Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.673
Revised: December 11, 2012
Accepted: December 20, 2012
Published online: February 7, 2013
Processing time: 165 Days and 21.6 Hours
AIM: To evaluate whether desferrioxamine decreases ischemia and perfusion injury aggravated by cold storage (CS) in a rat liver perfusion model.
METHODS: Isolated rat livers were kept in CS in University of Wisconsin Solution for 20 h at 4 °C, then exposed to 25 min of warm ischemia (WI) at 37 °C followed by 2 h of warm perfusion (WP) at 37 °C with oxygenated (95% oxygen and 5% carbon dioxide) Krebs-Henseleit buffer. Desferrioxamine (DFO), an iron chelator, was added at different stages of storage, ischemia and perfusion: in CS only, in WI only, in WP only, in WI and perfusion, or in all stages. Effluent samples were collected after CS and after WI. Perfusate samples and bile were collected every 30 min (0, 0.5, 1, 1.5 and 2 h) during liver perfusion. Cellular injury was assessed by the determination of lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) in the effluent and perfusate samples. Total iron was analysed in the perfusate samples. After WP, the liver was collected for the determination of liver swelling (wet to dry ratio) and liver morphological examination (hematoxylin and eosin staining).
RESULTS: Increased CS time caused increased liver dysfunction during WP. After 2 h of WP, liver injury was indicated by increased release of AST (0.5 h CS: 9.4 ± 2.2 U/g liver vs 20 h CS: 45.9 ± 10.8 U/g liver, P < 0.05) and LDH (0.5 h CS: 59 ± 14 U/g liver vs 20 h CS: 297 ± 71 U/g liver, P < 0.05). There was an associated increase in iron release into the perfusate (0.5 h CS: 0.11 ± 0.03 μmoL/g liver vs 20 h CS: 0.58 ± 0.10 μmoL/g liver, P < 0.05) and reduction in bile flow (0.5 h CS: 194 ± 12 μL/g vs 20 h CS: 71 ± 8 μL/g liver, P < 0.05). When DFO was added during WI and WP following 20 h of CS, release of iron into the perfusate was decreased (DFO absent 0.58 ± 0.10 μmoL/g liver vs DFO present 0.31 ± 0.06 μmoL/g liver, P < 0.05), and liver function substantially improved with decreased release of AST (DFO absent 45.9 ± 10.8 U/g liver vs DFO present 8.1 ± 0.9 U/g liver, P < 0.05) and LDH (DFO absent 297 ± 71 U/g liver vs DFO present 56 ± 7 U/g liver, P < 0.05), and increased bile flow (DFO absent 71 ± 8 μL/g liver vs DFO present 237 ± 36 μL/g liver, P < 0.05). DFO was also shown to improve liver morphology after WP. Cellular injury (the release of LDH and AST) was significantly reduced with the addition of DFO in CS medium but to a lesser extent compared to the addition of DFO in WP or WI and perfusion. There was no effect on liver swelling or bile flow when DFO was only added to the CS medium.
CONCLUSION: DFO added during WI and perfusion decreased liver perfusion injury aggravated by extended CS.
- Citation: Arthur PG, Niu XW, Huang WH, DeBoer B, Lai CT, Rossi E, Joseph J, Jeffrey GP. Desferrioxamine in warm reperfusion media decreases liver injury aggravated by cold storage. World J Gastroenterol 2013; 19(5): 673-681
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/673.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.673
Hypothermia is used extensively to preserve many types of donor organs for transplantation and protects organs, in part, by reduced requirements for metabolic energy[1]. Donor livers are flushed and then stored at 4 °C in specialized storage solutions (e.g., University Wisconsin solution). However, preservation injury can cause primary graft dysfunction and increased morbidity and mortality in liver transplant recipients[2]. When cold storage (CS) time is extended beyond 10 h, there is a significant increase in primary graft dysfunction and long term biliary complications following liver transplantation[3-6].
The mechanisms responsible for donor liver dysfunction after preservation are not fully understood but one important potential contributor to liver damage is redox active iron. Redox active iron has been identified as a contributor to isolated hepatocyte death and liver damage during hypothermia[7-10]. Redox active iron can react with hydrogen peroxide and initiate radical reactions which damage cellular macromolecules, such as DNA, proteins and membrane lipids. Chelation of iron by specialised proteins usually prevents the generation of radical species in vivo. However, when cells[7,9,11] or organs[12-14] are cooled there is an increase in redox active iron and this increase has been linked to cell death during hypothermia[7,9,10].
One approach to abrogating the activity of redox active iron is to chelate free iron with iron chelators such as desferrioxamine (DFO)[7,9,15]. When DFO was added to CS media there was reduced hepatocyte and endothelial cell death during CS, and liver damage on re-perfusion[7,11,14,16]. However, it is unlikely that DFO is taken up intracellularly by liver cells during storage at 4 °C[7,17,18], so intracellular redox active iron has the potential to predispose the donor liver to injury during implantation [warm ischemia (WI)] and subsequent re-perfusion[14]. DFO enters tissue by endocytosis at 37 °C and therefore the presence of DFO during WI and re-perfusion may offer additional protection from CS injury. To test this hypothesis, isolated rat livers were perfused after CS, with DFO added to the preservation media during CS, WI and WP. This strategy significantly protected livers from CS damage.
Krebs-Henseleit buffer (KHB), DFO and reduced glutathione were purchased from SIGMA and Aldrich, Sydney, Australia. Hartmann’s solution was obtained from Baxter Health Care Pty Ltd, Old Toongabbie, Australia, University of Wisconsin solution (UWS) from Bristol-Myers Squibb Company, New York, United States, gentamicin from Pfizer, West Ryde, Australia and Actrapid human insulin from Novo Nordisk Pharmaceuticals Pty Ltd, Baulkham Hills, Australia. Dexamethasone sodium phosphate was from DBL, Rowville, Australia.
The use of animals was approved by the Animal Ethics Committee of the University of Western Australia. All animals received food and water before graft retrieval. Adult male rats (Strain PVG, average weight 272 ± 5 g, n = 35) were anesthetized with halothane. The gastroduodenal vein, splenic vein, right renal artery, right and left adrenal veins were ligated and the bile duct was cannulated. The liver was flushed with 20 mL of cold Hartmann’s solution (control) or UWS with supplements (reduced glutathione, dexamethasone, insulin and gentamicin, pH 7.35-7.4 on ice) and heparin (5 U/mL) via the aorta. The liver was excised and placed in UWS with supplements for 20 h at 2-4 °C. After CS the suprahepatic vena cava (outlet) and portal vein (inlet) were cannulated, and the liver was connected to a rat liver perfusion system while it was in the CS medium. In the control group, after the liver was excised, the liver was connected to a rat liver perfusion system while it was in cold saline for up to 0.5 h (CS0.5 h). The liver was then transferred to the perfusion chamber (37 °C) and flushed with 20 mL of Hartmann’s solution and heparin (5 U/mL). The first 6.5 mL of effluent and the CS medium were collected. To simulate WI during liver implantation, the liver was covered with damp gauze and left at 37 °C chamber for 25 min.
After WI, the liver was perfused using a water-jacketed perfusion system (Radnoti Glass Technology, Inc., Monrovia, United States) with oxygenated (95% oxygen and 5% carbon dioxide) KHB at 20 mL/min at 37 °C and the first 6.5 mL of perfusate was collected. After 7.5 min of perfusion, the perfusate was re-circulated and filtered through a pre-filter and filter (0.8 μm/0.2 μm, 32 mm OD, Pall Life Sciences, Australia) and the liver was then perfused for 2 h. During liver perfusion, perfusate (1.5 mL) and bile were collected at 0, 0.5, 1, 1.5 and 2 h and kept on ice. Perfusates were centrifuged at 10 000 g for 10 min at 4 °C and the supernatant was analysed for lactate dehydrogenase (LDH), aspartate aminotransferase (AST) and total iron.
After 2 h of perfusion, the liver was pat-dried and weighed. Tissue (approximately 0.5 g) was dried at 80 °C for 72 h for the calculation of wet to dry ratio and a portion of tissue was snap frozen in liquid nitrogen for subsequent analysis. The remaining liver was fixed in 10% formaldehyde for histopathological examination.
Perfusate LDH levels were measured by the method of Passonneau[19]. AST enzyme activity was measured by automated biochemical analyser (Hitachi 917, Roche Diagnostics). Total iron levels were measured by Inductively Coupled Plasma-Mass Spectrometry (Varian 820, California, United States) with aqueous standards used for the calibration curve. LDH, AST and total iron were expressed relative to dry liver weight.
Liver sections were stained with hematoxylin and eosin and then examined independently by a pathologist (B DeBoer) and a hepatologist (GP Jeffrey). A scale for morphological classification of hepatic injury was used as previously described[20]. The 9 point scale was: (1) normal rectangular structure; (2) round hepatocytes with an increase of sinusoidal spaces; (3) vacuolization in zone 3; (4) vacuolization in zone 2; (5) vacuolization in zone 1; (6) vacuolization and nuclear pyknosis in zone 3; (7) vacuolization and nuclear pyknosis in zone 2; (8) vacuolization and nuclear pyknosis in zone 1; and (9) necrosis.
There were seven experimental groups and each group consisted of 5 rats. DFO (1 mmol/L) was tested in five experimental groups with different combinations of DFO during CS, WI, WP and perfusion (WIP), or CS, WI and perfusion (CS WIP) (Table 1). The required amount of DFO was weighed and added to the media prior to use and the concentration (1 mmol/L) was based on our previous isolated rat hepatocyte study[7].
| Addition of DFO | |||||
| Experimental group | Number per group | CS time (h) in UWS | CS | WI | WP |
| CS0.5 h | 5 | - | - | - | - |
| CS20 h | 5 | 20 | - | - | - |
| DFO (CS) | 5 | 20 | DFO | - | - |
| DFO (WI) | 5 | 20 | - | DFO | - |
| DFO (WP) | 5 | 20 | - | - | DFO |
| DFO (WIP) | 5 | 20 | - | DFO | DFO |
| DFO (CS WIP) | 5 | 20 | DFO | DFO | DFO |
All analyses were carried out using R 2.10.1[21], using the package base and agricolae[22]. A one-way between ANOVA was used to compare the outcome of each measurement (the levels of LDH, AST, liver wet to dry ratio and bile flow) and treatment condition (Table 1). Least Square Difference (LSD) was used for Post Hoc testing. Differences are considered to be significant if P < 0.05. Data is reported as mean ± SE.
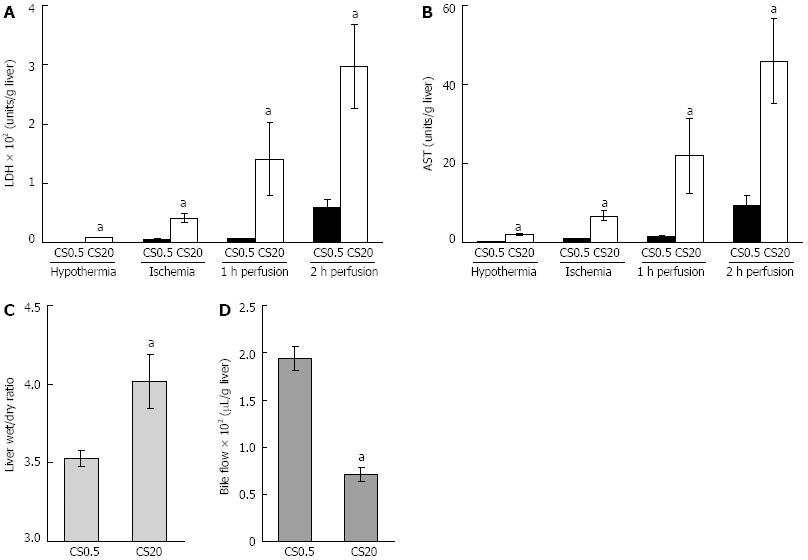
The degree to which liver damage occurred during CS, WI and WP was measured by the release of LDH and AST. Liver damage was evident immediately after extended CS. The LDH level was 7.9 ± 0.6 U/g liver and the AST level was 1.9 ± 0.1 U/g liver after 20 h of CS compared to 0.7 ± 0.1 U/g liver and 0.1 ± 0.0 U/g liver in the controls (CS0.5 in Figure 1). Increased damage was also evident after WI, with 9 fold higher LDH and 8 fold higher AST release in extended CS, relative to the controls (CS0.5 in Figure 1). The bulk (more than 95%) of LDH and AST release occurred during WP of livers subjected to extended CS (Figure 1). After 2 h of perfusion, LDH levels were 297 ± 71 U/g liver and AST levels were 45.9 ± 10.8 U/g liver in livers subjected to 20 h of CS compared to 59 ± 14 U/g liver and 9.4 ± 2.2 U/g liver respectively in livers of the control group. Twenty hours of CS resulted in a 14% increase in liver swelling and a 63% decrease in bile flow (Figure 1). Taken together, these data indicate that 20 h of CS caused a detrimental effect on liver function during subsequent WI and WP.
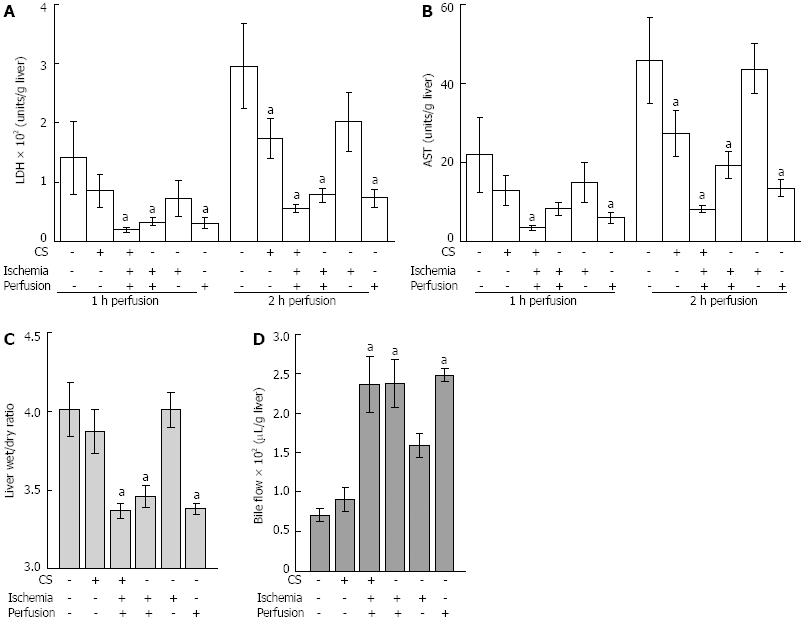
DFO was first added to CS medium only. There was no significant improvement in LDH levels or AST levels during WI. However during WP there was a 40% reduction of LDH and AST levels after 2 h of perfusion compared to livers that had not been exposed to DFO during CS (Figure 2). There was no effect on liver swelling or bile flow (Figure 2).
To test if either redox active iron was not completely chelated by DFO during CS, or additional redox active iron release occurred during WI and WP, DFO was added to solutions during all stages of the experiment. This resulted in a significant reduction of AST levels from 6.7 ± 1.3 U/g liver to 3.4 ± 0.5 U/g liver compared with no DFO during WI (P < 0.05). LDH levels were not significantly affected (DFO absent 39.9 ± 7.9 U/g liver vs DFO present 24.4 ± 2.5 U/g liver). In contrast, after 2 h of WP there was a large reduction of AST levels and LDH levels from 45.9 ± 10.8 U/g liver to 8.1 ± 0.9 U/g liver and 297 ± 71 U/g liver to 56 ± 7 U/g liver respectively when compared to no DFO (Figure 2). These levels were similar to the controls (CS0.5 in Figure 1). Consistent DFO effects were observed with improved liver swelling, decreased from 4.01 ± 0.17 to 3.37 ± 0.05 (wet weight to dry weight ratio) and bile flow increased from 71 ± 8 μL/g liver to 237 ± 36 μL/g liver (Figure 2).
To test the possibility that DFO had its predominant protective effect during WI and/or WP, DFO was added to either WI or WP media alone. DFO had no effect when added during WI alone, however there was a protective effect when it was added to perfusion medium alone (Figure 2). There was a reduction of LDH from 297 ± 71 U/g liver to 72 ± 15 U/g liver and AST from 45.9 ± 10.8 U/g liver to 13.4 ± 2.2 U/g liver compared to livers not treated with DFO (Figure 2). Furthermore, liver swelling was prevented and bile flow was maintained (Figure 2). Of note, the protective effect was comparable to that observed when DFO was present during all three stages of the experiment and this is consistent with the concept that redox active iron continued to be a major cause of liver damage during WI and WP following extended CS.
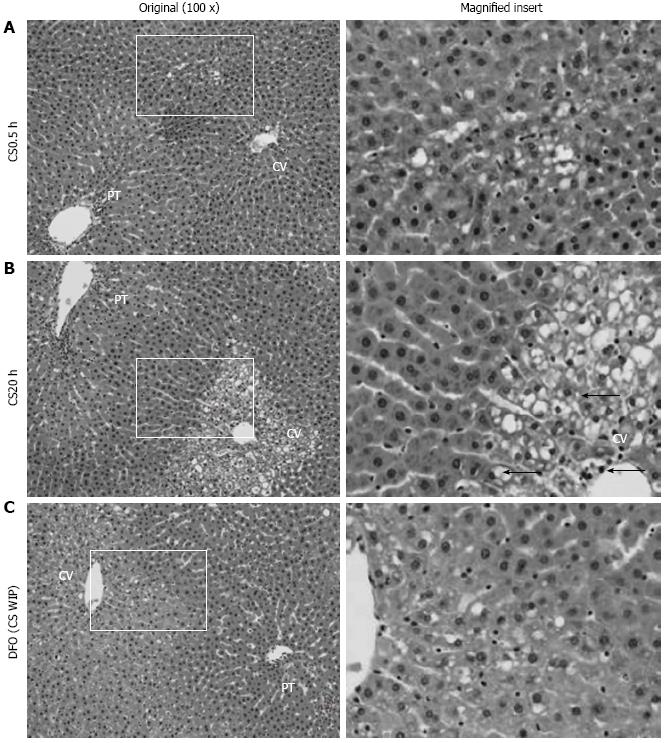
To further assess the protective effect of DFO, liver morphology was examined following 2 h of WP and assessed using a nine point scale ranging from 1 (normal structure) to 9 (necrosis) (Figure 3). Relative to the controls (CS0.5 h, morphological classification of 3.0 ± 0.1, mean ± SE, n = 4), extended CS resulted in extensive vacuolization and marked pyknosis (morphological classification of 5.5 ± 0.9, mean ± SE, n = 4, P < 0.05). DFO was largely effective in preventing changes to liver morphology (morphological classification of 3.0 ± 0.4, mean ± SE, n = 4, P < 0.05 relative to no DFO).
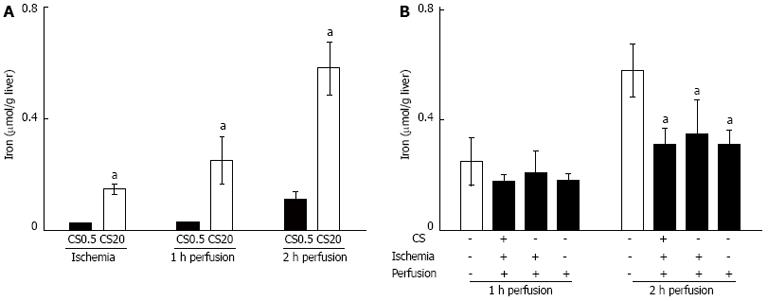
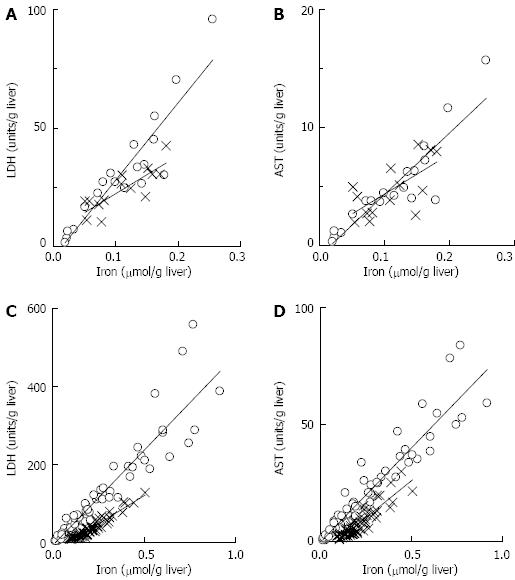
The effectiveness of DFO in preventing liver damage indicated redox active iron was likely a major contributor to liver damage. To further investigate this, iron release was measured following WI and during perfusion. Following WI and 20 h of CS there was 0.15 ± 0.02 μmoL iron/g liver compared with 0.02 ± 0.01 μmoL iron/g liver in the controls (CS0.5 in Figure 4, P < 0.05). After 2 h of WP, iron release after 20 h of CS was 0.58 ± 0.10 μmoL iron/g liver which was significantly higher than 0.11 ± 0.03 μmoL/g liver in the controls (CS0.5 in Figure 4, P < 0.05). Iron concentrations were significantly correlated with LDH release and AST release during WI (Figure 5). During WP, the concentration of iron was also correlated with LDH release and AST release (Figure 5). The significant correlation between iron and the damage markers LDH and AST was consistent with the hypothesis that redox active iron was causing liver damage.
The presence of DFO altered iron concentrations in WP media. When DFO was added during CS, WI and perfusion, iron concentrations were significantly decreased from 0.58 ± 0.10 μmoL/g liver (no DFO) to 0.31 ± 0.06 μmoL/g liver (with DFO, P < 0.05) (Figure 4). There was also a significant relationship between the concentration of iron and liver damage during both WI and WP (Figure 5). However, the slope of the line describing the relationship was significantly lower in the presence of DFO compared to the absence of DFO during WP (Figure 5). Consequently, for the same concentration of iron, there was less damage in the presence of DFO relative to absence of DFO. One explanation for this observation may be that by chelating iron during WI or perfusion, DFO prevented ongoing liver damage caused by redox active iron.
It is well established that extended CS causes increased liver damage and that the presence of iron chelators in storage media can substantially protect hepatocytes and livers from damage during cold incubation or storage[9,14,23,24]. In this study, extended CS also aggravated liver damage during the simulated transplantation protocol involving WI (mimicking implantation) and WP. The presence of DFO during CS only partial decreased the subsequent vulnerability of livers to the simulated transplantation protocol. The novel finding of this study was that the presence of DFO during WI and WP could substantially decrease the vulnerability of livers to extended CS.
Liver damage during WP was related to events occurring during CS, such that extended CS sensitised the liver to subsequent WI and perfusion injury. Redox active iron has been identified as a contributor to hepatocyte death[10] and liver damage during CS[7,9,10]. Data from this study provided evidence that redox active iron was involved in sensitising the liver during extended CS, as the presence of DFO during CS did partially protect against subsequent liver damage. There is evidence that DFO is not able to cross membranes during incubation at 4 °C[7,18], so by what mechanism did DFO partially protect the liver in this present model? Iron is present in UWS solution as measured in our laboratory (4.43 ± 0.05 μmoL/L, n = 4) and in another study[25], so the protective effect of DFO added during CS could be a result of chelation of extracellular iron. It has also been suggested that DFO is able to reduce intracellular iron by draining cytosolic iron to the extracellular medium[26]. Consistent with this concept, we have previously shown that chelation of extracellular iron protects hepatocytes during cold incubation[7]. Therefore, during extended CS, extracellular iron could have contributed to the sensitisation of livers to subsequent WI and perfusion injury.
Intracellular redox active iron could have also contributed to the sensitization of livers to subsequent WI and perfusion injury. Intracellular redox active iron has been shown to increase during cold incubation/storage in isolated hepatocytes[9,11] and whole liver[14]. Additionally, we have linked increased intracellular redox active iron to hepatocyte cell death during extended cold incubation[7]. In previous experiments not involving CS, redox active iron has been implicated in renal ischemia and perfusion injury[12,27] and ischemia and perfusion injury in livers[28]. Therefore, an increase in the concentration of intracellular redox active iron caused by the extended CS would exacerbate an ongoing cycle of redox active iron release during WI and WP. This explanation would account for the added effectiveness of DFO during WI and WP.
The findings of this study have implications for liver transplantation. There has been a focus on protecting livers during CS by developing various cryoprotective media or improving liver function prior to transplant using perfusion systems. This data indicates there may be a further opportunity to enhance liver preservation during transplantation and following transplantation by chelating redox active iron. This concept will need further testing in liver transplant models as the in vitro model used in this study does not fully encapsulate the complexity of the recipients’ response to the transplant procedure and drug intervention. From a clinical application perspective it is worth noting that DFO is already approved for a variety of clinical applications such as treating iron overload patients[29,30].
Cold donor organ preservation techniques were developed to reduce cellular metabolic activity and maintain cellular viability in donor organs. However, livers stored beyond about 12 h are generally considered to be unsuitable for transplantation.
Extending the time of cold storage (CS) increases the susceptibility of livers to ischemia and reperfusion injury during transplantation. Preventing ischemia and reperfusion injury would permit extended times of CS.
Desferrioxamine (DFO), an iron chelator, is often included in CS media to prevent oxidative stress caused by redox active iron. The novel finding of this study was that including DFO during warm ischemia and warm perfusion could substantially decrease the vulnerability of rat livers to an extended time (20 h) of CS.
This study shows that it is possible to extend the time in which livers can be kept in CS. Extended CS times would improve the numbers of livers available for transplant.
This is an experimental study of correct metodologia. The bibliography is sorted correctly.
P- Reviewer Lopez-Andujar R S- Editor L- Editor A E- Editor Zhang DN
| 1. | Llarrull MS, Pizarro MD, Scandizzi AL, Bottai H, Guibert EE, Rodriguez JV. Cold preservation of isolated hepatocytes in UW solution: experimental studies on the respiratory activity at 0 degrees C. Cryo Letters. 2007;28:313-328. [PubMed] |
| 2. | Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 413] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 3. | Defamie V, Laurens M, Patrono D, Devel L, Brault A, Saint-Paul MC, Yiotakis A, Barbry P, Gugenheim J, Crenesse D. Matrix metalloproteinase inhibition protects rat livers from prolonged cold ischemia-warm reperfusion injury. Hepatology. 2008;47:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Li L, Li CM, Zhang BY, Hu MD, Li XY, Ran JH, Huang M. Apoptosis of rat liver in cold preservation with custom-designed KYL solution. Hepatobiliary Pancreat Dis Int. 2007;6:497-503. [PubMed] |
| 5. | Shimizu H, Kataoka M, Ohtsuka M, Ito H, Kimura F, Togawa A, Yoshidome H, Kato A, Miyazaki M. Extended cold preservation of the graft liver enhances neutrophil-mediated pulmonary injury after liver transplantation. Hepatogastroenterology. 2005;52:1172-1175. [PubMed] |
| 6. | Vajdová K, Smreková R, Mislanová C, Kukan M, Lutterová M. Cold-preservation-induced sensitivity of rat hepatocyte function to rewarming injury and its prevention by short-term reperfusion. Hepatology. 2000;32:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Arthur PG, Niu X, Rigby P, Steer JH, Jeffrey GP. Oxidative stress causes a decline in lysosomal integrity during hypothermic incubation of rat hepatocytes. Free Radic Biol Med. 2008;44:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Ozaki M, Fuchinoue S, Teraoka S, Ota K. Mobilization of low-molecular-weight iron and peroxidative damage during ischemia and reoxygenation of the rat liver. Transplant Proc. 1994;26:918-921. [PubMed] |
| 9. | Rauen U, Petrat F, Li T, De Groot H. Hypothermia injury/cold-induced apoptosis--evidence of an increase in chelatable iron causing oxidative injury in spite of low O2-/H2O2 formation. FASEB J. 2000;14:1953-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Rauen U, Kerkweg U, Weisheit D, Petrat F, Sustmann R, de Groot H. Cold-induced apoptosis of hepatocytes: mitochondrial permeability transition triggered by nonmitochondrial chelatable iron. Free Radic Biol Med. 2003;35:1664-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Kerkweg U, Li T, de Groot H, Rauen U. Cold-induced apoptosis of rat liver cells in University of Wisconsin solution: the central role of chelatable iron. Hepatology. 2002;35:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Huang H, Salahudeen AK. Cold induces catalytic iron release of cytochrome P-450 origin: a critical step in cold storage-induced renal injury. Am J Transplant. 2002;2:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Pincemail J, Sergent O, Detry O, Gaspar Y, Cheramy-Bien JP, Cillard J, Meurisse M, Defraigne JO. Intracellular free iron content of rat liver tissue after cold ischemia. Transplant Proc. 2002;34:759-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Wyllie S, Seu P, Gao FQ, Goss JA. Deregulation of iron homeostasis and cold-preservation injury to rat liver stored in University of Wisconsin solution. Liver Transpl. 2003;9:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother. 2001;55:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 320] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Vreugdenhil PK, Rankin MA, Southard JH. Cold storage sensitizes hepatocytes to oxidative stress injury. Transpl Int. 1997;10:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Cable H, Lloyd JB. Cellular uptake and release of two contrasting iron chelators. J Pharm Pharmacol. 1999;51:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Lloyd JB, Cable H, Rice-Evans C. Evidence that desferrioxamine cannot enter cells by passive diffusion. Biochem Pharmacol. 1991;41:1361-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Passonneau JV, Lowry OH. Chapter 7: A collection of enzyme assays. Totowa, New Jersey: Humana Press 1993; 281. |
| 20. | Bessems M, Doorschodt BM, van Vliet AK, van Gulik TM. Improved rat liver preservation by hypothermic continuous machine perfusion using polysol, a new, enriched preservation solution. Liver Transpl. 2005;11:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | The R Core Team. R: A Language and Environment for Statistical Computing [Computer program]. Vienna: R Foundation for Statistical Computing 2010; . |
| 22. | De Mediburu F. Agricolae: Statistical Procedures for Agricultural Research. R package version 1.09-9 [Computer program]. Vienna: R Foundation for Statistical Computing 2010; . |
| 23. | Bahde R, Palmes D, Gemsa O, Minin E, Stratmann U, de Groot H, Rauen U, Spiegel HU. Attenuated cold storage injury of rat livers using a modified HTK solution. J Surg Res. 2008;146:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Huang H, He Z, Roberts LJ, Salahudeen AK. Deferoxamine reduces cold-ischemic renal injury in a syngeneic kidney transplant model. Am J Transplant. 2003;3:1531-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Evans PJ, Tredger JM, Dunne JB, Halliwell B. Catalytic metal ions and the loss of reduced glutathione from University of Wisconsin preservation solution. Transplantation. 1996;62:1046-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Tenopoulou M, Doulias PT, Barbouti A, Brunk U, Galaris D. Role of compartmentalized redox-active iron in hydrogen peroxide-induced DNA damage and apoptosis. Biochem J. 2005;387:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | de Vries B, Snoeijs MG, von Bonsdorff L, Ernest van Heurn LW, Parkkinen J, Buurman WA. Redox-active iron released during machine perfusion predicts viability of ischemically injured deceased donor kidneys. Am J Transplant. 2006;6:2686-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Omar R, Nomikos I, Piccorelli G, Savino J, Agarwal N. Prevention of postischaemic lipid peroxidation and liver cell injury by iron chelation. Gut. 1989;30:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Pippard MJ, Callender ST. The management of iron chelation therapy. Br J Haematol. 1983;54:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Miyajima H, Takahashi Y, Kamata T, Shimizu H, Sakai N, Gitlin JD. Use of desferrioxamine in the treatment of aceruloplasminemia. Ann Neurol. 1997;41:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |