than in patients treated without Bev (43.3% vs 63.7%, P < 0.001; 16.8% vs 46.5%, P < 0.00, respectively).
CONCLUSION: Bev can be safely administered before hepatic resection in patients with CLM, and has a protective effect against hepatic injury in patients treated with oxaliplatin chemotherapy.
- Citation: Li DB, Ye F, Wu XR, Wu LP, Chen JX, Li B, Zhou YM. Preoperative administration of bevacizumab is safe for patients with colorectal liver metastases. World J Gastroenterol 2013; 19(5): 761-768
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/761.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.761
The liver is the most common metastatic site of colorectal cancer (CC). Approximately 50% of CC patients develop colorectal liver metastases (CLM) over the course of the disease[1]. Hepatectomy is the most important modality in the treatment of CLM, offering a 5-year survival rate of approximately 30%-65%[2]. However, the long-term survival of CLM patients remains poor due to the high rate of recurrence and metastases after surgery. It is reported that neoadjuvant chemotherapy before hepatectomy is associated with improved survival of CLM patients[3,4].
Bevacizumab (Bev) is a monoclonal antibody against vascular endothelial growth factor (VEGF) and can inhibit the growth of human tumor xenografts. In addition to inhibiting the antiangiogenic effect, Bev may also improve the delivery of chemotherapy by altering the tumor vasculature and decreasing the elevated interstitial pressure in tumors[5]. Randomized clinical trials[6,7] have demonstrated that the addition of Bev to fluorouracil-based combination chemotherapy can improve overall survival, the response rate and duration of response, and prolong time to disease progression in CLM patients with metastatic colorectal cancer. For this reason, some researchers advocate the use of Bev in the neoadjuvant setting before surgery for resectable CLM[8-11]. However, Bev is known to be associated with bleeding, thrombosis, gastrointestinal perforation, impaired wound healing and liver regeneration[8], thus alerts surgeons to the safe use of Bev at the time of hepatic surgery.
Chemotherapy-specific liver injuries have been more frequently reported as a disadvantage with the use of oxaliplatin in particular, because it is associated with the occurrence of sinusoidal dilation, a distinctive type of hepatic and vascular injury characteristic of hepatic veno-occlusive disease[12]. Nakano et al[13] reported that such injury was associated with increased morbidity and mortality after hepatectomy in CLM patients. Although the etiology of sinusoidal dilation remains unclear, it has been documented that VEGF is one of the causative cytokines for the development of sinusoidal dilation in patients undergoing bone marrow transplantation[14]. VEGF blockade by Bev may therefore attenuate sinusoidal injury[15].
Although several recent studies[8-10,15-24] have commented on the impact of preoperative Bev administration on the safety and/or oxaliplatin-associated liver injury after CLM resection, none of these studies were randomized controlled trials. A meta-analysis is therefore required to provide an improved level of evidence on this subject.
A Medline, Embase, Ovid and Cochrane database search was performed to identify all studies published up to March 2012 that assessed the influence of preoperative Bev administration on the outcome after CLM resection. The following Mesh search headings were used: “colorectal cancer”, “liver metastases”, “bevacizumab”, “hepatic resection”, “hepatectomy” and “neoadjuvant chemotherapy”. A manual search of the reference lists of relevant papers was also carried out to identify additional trials.
Two reviewers (Li B and Wu LP) independently appraised each article and extracted the following parameters: first author, year of publication, study population characteristics, study design, number of patients in each arm, sex, age, inclusion and exclusion criteria, and outcomes of interest. All relevant text, tables and figures were reviewed for data extraction. Discrepancies between the reviewers were resolved through discussion and consensus.
Morbidity and mortality were defined as those events occurring within 30 or 90 d after surgery. Severe complications were defined as events requiring intensive care management or surgical, endoscopic, or radiologic interventions[25].
Only trials that compared postoperative outcomes after hepatectomy between CLM patients treated with neoadjuvant chemotherapy with and without Bev administration before surgery were included. Abstracts, letters, proceedings from scientific meetings, editorials and expert opinions, reviews without original data, case reports, studies lacking control groups, repetitive data, non-English language papers and animal studies were excluded. To avoid drug interactions, studies involving other targeted molecular therapies were also excluded.
The meta-analysis was performed using the Review Manager (RevMan) software version 5.0 (The Cochrane Collaboration, Software Update, Oxford, United Kingdom). The Mantel-Haenszel method was used to combine odds ratio with 95%CI for the outcomes of interest, including overall morbidity, major complications, Bev-related complications, general complications, mortality, and incidence and severity of sinusoidal dilation. Heterogeneity between trials was assessed by calculating the Q and I2 statistic. If I2 > 10%, a random-effects approach instead of a fixed-effects analysis was undertaken. Publication bias was assessed visually using a funnel plot. Statistical significance was defined as P < 0.05.
Thirteen nonrandomized studies published between 2007 and 2012 met the inclusion criteria and were suitable for meta-analysis[8-10,15-24]. The characteristics of the included studies are summarized in Table 1. Sample size ranged from 31 to 274, with a total of 1431 participants.
| Ref. | Country | Group | No. of patients | M/F | Age (yr) | No. of lesions | Interval between Bev-treatment and surgery | MR |
| D'Angelica et al[8] | United States | Bev – | 32 | 9/23 | 51 (34-77)1 | 1 (1-11)1 | - | 17 |
| Bev + | 16 | - | - | - | 6.9 (3-15) wk1 | - | ||
| Reddy et al[9] | United States | Bev – | 57 | 37/20 | 60 (51-68)1 | 2 (1-4)1 | - | 39 |
| Bev + | 39 | 25/14 | 55 (49-64)1 | 2 (1-3)1 | 10 (8-13) wk1 | 27 | ||
| Mahfud et al[10] | Multicenter | Bev – | 45 | 19/26 | 62 (59-65)1 | 6 (3-8)1 | - | 25 |
| Bev + | 45 | 31/14 | 58 (54-61)1 | 4 (3-5)1 | 9 wk1 | 19 | ||
| Ribero et al[15] | United States | Bev – | 43 | 26/17 | 57 (26-80)1 | 2 (1-8)1 | - | - |
| Bev + | 62 | 36/26 | 53.5 (34-85)1 | 2 (1-21)1 | ≥ 6 wk | - | ||
| Kesmodel et al[16] | United States | Bev – | 44 | 30/14 | 58 (31-80)1 | 2 (1-9)1 | - | 30 |
| Bev + | 81 | 48/33 | 57 (29-84)1 | 3 (1-31)1 | 58 (31-117) d1 | 47 | ||
| Zorzi et al[17] | United States | Bev – | 13 | - | - | - | - | - |
| Bev + | 19 | - | - | - | - | - | ||
| Aussilhou et al[18] | France | Bev – | 20 | - | - | - | - | 20 |
| Bev + | 11 | - | - | - | - | 9 | ||
| Klinger et al[19] | Austria | Bev – | 50 | 34/16 | 62.41 | - | - | - |
| Bev + | 56 | 32/24 | 63.01 | - | 2-5 wk | - | ||
| Pessaux et al[20] | France | Bev – | 21 | 13/8 | 63.3 ± 11.72 | 3 ± 32 | - | 4 |
| Bev + | 21 | 10/11 | 65 ± 8.22 | 3.8 ± 2.52 | 11.7 ± 4.7 wk2 | 5 | ||
| Rubbia-Brandt et al[21] | Multicenter | Bev – | 204 | - | - | - | - | - |
| Bev + | 70 | - | - | - | - | - | ||
| Tamandl et al[22] | Austria | Bev – | 112 | 35/77 | 63.6 (28.9-84.2)1 | 2 (1-10)1 | - | 17 |
| Bev + | 102 | 39/63 | 63.3 (31.4-81.6)1 | 2 (1-10)1 | 34 (17-99) d1 | 28 | ||
| Wicherts et al[23] | Multicenter | Bev – | 97 | 61/36 | 62 ± 112 | 4.5 ± 4.62 | - | 38 |
| Bev + | 67 | 42/25 | 58 ± 112 | 5.6 ± 4.52 | 8 (3-19) wk1 | 31 | ||
| van der Pool et al[24] | The Netherlands | Bev – | 53 | 33/20 | 62 (41-79)1 | 3 (1-7)1 | - | 14 |
| Bev + | 51 | 29/22 | 64 (41-77)1 | 2 (1-8)1 | 11 (5-38) wk1 | 9 |
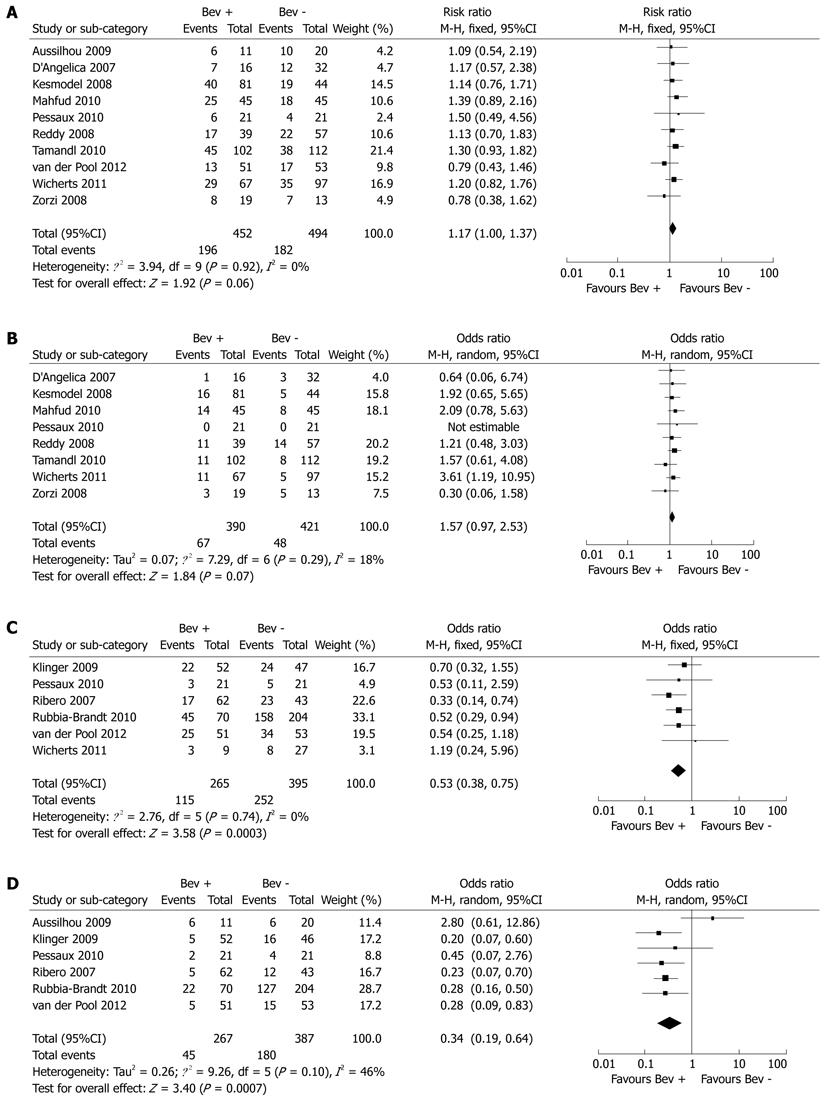
Results from overall meta-analysis are outlined in Table 2.
| Outcome of interest | No. of studies | No. of patients | OR | 95%CI | P-value | I2(%) |
| Overall morbidity | 10[8-10,16-18,20,22-24] | Bev + = 452, Bev - = 494 | 1.17 | 1.00, 1.37 | 0.06 | 0 |
| Severe complication | 8[8-10,16,17,20,22,23] | Bev + = 390, Bev - = 421 | 1.57 | 0.97, 2.53 | 0.07 | 18 |
| Wound complication | 6[8-10,16,22,23] | Bev + = 269, Bev - = 296 | 1.43 | 0.82, 2.50 | 0.21 | 0 |
| Bleeding/thromboembolic complication | 5[8-10,23,24] | Bev + = 218, Bev - = 284 | 0.99 | 0.41, 2.38 | 0.98 | 0 |
| Cardiovascular complication | 2[16,23] | Bev + = 148, Bev - = 141 | 1.14 | 0.23, 5.56 | 0.88 | 0 |
| Pulmonary complication | 6[8,16,18,20,23,24] | Bev + = 247, Bev - = 267 | 1.10 | 0.60, 2.02 | 0.77 | 0 |
| Renal or urinary complication | 6[8,16,18,20,23,24] | Bev + = 247, Bev - = 267 | 0.73 | 0.24, 2.23 | 0.58 | 0 |
| Hepatic dysfunction | 5[16-18,20,23] | Bev + = 223, Bev - = 219 | 0.48 | 0.22, 1.05 | 0.07 | 0 |
| Mortality | 10[8-10,16-18,20,22-24] | Bev + = 452, Bev - = 494 | 0.63 | 0.16, 2.56 | 0.52 | 0 |
| Overall sinusoidal dilation | 6[15,19-21,23,24] | Bev + = 265, Bev - = 395 | 0.53 | 0.38, 0.75 | < 0.001 | 0 |
| Moderate or severe sinusoidal dilation | 6[15,18-21,24] | Bev + = 267,Bev - = 387 | 0.34 | 0.19,0.64 | < 0.001 | 46 |
Ten studies reported on overall morbidity, which was found to be comparable in the Bev + group and Bev - group (43.3% vs 36.8%, P = 0.06) (Figure 1A). Similarly, there was no significant difference in severe complications between the Bev + and Bev - groups (17.1% vs 11.4%, P = 0.07) (Figure 1B). Nor was there a significant difference in cardiovascular, pulmonary and renal or urinary complications between the Bev + and Bev - groups (2.7% vs 2.1%, P = 0.88; 10.1% vs 9.3%, P = 0.67; 1.2% vs 2.2%, P = 0.58, respectively).
Bev-related complications including wound and thromboembolic/bleeding events were also similar in the Bev + and Bev - groups (14.4% vs 8.1%, P = 0.21; 4.1% vs 3.8%, P = 0.98, respectively). Four studies reported other types of Bev-related complications[16,22-24]. Kesmodel et al[16] reported hypertension in nine patients and proteinuria in two patients. van der Pool et al[24] reported hypertension in one patient. In one study, mild arterial hypertension occurred before surgery in one patient, necessitating dose reduction and treatment with beta-blocker therapy. No bowel perforations occurred in 13 patients with primary colorectal tumor in situ who received Bev. Anastomotic leakage with localized peritonitis occurred in one of seven patients who underwent synchronous colorectal and hepatic resections[23]. In another report by Tamandl et al[22], one patient developed anastomotic dehiscence after combined hepatic surgery and right colectomy.
Five studies reported on hepatic dysfunction, which was nonsignificantly less frequent in the Bev + group as compared with the Bev - group (5.3% vs 9.5%, P = 0.07).
Ten studies reported on postoperative mortality. There were 3 (0.6%) deaths in the Bev + group, which was similar to that in the Bev - group (5 deaths, 1.0%).
Seven studies evaluated the effect of Bev for CLM on nontumorous liver histology, and one study reported a significant difference in neoadjuvant treatment regimens between patient groups. To ensure homogeneity within groups, only patients treated with oxaliplatin were included for analysis[23]. Pooled analysis showed that Bev significantly reduced the incidence (Bev + 43.3% vs Bev - 63.7%, P < 0.001) and severity (Bev + 16.8% vs Bev - 46.5%, P < 0.001) of sinusoidal dilation (Figure 1C, D).
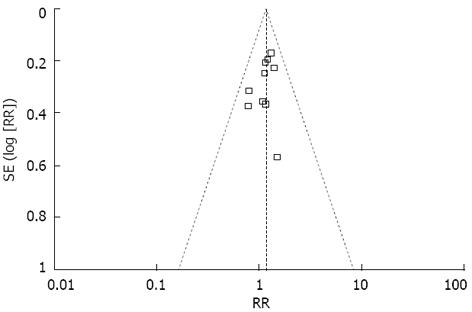
A funnel plot of the studies included in the meta-analysis reporting on overall morbidity is shown in Figure 2. None of the studies lay outside the limits of the 95%CI, and there was no evidence of publication bias.
Liver regeneration is an important component of the recovery process that occurs after various forms of hepatic injury, including partial hepatectomy (PH)[26]. Angiogenesis, the formation of new blood vessels, is a fundamental process in liver regeneration and repair. VEGF is considered a key regulator of normal and pathological angiogenesis. VEGF increases vascular dilatation and permeability, and induces the migration and proliferation of endothelial cells. These activities are mediated via two receptors for VEGF: kinase insert domain-containing receptor, and fms-like tyrosine kinase-1 receptor[27,28]. Endogenous expression of VEGF in hepatocytes and its receptors in endothelial cells has been shown to increase after PH[26].VEGF treatment protected the liver against chemically induced cytotoxicity, associated with a marked increase in the proliferation of hepatocytes and sinusoidal endothelial cells (SEC)[28,29]. In addition, exogenous VEGF administration promoted the increase of vessel density, vessel diameter, intrasinusoidal space, liver body weight ratio and hepatocyte proliferation after PH in the rat model. Conversely, these effects were completely suppressed by anti-VEGF treatment[30]. These results suggest that VEGF plays an important role in liver regeneration. Therefore, the safety of VEGF inhibitor administration at the time of hepatic surgery needs to be addressed.
The present meta-analysis shows that both overall and severe complications were not significantly different between the Bev + and Bev - groups. In addition, Bev treatment did not seem to increase the risk of Bev-related complications (wound and bleeding/thromboembolic events), hepatic dysfunction, and postoperative deaths. On the other hand, Wicherts et al[23] reported that liver functional recovery parameters including prothrombin time and serum total bilirubin level were equivalent between the Bev + and Bev - groups in the postoperative period, suggesting that Bev administration should be safe before hepatic resection.
To increase the safety of surgery, preoperative portal vein embolization (PVE) has been used for major hepatic resection in CLM patients, because PVE can induce homolateral atrophy and contralateral compensatory hypertrophy of the remnant liver, thus decreasing the risk of postoperative liver failure[31]. In a relatively large cohort study of 100 patients, Covey et al[32] found that the mean growth of non-embolized hemiliver was comparable in patients treated with and without neoadjuvant chemotherapy (22% ± 3% vs 26% ± 3%) with a similar number of patients with less than 5% growth of liver (4 vs 6) after PVE. Similar findings were also reported by other authors[33,34]. Zorzi et al[17] found that chemotherapy with Bev did not alter non-embolized liver hypertrophy. In contrast, a study conducted by Aussilhou et al[18] demonstrated that the hypertrophy of the future liver remnant after PVE was impaired in patients treated with Bev. These inconsistent results may be attributed to different durations of chemotherapy used in the two studies. It was reported that postoperative morbidity was correlated with the number of cycles of chemotherapy before surgery. Karoui et al[35] reported that morbidity in patients who received six or more cycles of chemotherapy was significantly higher that that in patients who received less than six cycles (54% vs 19%, P = 0.047). Indeed, almost 80% of the patients in the series by Aussilhou et al[18] received six or more cycles of chemotherapy compared with 53% in the study by Zorzi et al[17].
Although oxaliplatin is often utilized as a chemotherapeutic agent in the treatment of CLM, it can exert adverse effects on the liver. Oxaliplatin-based chemotherapy has been shown to cause hepatic sinusoidal dilation[12]. Our study has shown that Bev can significantly reduce the incidence and severity of sinusoidal dilation, thus supporting the use of Bev for CLM. An explanation for the above protective effect remains unclear. Increased expression of matrix metalloproteinase (MMP)-9 and MMP-2 by SEC was found to play an important role in sinusoidal dilation development in the monocrotaline-induced rat model[36]. An in vitro study[37] suggested that VEGF could up-regulate MMP-9 expression. It is postulated that VEGF blockade by Bev may attenuate sinusoidal injury by down-regulating MMP-9[15].
There is no consensus on the optimal time interval between discontinuation of Bev and hepatic surgery. D’Angelica et al[8] found that postoperative complications were more common in patients who received Bev within 8 wk. Similarly, Reddy et al[9] noted that patients who underwent hepatectomy within 8 wk of Bev treatment may be at a higher risk of overall, severe and hepatic complications after surgery. Whereas Kesmodel et al[16] failed to confirm that the time interval from discontinuation of Bev (≥ 60 vs < 60 d) to surgery was associated with an increased likelihood of developing complications. In addition, a subgroup analysis of patients who received Bev also demonstrated that there was no significant difference in complication rates between patients who received Bev 31-45 d, 46-60 d and greater than 60 d before surgery (P = 0.21). In another report, Mahfud et al[10] showed that the occurrence of postoperative complications was similar in patients who had received Bev for < 6 wk and in those who had taken Bev ≥ 6 wk before liver resection. Based on the evidence that the median half-life of Bev in humans is approximately 21 d (range 11-50 d), some authors recommend waiting at least 6-8 wk from discontinuation of Bev to surgery[8,9,16].
The main limitation of this meta-analysis was that all evidence came from nonrandomized trials which could introduce potential bias in data collection and analysis. However, there is evidence that nonrandomized studies may generally give valid results[38].
In conclusion, our meta-analysis has shown that Bev can be safely administered before hepatic resection in CLM patients, knowing that it has a protective effect against oxaliplatin-related liver injury. The issue of optimal timing of hepatectomy in patients treated with Bev should be addressed in future prospective multicenter trials.
Bevacizumab (Bev), a monoclonal antibody that targets vascular endothelial growth factor (VEGF) A, has recently been added to standard neoadjuvant chemotherapy in patients with colorectal liver metastases (CLM). However, Bev is reportedly associated with bleeding, thrombosis, gastrointestinal perforation, impaired wound healing and liver regeneration. Therefore, the safety of Bev at the time of hepatic surgery needs to be addressed.
The study evaluated the impact of preoperative administration of neoadjuvant Bev on the outcome of patients undergoing resection for CLM using a meta-analysis of all relevant studies.
Findings from this meta-analysis suggest that preoperative Bev does not seem to increase postoperative morbidity after hepatic surgery of CLM and has a protective effect against hepatic injury in patients treated with oxaliplatin chemotherapy.
Bev can be safely administered in CLM patients before hepatic resection.
Bev, a monoclonal antibody against VEFG, can inhibit the growth of human tumor xenografts.
The topic is interesting and the methodology of the meta-analysis is appropriate.
| 1. | Mohammad WM, Balaa FK. Surgical management of colorectal liver metastases. Clin Colon Rectal Surg. 2009;22:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Liu JH, Hsieh YY, Chen WS, Hsu YN, Chau GY, Teng HW, King KL, Lin TC, Tzeng CH, Lin JK. Adjuvant oxaliplatin- or irinotecan-containing chemotherapy improves overall survival following resection of metachronous colorectal liver metastases. Int J Colorectal Dis. 2010;25:1243-1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Tanaka K, Adam R, Shimada H, Azoulay D, Lévi F, Bismuth H. Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg. 2003;90:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Benoist S, Nordlinger B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol. 2009;16:2385-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1454] [Article Influence: 66.1] [Reference Citation Analysis (2)] |
| 6. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [PubMed] |
| 7. | Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697-3705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 663] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 8. | D’Angelica M, Kornprat P, Gonen M, Chung KY, Jarnagin WR, DeMatteo RP, Fong Y, Kemeny N, Blumgart LH, Saltz LB. Lack of evidence for increased operative morbidity after hepatectomy with perioperative use of bevacizumab: a matched case-control study. Ann Surg Oncol. 2007;14:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 9. | Reddy SK, Morse MA, Hurwitz HI, Bendell JC, Gan TJ, Hill SE, Clary BM. Addition of bevacizumab to irinotecan- and oxaliplatin-based preoperative chemotherapy regimens does not increase morbidity after resection of colorectal liver metastases. J Am Coll Surg. 2008;206:96-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Mahfud M, Breitenstein S, El-Badry AM, Puhan M, Rickenbacher A, Samaras P, Pessaux P, Lopez-Ben S, Jaeck D, Figueras J. Impact of preoperative bevacizumab on complications after resection of colorectal liver metastases: case-matched control study. World J Surg. 2010;34:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 11. | Neeff HP, Drognitz O, Klock A, Illerhaus G, Opitz OG, Hopt UT, Makowiec F. Impact of preoperative targeted therapy on postoperative complications after resection of colorectal liver metastases. Int J Colorectal Dis. 2012;27:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 773] [Article Influence: 35.1] [Reference Citation Analysis (2)] |
| 13. | Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, Bachellier P, Jaeck D. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 353] [Article Influence: 19.6] [Reference Citation Analysis (2)] |
| 14. | Iguchi A, Kobayashi R, Yoshida M, Kobayashi K, Matsuo K, Kitajima I, Maruyama I. Vascular endothelial growth factor (VEGF) is one of the cytokines causative and predictive of hepatic veno-occlusive disease (VOD) in stem cell transplantation. Bone Marrow Transplant. 2001;27:1173-1180. [PubMed] |
| 15. | Ribero D, Wang H, Donadon M, Zorzi D, Thomas MB, Eng C, Chang DZ, Curley SA, Abdalla EK, Ellis LM. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 283] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 16. | Kesmodel SB, Ellis LM, Lin E, Chang GJ, Abdalla EK, Kopetz S, Vauthey JN, Rodriguez-Bigas MA, Curley SA, Feig BW. Preoperative bevacizumab does not significantly increase postoperative complication rates in patients undergoing hepatic surgery for colorectal cancer liver metastases. J Clin Oncol. 2008;26:5254-5260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Zorzi D, Chun YS, Madoff DC, Abdalla EK, Vauthey JN. Chemotherapy with bevacizumab does not affect liver regeneration after portal vein embolization in the treatment of colorectal liver metastases. Ann Surg Oncol. 2008;15:2765-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 18. | Aussilhou B, Dokmak S, Faivre S, Paradis V, Vilgrain V, Belghiti J. Preoperative liver hypertrophy induced by portal flow occlusion before major hepatic resection for colorectal metastases can be impaired by bevacizumab. Ann Surg Oncol. 2009;16:1553-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Klinger M, Eipeldauer S, Hacker S, Herberger B, Tamandl D, Dorfmeister M, Koelblinger C, Gruenberger B, Gruenberger T. Bevacizumab protects against sinusoidal obstruction syndrome and does not increase response rate in neoadjuvant XELOX/FOLFOX therapy of colorectal cancer liver metastases. Eur J Surg Oncol. 2009;35:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 20. | Pessaux P, Panaro F, Casnedi S, Zeca I, Marzano E, Bachellier P, Jaeck D, Chenard MP. Targeted molecular therapies (cetuximab and bevacizumab) do not induce additional hepatotoxicity: preliminary results of a case-control study. Eur J Surg Oncol. 2010;36:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (2)] |
| 21. | Rubbia-Brandt L, Lauwers GY, Wang H, Majno PE, Tanabe K, Zhu AX, Brezault C, Soubrane O, Abdalla EK, Vauthey JN. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 22. | Tamandl D, Gruenberger B, Klinger M, Herberger B, Kaczirek K, Fleischmann E, Gruenberger T. Liver resection remains a safe procedure after neoadjuvant chemotherapy including bevacizumab: a case-controlled study. Ann Surg. 2010;252:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 23. | Wicherts DA, de Haas RJ, Sebagh M, Saenz Corrales E, Gorden DL, Lévi F, Paule B, Azoulay D, Castaing D, Adam R. Impact of bevacizumab on functional recovery and histology of the liver after resection of colorectal metastases. Br J Surg. 2011;98:399-407. [PubMed] [DOI] [Full Text] |
| 24. | van der Pool AE, Marsman HA, Verheij J, Ten Kate FJ, Eggermont AM, Ijzermans JN, Verhoef C. Effect of bevacizumab added preoperatively to oxaliplatin on liver injury and complications after resection of colorectal liver metastases. J Surg Oncol. 2012;106:892-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26131] [Article Influence: 1187.8] [Reference Citation Analysis (2)] |
| 26. | Assy N, Spira G, Paizi M, Shenkar L, Kraizer Y, Cohen T, Neufeld G, Dabbah B, Enat R, Baruch Y. Effect of vascular endothelial growth factor on hepatic regenerative activity following partial hepatectomy in rats. J Hepatol. 1999;30:911-915. [PubMed] |
| 27. | Endo D, Kogure K, Hasegawa Y, Maku-uchi M, Kojima I. Activin A augments vascular endothelial growth factor activity in promoting branching tubulogenesis in hepatic sinusoidal endothelial cells. J Hepatol. 2004;40:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (3)] |
| 28. | Marino G, Piazzese E, Gruttadauria S, Nicotra G, Guarnaccia M, Emmanuele G, Bartoloni G, Messina A, Travali S, Famulari C. New model of liver regeneration induced through use of vascular endothelial growth factor. Transplant Proc. 2006;38:1193-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 29. | Namisaki T, Yoshiji H, Kojima H, Yoshii J, Ikenaka Y, Noguchi R, Sakurai S, Yanase K, Kitade M, Yamazaki M. Salvage effect of the vascular endothelial growth factor on chemically induced acute severe liver injury in rats. J Hepatol. 2006;44:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 30. | Bockhorn M, Goralski M, Prokofiev D, Dammann P, Grünewald P, Trippler M, Biglarnia A, Kamler M, Niehues EM, Frilling A. VEGF is important for early liver regeneration after partial hepatectomy. J Surg Res. 2007;138:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 31. | Pamecha V, Glantzounis G, Davies N, Fusai G, Sharma D, Davidson B. Long-term survival and disease recurrence following portal vein embolisation prior to major hepatectomy for colorectal metastases. Ann Surg Oncol. 2009;16:1202-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 32. | Covey AM, Brown KT, Jarnagin WR, Brody LA, Schwartz L, Tuorto S, Sofocleous CT, D’Angelica M, Getrajdman GI, DeMatteo R. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 33. | Goéré D, Farges O, Leporrier J, Sauvanet A, Vilgrain V, Belghiti J. Chemotherapy does not impair hypertrophy of the left liver after right portal vein obstruction. J Gastrointest Surg. 2006;10:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Nafidi O, Désy D, Létourneau R, Côté J, Plasse M, Vandenbroucke F, Roy A, Dagenais M, Lapointe RW. Hypertrophy of the non-embolized liver after chemotherapy. HPB (Oxford). 2009;11:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, Rougier P, Nordlinger B. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 522] [Article Influence: 26.1] [Reference Citation Analysis (5)] |
| 36. | Deleve LD, Wang X, Tsai J, Kanel G, Strasberg S, Tokes ZA. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 37. | Wang H, Keiser JA. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res. 1998;83:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 310] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 38. | Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878-1886. [PubMed] |
P- Reviewer Krivokapic Z S- Editor Wen LL L- Editor Webster JR E- Editor Zhang DN