Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.706
Revised: November 19, 2012
Accepted: November 24, 2012
Published online: February 7, 2013
AIM: To compare the efficacy of modified percutaneous transhepatic variceal embolization (PTVE) with 2-octyl-cyanoacrylate (2-OCA) and endoscopic variceal obturation (EVO) with an injection of 2-OCA for prophylaxis of gastric variceal rebleeding.
METHODS: In this retrospective study, the medical records of liver cirrhosis patients with gastric variceal bleeding who underwent either endoscopic 2-OCA (EVO) or modified PTVE using 2-OCA at Shandong Provincial Hospital from January 2006 to December 2008 were reviewed. Patient demographics, rebleeding rate, survival rate, and complications were compared between the two groups (PTVE and EVO). All results were expressed as mean ± SD, or as a percentage. Quantitative variables were compared by two sample Student t tests, and qualitative variables were compared by the Fisher exact test or the χ2 test (with Yates correction) where appropriate. A P value less than 0.05 was considered significant. Statistical computation was performed using SPSS 13.0 software.
RESULTS: A total of 77 patients were included; 45 patients who underwent EVO and 32 patients who received PTVE. During the follow-up (19.78 ± 7.70 mo in the EVO group, vs 21.53 ± 8.56 mo in the PTVE group) rebleeding occurred in 17 patients in the EVO group and in 4 patients in the PTVE group (37.78% vs 12.5%, P = 0.028). The cumulative rebleeding-free rate was 75%, 59%, and 49% in 1, 2, and 3 years respectively for EVO, and 93%, 84%, and 84% for PTVE (P = 0.011). Cox analysis was used to identify independent factors that predicted rebleeding after treatment. Variables including age, gender, cause, Child-Pugh classification, size of gastric varices (GV), location of GV, and treatment methods were analyzed. It was revealed that Child-Pugh classification [risk ratio (RR) 2.10, 95%CI: 1.03-4.28, P = 0.040], choice of treatment (RR 0.25, 95%CI: 0.08-0.80, P = 0.019), and size of GV (RR 2.14, 95%CI: 1.07-4.28, P = 0.032) were the independent factors for predicting rebleeding. Follow-up computed tomography revealed that cyanoacrylate was retained in the varices and in the feeding veins of PTVE patients. During the follow-up, eight patients in the EVO group and four patients in the PTVE group died. The cumulative survival rates at 1, 2, and 3 years were 93%, 84%, and 67% respectively in the EVO group, and 97%, 88%, and 74% respectively in the PTVE group. The survival rates were not significantly different between the two groups (P = 0.432). Cox analysis showed that the Child-Pugh classification was the most significant prognostic factor of survival (RR 2.77, 95%CI: 1.12-6.80, P = 0.027). The incidence of complications was similar in both groups.
CONCLUSION: With extensive and permanent obliteration of gastric varices and its feeding veins, PTVE with 2-OCA is superior to endoscopic 2-OCA injection for preventing gastric variceal rebleeding.
- Citation: Wang J, Tian XG, Li Y, Zhang CQ, Liu FL, Cui Y, Liu JY. Comparison of modified percutaneous transhepatic variceal embolization and endoscopic cyanoacrylate injection for gastric variceal rebleeding. World J Gastroenterol 2013; 19(5): 706-714
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/706.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.706
Although the incidence of bleeding from gastric varices (GV) is lower than from esophageal varices (EV), once it occurs the outcome is worse, and with higher mortality[1-3]. After an episode of acute variceal bleeding, patients are at high risk for recurrent bleeding and death. Thus, prevention of recurrent bleeding is essential[4,5]. However, rupture from gastric varices, especially varices located in the gastric fundus, poses particular therapeutic challenges because of the location and rapid blood flow. The current treatment methods for gastric varices are far from ideal.
Endoscopic variceal obturation (EVO) with the injection of cyanoacrylate has been widely adopted and proved to be effective in the emergency hemostasis of bleeding from gastric varices since it was proposed in 1986[6]. At present, this method is the first-line treatment for gastric variceal bleeding recommended by Baveno IV consensus and AASLD guidelines[7,8]. However, the long-term rebleeding rate after endoscopic cyanoacrylate injection is still high[9-12]. Additionally, there is also a potential risk of systemic embolism in patients with underlying gastrorenal shunts, and other serious complications such as sepsis, fistula, and pericarditis[13-16].
Balloon-occluded retrograde transvenous obliteration (BRTO) has become the standard treatment for gastric varices in Japan. However, patients without catheterizable gastrorenal shunts cannot be treated by BRTO[17,18]. Therefore for these patients, percutaneous transhepatic variceal embolization (PTVE) with N-butyl-2-cyanoacrylate was introduced and showed satisfactory results[19,20] in a small series of patients.
Based on our previous reports of modified PTVE with 2-octyl cyanoacrylate (2-OCA) for bleeding EV[21,22], we have performed modified PTVE with 2-OCA to prevent gastric variceal rebleeding in recent years.
In this retrospective study, 77 patients with prior bleeding from gastric varices who underwent EVO or modified PTVE for prevention of rebleeding were analyzed. Rebleeding rate, survival, and complications were compared between these two procedures. To our knowledge, at the time of writing, no other report in the literature has compared these two procedures in the prevention of gastric variceal rebleeding.
The medical records of liver cirrhosis patients with gastric variceal bleeding who underwent either endoscopic 2-OCA injection or modified PTVE using 2-OCA in our hospital from January 2006 to December 2008 were reviewed. Local ethics committee approval was obtained for the chart review.
The inclusion criteria: (1) diagnosis of liver cirrhosis by biopsy or clinical examination and imaging, including ultrasound, computed tomography (CT), or magnetic resonance imaging; (2) patient suffered bleeding within 6 mo before being admitted or acute bleeding with achieved hemostasis by pharmacological treatment; (3) endoscopically-confirmed bleeding from gastric varices: active spurting or oozing of blood from gastric varices during endoscopy, blood clot coating on gastric varices or the presence of erosive spots on gastric varices, with no other potential source of bleeding; and (4) patient aged between 20-65 years.
Exclusion criteria: (1) hepatocellular carcinoma or other malignancies; (2) a history of transjugular intrahepatic portosystemic shunt (TIPS), surgery, or endoscopic therapy for esophagogastric variceal bleeding; (3) portal vein thrombosis; or (4) infection.
Choice of treatment method was based on the patients’ intentions after being given a sufficient explanation of the two treatment methods. Informed written consent was obtained from each patient.
Endoscopic intra variceal injection of 2-octyl cyanoacrylate (Baiyun Medical Adhesive Corporation, Guangzhou, China) was performed using a video endoscope (XQ230, Olympus Optical, Tokyo, Japan) and a 23-gauge disposable injection needle (Medwork Medical Products and Services GmbH). Each injection contained a 0.5-2.0 mL mixture of 2-OCA and Lipiodol (1:1). The injection was aimed at the varices that were either bleeding, possessed red color signs, or were the most prominent. Obliteration was assessed by blunt palpation with a catheter, and presence of hardness indicated a complete obliteration. If the varices remained soft, additional injections were done until all gastric varices became hardened. If necessary, additional injections of cyanoacrylate were performed 2-3 wk after the initial session.
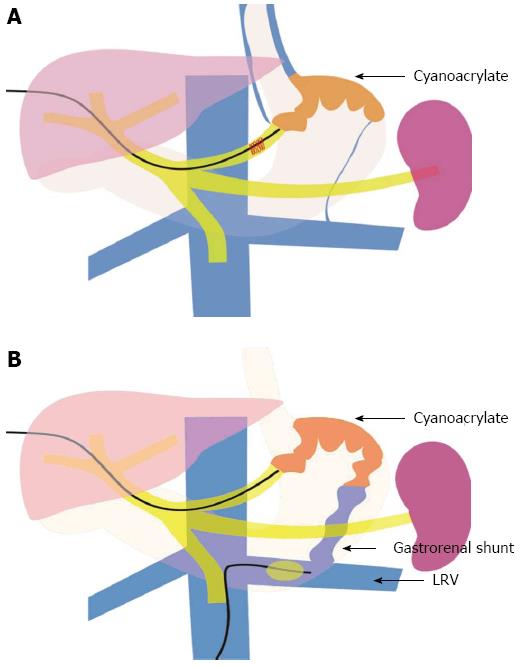
PTVE was performed alone, or combined with left renal vein obstruction with a balloon catheter if a large gastrorenal shunt was present (Figure 1). Shortly after a percutaneous transhepatic puncture of the intrahepatic branch of the portal vein, a 5F cobra catheter (Cook, Bloomington, IN) was inserted into the splenic vein and a splenoportography was performed to evaluate the gastric varices, the feeding veins, and draining veins. The main gastric feeding vein (e.g., the left gastric vein, short gastric vein, or posterior gastric vein) was then selected with the 5F catheter, and a venography was performed to assess blood flow velocity and the size of varices (the gastrorenal shunt’s size being of particular interest).
Based on these data, the embolization of 2-OCA was carried out in the following ways: (1) PTVE alone: In patients without large gastrorenal shunts, the blood flow in gastric varices was slow, and the contrast material could stay in the varices for more than 5 s after injection. In these patients, the cyanoacrylate was directly injected into the gastric varices; and (2) PTVE combined with left renal vein obstruction with a balloon catheter: In patients with large gastrorenal shunts, there was rapid blood flow in the varices and the contrast material disappeared within 3-5 s after injection. For these patients, a 6F balloon catheter (Cook, Bloomington, IN) with a diameter of 15 to 20 mm was inserted into the left renal vein via the right femoral vein to reduce the blood flow of the gastrorenal shunt and varices, and then the cyanoacrylate was injected. In these two ways, the cyanoacrylate could obliterate the entire varices and feeding veins, while cyanoacrylate migration to the systemic circulation could be avoided.
When the cyanoacrylate was flowing into all the gastric varices, the catheter was immediately withdrawn. Splenoportography was again performed to assess the obliteration of the varices. If other feeding veins (such as the short or posterior gastric veins) were present, the procedure would be repeated until the gastric varices and feeding veins were completely filled with cyanoacrylate. Finally, the 5F sheath system was withdrawn, and the puncture tract was embolized with microcoils. Low molecular heparin (100 IU/kg body weight, daily) was subcutaneously administered 24 h after the procedure for 5 to 7 d to prevent portal venous thrombosis.
Follow-up endoscopy was performed for the two groups at intervals of 1, 3, and 6 mo after the procedures, and then every 6-12 mo or when it was considered clinically necessary. In patients with rebleeding, endoscopy was performed to identify the cause of the bleeding. Portal venography was performed at 1 mo after the procedure, and every 6 mo thereafter with 3-dimensional multi-detector row CT (GE Medical systems, Milwaukee, WI) to observe the formation of portal vein thrombosis, the location and extent of cyanoacrylate glue, variceal recanalization, and the occurrence of collateral vessels. Rebleeding, survival, and complications were recorded.
Recurrent bleeding was defined as the presence of hematemesis or melena, with the bleeding source being endoscopically proven to originate from gastric or EV, or other resources after the index treatment. Bleeding from gastric varices was distinguished from that of EV on the basis of whether active bleeding or erosive spots were present on the gastric varices themselves.
Complications were defined as any untoward events that required active treatment or prolonged hospitalization.
All results were expressed as mean ± SD, or as a percentage. Quantitative variables were compared by two sample Student t tests, and qualitative variables were compared by the Fisher exact test or the chi-squared test (with Yates correction) where appropriate. The Kaplan-Meier estimation was used to examine recurrence and rebleeding of gastric varices and rate of survival. Comparisons were performed using the log-rank test. A Cox’s analysis was performed to detect possible independent predictors for variceal rebleeding and death. A P value less than 0.05 was considered significant. Statistical computation was performed using SPSS 13.0 software.
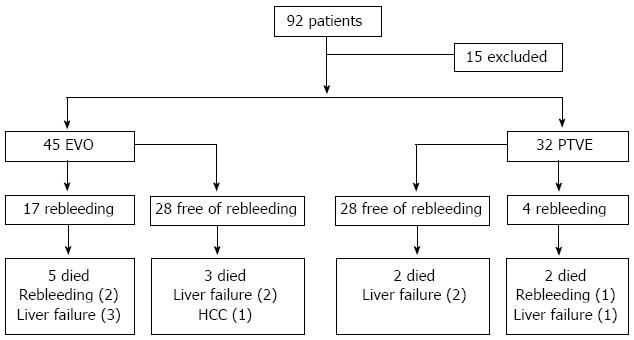
From January 2006 to December 2008, EVO or PTVE was performed in a total of 92 cirrhotic patients with a history of gastric variceal bleeding in Shandong Provincial Hospital. Of the 92 patients, six had hepatocellular carcinoma, and nine had previously received TIPS or shunt surgery; these 15 patients were excluded. Of the remaining 77 patients, EVO was performed in 45, and PTVE was performed in 32 (Figure 2). The clinical characteristics of the 77 patients in the two groups were retrospectively reviewed from a computerized database of our hospital. Gastric varices were subdivided by Sarin classification[4], the form of gastric varices was classified according to Hashizume classification[23], and liver function was estimated based on the Child-Pugh classification[24]. The follow-up records were carefully reviewed. The median follow-up period was 19.78 ± 7.70 mo (range, 3 to 41 mo) in the EVO group and 21.53 ± 8.56 mo (range, 6 to 44 mo) in the PTVE group (P = 0.350). Table 1 shows the patient characteristics in the two groups, for which there was no significant difference.
| EVO (n = 45) | PTVE (n = 32) | P value | |
| Sex (M/F) | 33/12 | 22/10 | 0.661 |
| Age (mean ± SD, yr) | 52.69 ± 8.99 | 50.65 ± 7.23 | 0.293 |
| Etiology of cirrhosis | 0.999 | ||
| Hepatitis B | 25 | 18 | |
| Hepatitis C | 9 | 6 | |
| Alcohol | 8 | 6 | |
| Others | 3 | 2 | |
| Child-Pugh class (A/B/C) | 14/21/10 | 9/17/6 | 0.851 |
| Bleeding onset | 1.000 | ||
| Recent variceal bleeding | 40 | 28 | |
| Acute variceal bleeding | 5 | 4 | |
| Blood transfusion (unit) | 4.50±3.32 | 5.33±3.60 | 0.542 |
| Form of GV (F1/F2/F3)1 | 12/23/10 | 7/13/12 | 0.343 |
| Location of GV2 | 0.467 | ||
| GOV2 | 29 | 18 | |
| IGV1 | 16 | 14 | |
| Ascites | 26 | 20 | 0.857 |
| Duration of follow-up (mo) | 19.78 ± 7.70 | 21.53 ± 8.56 | 0.350 |
The outcomes of the procedures are shown in Table 2. In the EVO group, 35 patients (77.78%) achieved complete obliteration of gastric varices. Of the 35 patients, one session of EVO was needed to achieve complete obliteration in 23 patients, and two or three sessions were required in 12 patients. The total volume of cyanoacrylate used in the initial session of each EVO group patient was 3.03 ± 1.04 mL (range, 1.5-5.5 mL). In the PTVE group, 18 patients underwent gastric variceal embolization by PTVE alone, while the other 14 patients underwent combined PTVE with left renal vein balloon obstruction. Thirty patients (93.75%) achieved complete obliteration, which was confirmed by a splenoportography after the PTVE procedure. The volume of cyanoacrylate used in the PTVE group was 6.69 ± 2.92 mL (range, 3-16 mL); more than in the EVO group (P = 0.000).
| EVO(n = 45) | PTVE(n = 32) | P value | |
| Status of GV | 0.290 | ||
| Disappeared | 17/45 | 16/32 | |
| Collapsed | 16/45 | 12/32 | |
| Remained | 12/45 | 4/32 | |
| Amount of cyanoacrylate (mL) | 3.03 ± 1.04 | 6.69 ± 2.92 | 0.000 |
| Rebleeding | 17/45 | 4/32 | 0.028 |
| Rebleeding from GV | 13/45 | 2/32 | 0.029 |
| Rebleeding from other sources | 4/45 | 2/32 | 1.000 |
| EV bleeding | 2 | 1 | 1.000 |
| PHG | 1 | 1 | 1.000 |
| Unknown | 1 | 0 | 1.000 |
| Death during follow-up | 8/45 | 4/32 | 0.751 |
| Causes of death | |||
| Progressive Liver failure | 5/45 | 3/32 | 1.000 |
| Rebleeding | 2/45 | 1/32 | 1.000 |
| HCC | 1/45 | 0/32 | 1.000 |
An endoscopic follow-up was performed in all patients in both groups. Generally, 1-3 mo after the injection, the cyanoacrylate plug began to slough off the submucosa in some patients, and the varices disappeared or collapsed after complete or partial expulsion of the glue within one year in the PTVE group. In patients who underwent EVO, the endoscopic findings were similar to that in the PTVE group. During the endoscopic follow-up, the gastric varices disappeared in 16 patients or shrunk in 12 patients in the PTVE group, and disappeared in 17 patients or shrunk in 16 patient in the EVO group (P = 0.290).
Twenty patients in the EVO group and fifteen patients in the PTVE group underwent contrast-enhanced CT and portal venography during follow-up. In the PTVE group, it was confirmed that the gastric varices, the perforating veins in the fundus, the perifundus veins, and all the feeding veins were filled with cyanoacrylate 1 mo after the procedure. 3-6 mo after PTVE, the amount of cyanoacrylate in the submucosal varices was reduced compared to previously. Twelve months later, the cyanoacrylate in the submucosal varices almost completely gone, but the cyanoacrylate in the perifundus varices and feeding veins remained the same as at the start. Portal venography showed that there was no blood flow in the eradicated varices with sufficient amount of cyanoacrylate after one year. In the EVO group, CT revealed that the cyanoacrylate was only scattered in the gastric varices and the perifundus varices, which was not as extensive and complete as in the PTVE group.
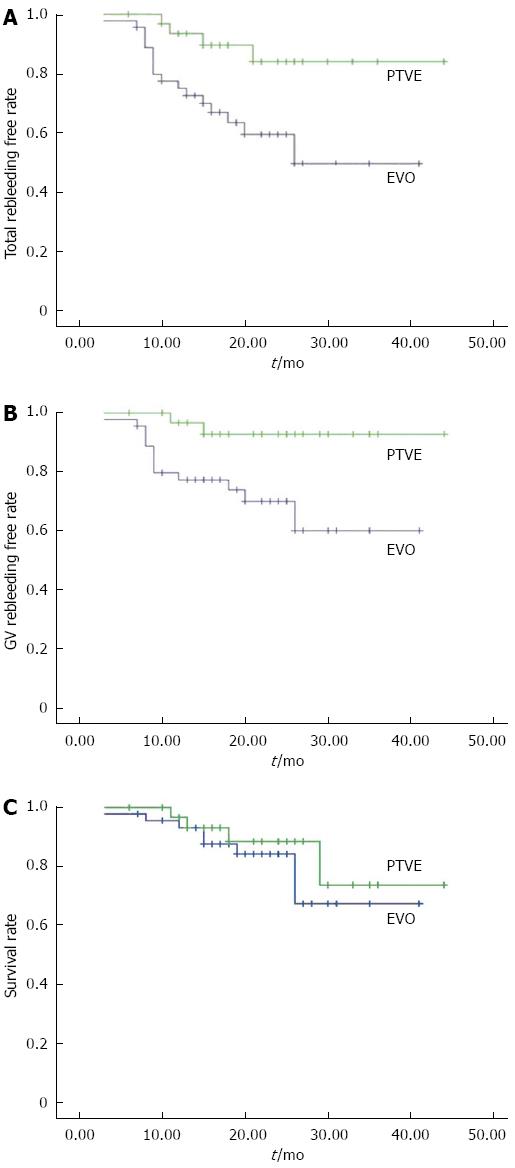
During the follow-up, rebleeding occurred in 17 patients (37.78%) in the EVO group and four patients (12.5%) in the PTVE group. The rebleeding rate was significantly lower in the PTVE group compared to the EVO group (P = 0.028). The cumulative rebleeding-free rate was 75%, 59%, and 49% in 1, 2, and 3 years respectively for the EVO group, and 93%, 84%, and 84% for the PTVE group (P = 0.011, Figure 3A).
The causes of rebleeding included: gastric variceal rebleeding (13 in EVO and 2 in PTVE), aggravated esophageal variceal bleeding (2 in EVO and 1 in PTVE), portal hypertensive gastropathy (1 in EVO and 1 in PTVE), and unknown reasons (1 in EVO and nil in PTVE). The cumulative rate free of GV rebleeding at 1, 2, and 3 years was 77%, 70%, and 60% respectively for the EVO group, and 97%, 93%, and 93% for the PTVE group (P = 0.012, Figure 3B). Of the 15 patients with rebleeding from GV, three died of uncontrolled bleeding, four received repeated EVO, three received TIPS, and five received surgery. No patient died of bleeding from EV or other sources.
Cox analysis was used to identify the independent factors that predicted rebleeding after treatment. Variables including age, gender, cause, Child-Pugh classification, size of GV, location of GV, and treatment methods were analyzed. It was revealed that the Child-Pugh classification (RR 2.10, 95%CI: 1.03-4.28, P = 0.040), choice of treatment (RR 0.25, 95%CI: 0.08-0.80, P = 0.019), and size of GV (RR 2.14, 95%CI: 1.07-4.28, P = 0.032) were the independent predicting factors for rebleeding.
During the follow-up, eight patients in the EVO group and four patients in the PTVE group died. The cumulative survival rates at 1, 2, and 3 years were 93%, 84%, and 67% respectively in the EVO group, and 97%, 88%, and 74% respectively in the PTVE group. The survival rates were not significantly different between the two groups (P = 0.432, Figure 3C). In the EVO group, five patients died of progression of hepatic failure, compared with three patients in the PTVE group. Two patients in the EVO group and one in the PTVE group died of uncontrolled rebleeding. The remaining patient in the EVO group died of hepatocellular carcinoma, which occurred after the procedure.
Seven variables (sex, age, the Child Pugh classification, etiology, choice of modality, form of GV, and location of GV) were taken into consideration in the multivariate analysis using the Cox regression model. Based on the Cox analysis, the Child-Pugh classification was the most significant prognostic factor of survival (RR 2.77, 95%CI: 1.12-6.80, P = 0.027).
Complications of the procedures are shown in Table 3. Twenty-nine patients experienced complications in the EVO group, compared to twenty-two patients in the PTVE group (P = 0.881). There was no significant difference between the groups. The common complications after treatment were fever and abdominal pain. 19 patients in the EVO group and 12 patients in the PTVE group suffered from fever (P = 0.857). Abdominal pain was encountered in 12 patients in the EVO group and 15 patients in the PTVE group (P = 0.112). The patients were treated with conventional medicinal therapy, with fever and abdominal pain usually being alleviated within 1-2 wk. One patient in the EVO group encountered pulmonary embolism, while no patient encountered systemic embolization in the modified PTVE group (P=1.000). Mild to moderate ascites appeared in 3 patients in the EVO group and 6 in the PTVE group (P = 0.152), and all of them were controlled by medication. Partial portal vein thrombosis appeared in two patients in the PTVE group, but the portal vein was patent under Doppler ultrasound. No patient died of complications in either group.
| EVO (n = 45) | PTVE (n = 32) | P value | |
| Total complications (%) | 29 | 22 | 0.881 |
| Bacteremia | 4 | 1 | 0.395 |
| Fever | 19 | 12 | 0.857 |
| Abdominal pain | 12 | 15 | 0.112 |
| Ulcer | 3 | 1 | 0.637 |
| SBP | 2 | 3 | 0.644 |
| Ascites | 3 | 6 | 0.152 |
| Portal vein thrombosis | 0 | 2 | 0.170 |
| Pulmonary embolism | 1 | 0 | 1.000 |
Although gastric varices tend to bleed less than EV, the mortality associated with gastric variceal hemorrhage is substantial[1-3] (Kim, 1997 #5; Sarin, 1992 #4; Ryan, 2004 #6) (Kim, 1997 #5; Sarin, 1992 #4; Ryan, 2004 #6). Furthermore, with the successful management of bleeding EV, as well as successful prophylaxis of first bleeding from EV, “secondary” gastric varices develop in 9% to 15% of patients, which have a higher frequency of bleeding compared with primary gastric varices[1,23]. Unlike EV, gastric varices pose particular therapeutic challenges because of their size and location. Standard endoscopic therapies used for EV, such as sclerotherapy and band ligation, are less effective for gastric varices and have been shown to be associated with high complication rates[6,7]. This leads to the need for more aggressive and costly interventions, such as TIPS and BRTO, however each has its limitations. The current methods for the treatment of gastric varices are far from ideal.
EVO with the injection of agents such as N-buyt1-2-cyanoacrylate for the treatment of GV has been widely adopted, and both the immediate and long-term efficacies have been confirmed in the treatment of GV[25-28]. However, the long-term rebleeding rate after EVO was still high[9-12]. Incomplete obliteration and recurrence of varices are considered to be important causes of rebleeding[29]. However, the unobliterated components of the GV are sometimes small, and a further intraluminal injection might be difficult due to the previously injected polymers. In such circumstances, GV obliteration might not be complete. Over time, the residual GV can become larger, and rebleeding can occur. Incomplete obliteration and recurrence of varices are considered to be important causes of rebleeding[9]. Additionally, although rare, fatal complications do occur[13-16].
PTVE with cyanoacrylate is a modified procedure of the classical percutaneous transhepatic obliteration[30-32]. Though this novel technique was used only in a small number of patients with gastric varices and without gastrorenal shunts who were not candidate for BRTO[19,20], it started a new treatment method for gastric varices. In recent years, based on the good results of modified PTVE with 2-OCA in the treatment of EV reported in our previous study[21,22], we introduced 2-OCA in modified PTVE to treat gastric varices. In this retrospective study, we compared modified PTVE with an endoscopic injection using 2-OCA in the management of gastric varices. Despite the retrospective nature of the study, there were no significant differences in age, sex, cause of disease, or severity of liver disease between the studied patients.
In our study, the rebleeding rate of EVO was 37.8%, while it was only 12.5% in patients who underwent PTVE with cyanoacrylate. This showed that PTVE with 2-OCA was superior to endoscopic 2-OCA injection with respect to preventing rebleeding. Regarding the risk factors of variceal rebleeding, it was revealed that liver function, size of GV, and treatment methods were independent factors that predicted the rebleeding risk of GV. Although the rebleeding rate of GV was lower in patients who underwent PTVE, this did not yield a decreased mortality compared with patients in the EVO group. Cox analysis showed the liver function in the Child-Pugh classification was the most significant factor.
There are two technical superiorities of PTVE over EVO, which may contribute to the lower variceal rebleeding rate of PTVE. The first is that PTVE can achieve a more extensive obliteration area than EVO. In the present study, with regards to the PTVE procedure, 2-OCA was injected into entire gastric varices, perforating veins in the fundus and perigastric veins, including all the feeding veins in most of the patients (30/32, 93.75%). In the EVO procedure, although we tried to achieve complete obliteration in one session, some patients needed repeated injections, and even then, the obliteration rate was only 77.7%. Contrast CT follow-up showed that the PTVE could obliterate all collaterals in the vicinity of the gastric fundus over a wider area and in deeper layers compared with EVO. Adequate 2-OCA injection is important for complete variceal obliteration. In our study, more cyanoacrylate was used in the PTVE procedure than in EVO, which also indicates a wider obliteration range in the PTVE group. The second technical superiority of PTVE over EVO is that 2-OCA can be permanently embolized in perforating veins in the fundus and perigastric veins, including all its feeding veins. In our study, follow-up endoscopy and CT scans showed that the cyanoacrylate in the submucosa could be released with time, and lead to the eradication of the varices after PTVE. However, CT scans revealed that the 2-OCA still stayed in the perifundus varices and perforating veins in the gastric fundus and all the afferent veins during follow-up. This might explain why PTVE achieved long-term obliteration, and prevented recanalization and rebleeding of gastric varices.
Complications were similar in patients who underwent EVO or modified PTVE. Fever and abdominal pain were the two common complications in both groups. They were given conventional treatment and the fever and abdominal pain were usually alleviated within 1-2 wk. Six patients in the PTVE group and three in the EVO group experienced mild to moderate ascites. They were given diuretics and or albumin transfusion, and no patients experienced refractory ascites. Partial portal vein thrombosis presented in two patients in PTVE group, but the portal vein was patent under Doppler ultrasound without serious consequences. In the present study, one patient in the EVO group encountered pulmonary embolism. However, no patient encountered systemic embolization in the modified PTVE group. The modification of PTVE contributed to this satisfactory result. Firstly, in patients without a large gastrorenal shunt, the blood flow in the gastric varices was slow, so there was enough time for 2-OCA to obliterate the varices, and it was unlikely that the tissue glue would migrate into the systemic circulation. Therefore, the 2-OCA could be injected into the gastric varices with percutaneous transhepatic variceal embolization alone. Secondly, in patients with a large gastrorenal shunt and rapid blood flow in the gastric varices, percutaneous transhepatic embolization combined with left renal vein balloon catheter obstruction was performed to prevent systemic embolization.
Some authors tried to obliterate the afferent vein of the gastric varices using TIPS combined with embolotherapy[33]. However, TIPS for gastric varices is not as effective as that for EV; moreover, stent failure and hepatic encephalopathy also limit its application[34-36]. BRTO embolized the gastric varices through the outflow vein (the gastrorenal shunt), and it is recognized as a safe and effective treatment for gastric varices with a gastrorenal shunt. However, patients without a catheterizable gastrorenal shunt are not suitable for BRTO. In our study, even in patients with a large gastrorenal shunt, the embolization was still performed by PTVE, in which the gastric varices were embolized through the inflow veins, such as the left gastric vein, short gastric vein and/or posterior gastric vein. We suppose that PTVE could achieve a more extensive obliteration range than BRTO. As antegrade transcatheter embolization, PTVE with 2-OCA could embolized the entire gastric varices and all its inflow veins (left gastric vein, short gastric vein and/or posterior gastric vein). But BRTO variceal embolization is performed by gastrorenal shunt (outflow vein), in which the inflow veins perhaps could not be completely embolized.
In conclusion, with extensive and permanent obliteration of both gastric varices and its feeding veins, PTVE with 2-OCA is a prospective modality for the treatment of gastric varices. It is superior to endoscopic 2-OCA injection in terms of preventing rebleeding. However, our study is a retrospective single-center study. A future prospective, randomized, and controlled trial is required.
We would like to thank Dr. Vijay Shah from Mayo Clinic for his critical review of the manuscript. Thanks to Dr. Edward C Mignot, Shandong University, for linguistic advice.
Although gastric varices tend to bleed less than esophageal varices (EV), the mortality associated with gastric variceal hemorrhage is substantial. Unlike EV, gastric varices pose particular therapeutic challenges because of their size and location. The current methods for the treatment of gastric varices are far from ideal.
At present, endoscopic variceal obturation with the injection of agents such as N-butyl-2-cyanoacrylate is the first-line treatment for gastric variceal bleeding. However, the unobliterated components of the gastric varices (GV) are sometimes small, and a further intraluminal injection might be difficult due to the previously injected polymers. In such circumstances, GV obliteration might not be complete. Over time, the residual GV can become larger, and rebleeding can occur. Incomplete obliteration and recurrence of varices are considered to be important causes of rebleeding.
Percutaneous transhepatic variceal embolization (PTVE) with cyanoacrylate is a modified procedure of the classical percutaneous transhepatic obliteration. With GV, the perifundus veins and all the feeding veins in the vicinity of the gastric fundus are sufficiently obliterated with cyanoacrylate. PTVE with 2-octyl-cyanoacrylate (2-OCA) can improve long-term efficacy by preventing gastric varices rebleeding. In this retrospective study, the authors compared modified PTVE with endoscopic injection using 2-OCA in the management of gastric varices.
With extensive and permanent obliteration of both GV and its feeding veins, PTVE with 2-OCA is a prospective modality for the treatment of GV. It is superior to endoscopic 2-OCA injection in terms of preventing rebleeding.
The authors report their results of a retrospective control study: modified PTVE with 2-OCA and endoscopic variceal obturation with the injection of 2-OCA for prophylaxis of gastric variceal rebleeding. Important data including the rebleeding rate, survival rate, complications, and prognostic predictors were reported. This study indicates that PTVE with 2-OCA can be a better option for secondary prophylaxis of the gastric variceal hemorrhage. However, given the small sample size and retrospective nature of this study, their results may not be representative of the broader population of patients with gastric variceal and a future prospective, randomized, and controlled trial is required.
| 1. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [PubMed] |
| 2. | Kim T, Shijo H, Kokawa H, Tokumitsu H, Kubara K, Ota K, Akiyoshi N, Iida T, Yokoyama M, Okumura M. Risk factors for hemorrhage from gastric fundal varices. Hepatology. 1997;25:307-312. [PubMed] |
| 3. | Ryan BM, Stockbrugger RW, Ryan JM. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004;126:1175-1189. [PubMed] |
| 4. | D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475-505. [PubMed] [DOI] [Full Text] |
| 5. | Bosch J, García-Pagán JC. Prevention of variceal rebleeding. Lancet. 2003;361:952-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 290] [Article Influence: 12.6] [Reference Citation Analysis (2)] |
| 6. | Soehendra N, Nam VC, Grimm H, Kempeneers I. Endoscopic obliteration of large esophagogastric varices with bucrylate. Endoscopy. 1986;18:25-26. [PubMed] [DOI] [Full Text] |
| 7. | de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167-176. [PubMed] [DOI] [Full Text] |
| 8. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Huang YH, Yeh HZ, Chen GH, Chang CS, Wu CY, Poon SK, Lien HC, Yang SS. Endoscopic treatment of bleeding gastric varices by N-butyl-2-cyanoacrylate (Histoacryl) injection: long-term efficacy and safety. Gastrointest Endosc. 2000;52:160-167. [PubMed] |
| 10. | Akahoshi T, Hashizume M, Shimabukuro R, Tanoue K, Tomikawa M, Okita K, Gotoh N, Konishi K, Tsutsumi N, Sugimachi K. Long-term results of endoscopic Histoacryl injection sclerotherapy for gastric variceal bleeding: a 10-year experience. Surgery. 2002;131:S176-S181. [PubMed] |
| 11. | Lo GH, Liang HL, Chen WC, Chen MH, Lai KH, Hsu PI, Lin CK, Chan HH, Pan HB. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 310] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | Hou MC, Lin HC, Lee HS, Liao WC, Lee FY, Lee SD. A randomized trial of endoscopic cyanoacrylate injection for acute gastric variceal bleeding: 0.5 mL versus 1.0 mL. Gastrointest Endosc. 2009;70:668-675. [PubMed] [DOI] [Full Text] |
| 13. | Greenwald BD, Caldwell SH, Hespenheide EE, Patrie JT, Williams J, Binmoeller KF, Woodall L, Haluszka O. N-2-butyl-cyanoacrylate for bleeding gastric varices: a United States pilot study and cost analysis. Am J Gastroenterol. 2003;98:1982-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Tan YM, Goh KL, Kamarulzaman A, Tan PS, Ranjeev P, Salem O, Vasudevan AE, Rosaida MS, Rosmawati M, Tan LH. Multiple systemic embolisms with septicemia after gastric variceal obliteration with cyanoacrylate. Gastrointest Endosc. 2002;55:276-278. [PubMed] [DOI] [Full Text] |
| 15. | Battaglia G, Morbin T, Patarnello E, Merkel C, Corona MC, Ancona E. Visceral fistula as a complication of endoscopic treatment of esophageal and gastric varices using isobutyl-2-cyanoacrylate: report of two cases. Gastrointest Endosc. 2000;52:267-270. [PubMed] [DOI] [Full Text] |
| 16. | Chen YY, Shen TC, Soon MS, Lai JH. Life-threatening pericarditis after N-butyl-2-cyanoacrylate injection for esophageal variceal bleeding: Case report. Gastrointest Endosc. 2005;61:487-489. [PubMed] |
| 17. | Cho SK, Shin SW, Lee IH, Do YS, Choo SW, Park KB, Yoo BC. Balloon-occluded retrograde transvenous obliteration of gastric varices: outcomes and complications in 49 patients. AJR Am J Roentgenol. 2007;189:W365-W372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Ninoi T, Nishida N, Kaminou T, Sakai Y, Kitayama T, Hamuro M, Yamada R, Nakamura K, Arakawa T, Inoue Y. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol. 2005;184:1340-1346. [PubMed] |
| 19. | Kwak HS, Han YM. Percutaneous transportal sclerotherapy with N-butyl-2-cyanoacrylate for gastric varices: technique and clinical efficacy. Korean J Radiol. 2008;9:526-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Kiyosue H, Matsumoto S, Yamada Y, Hori Y, Okino Y, Okahara M, Mori H. Transportal intravariceal sclerotherapy with N-butyl-2-cyanoacrylate for gastric varices. J Vasc Interv Radiol. 2004;15:505-509. [PubMed] |
| 21. | Zhang CQ, Liu FL, Liang B, Sun ZQ, Xu HW, Xu L, Feng K, Liu ZC. A modified percutaneous transhepatic variceal embolization with 2-octyl cyanoacrylate versus endoscopic ligation in esophageal variceal bleeding management: randomized controlled trial. Dig Dis Sci. 2008;53:2258-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Zhang CQ, Liu FL, Liang B, Xu HW, Xu L, Feng K, Liu ZC. A modified percutaneous transhepatic varices embolization with 2-octyl cyanoacrylate in the treatment of bleeding esophageal varices. J Clin Gastroenterol. 2009;43:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Hashizume M, Kitano S, Yamaga H, Koyanagi N, Sugimachi K. Endoscopic classification of gastric varices. Gastrointest Endosc. 1990;36:276-280. [PubMed] |
| 24. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 25. | Lo GH, Lai KH, Cheng JS, Chen MH, Chiang HT. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001;33:1060-1064. [PubMed] [DOI] [Full Text] |
| 26. | Tan PC, Hou MC, Lin HC, Liu TT, Lee FY, Chang FY, Lee SD. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology. 2006;43:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Procaccini NJ, Al-Osaimi AM, Northup P, Argo C, Caldwell SH. Endoscopic cyanoacrylate versus transjugular intrahepatic portosystemic shunt for gastric variceal bleeding: a single-center U.S. analysis. Gastrointest Endosc. 2009;70:881-887. [PubMed] [DOI] [Full Text] |
| 28. | Kind R, Guglielmi A, Rodella L, Lombardo F, Catalano F, Ruzzenente A, Borzellino G, Girlanda R, Leopardi F, Pratticò F. Bucrylate treatment of bleeding gastric varices: 12 years’ experience. Endoscopy. 2000;32:512-519. [PubMed] |
| 29. | Rockey DC. Management of gastric varices. Gastroenterology. 2001;120:1875-1876; discussion 1875-1876. [PubMed] |
| 30. | Terabayashi H, Ohnishi K, Tsunoda T, Nakata H, Saito M, Tanaka H, Iida S, Nomura F, Okuda K. Prospective controlled trial of elective endoscopic sclerotherapy in comparison with percutaneous transhepatic obliteration of esophageal varices in patients with nonalcoholic cirrhosis. Gastroenterology. 1987;93:1205-1209. [PubMed] |
| 31. | Uflacker R. Percutaneous transhepatic obliteration of gastroesophageal varices using absolute alcohol. Radiology. 1983;146:621-625. [PubMed] |
| 32. | Bengmark S, Börjesson B, Hoevels J, Joelsson B, Lunderquist A, Owman T. Obliteration of esophageal varices by PTP: a follow-up of 43 patients. Ann Surg. 1979;190:549-554. [PubMed] |
| 33. | Xiao T, Chen L, Chen W, Xu B, Long Q, Li R, Li L, Peng Z, Fang D, Wang R. Comparison of transjugular intrahepatic portosystemic shunt (TIPS) alone versus TIPS combined with embolotherapy in advanced cirrhosis: a retrospective study. J Clin Gastroenterol. 2011;45:643-650. [PubMed] [DOI] [Full Text] |
| 34. | Sanyal AJ, Freedman AM, Luketic VA, Purdum PP, Shiffman ML, DeMeo J, Cole PE, Tisnado J. The natural history of portal hypertension after transjugular intrahepatic portosystemic shunts. Gastroenterology. 1997;112:889-898. [PubMed] |
| 35. | Barange K, Péron JM, Imani K, Otal P, Payen JL, Rousseau H, Pascal JP, Joffre F, Vinel JP. Transjugular intrahepatic portosystemic shunt in the treatment of refractory bleeding from ruptured gastric varices. Hepatology. 1999;30:1139-1143. [PubMed] [DOI] [Full Text] |
| 36. | ter Borg PC, Hollemans M, Van Buuren HR, Vleggaar FP, Groeneweg M, Hop WC, Laméris JS. Transjugular intrahepatic portosystemic shunts: long-term patency and clinical results in a patient cohort observed for 3-9 years. Radiology. 2004;231:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
P- Reviewer Kim HC S- Editor Gou SX L- Editor Rutherford A E- Editor Zhang DN