INTRODUCTION
Smoothelin, a constituent of the smooth muscle cell cytoskeleton, has been described as a marker of end-stage differentiation of smooth muscle cells because it has been found only in contractile smooth muscle cells[1-3]. Smoothelin has two major isoforms in adults, which are expressed in a tissue specific manner: a 59 kDa isoform, smoothelin-A, which is expressed in visceral and urogenital tissues, such as the digestive tract, bladder, and prostate; and a 110 kDa isoform, smoothelin-B, which is expressed in blood vessel walls[4]. Transient synthesis of a third smoothelin isoform has been detected in embryonic striated muscle cells in chicken[5]. In cultured smooth muscle cells, smoothelin colocalizes with α-smooth muscle actin stress fibers[3] and smoothelin can bind to α-smooth muscle actin, which suggested a direct role of smoothelin in contraction[6]. In contrast, cells with smooth muscle cell-like features, such as myofibroblasts, do not express smoothelin[3]. Myofibroblasts are contractile cells that express α-smooth muscle actin[7]. These cells are involved in tissue repair processes and, particularly, in extracellular matrix deposition and remodeling[8]. In normal connective tissues, myofibroblasts are rare[9]. After tissue injury, myofibroblasts appear, and it is commonly accepted that most are derived from locally recruited connective tissue fibroblasts[10]. In the liver, the most important cells involved in fibrogenesis are hepatic stellate cells and portal fibroblasts, which are able to differentiate into myofibroblasts and are responsible for matrix deposition[11]. However, the involvement of different cell types with various origins, such as smooth muscle cells, circulating cells or bone marrow-derived cells has also been suggested in the establishment of liver fibrosis/cirrhosis[12-16]. However, the degree to which this process contributes to fibrosis remains a matter of intense debate and is likely to be context-dependent. To determine the possible contribution of smooth muscle cells to the appearance of myofibroblasts during fibrotic processes, we studied the expression of smoothelin in different diseases that show myofibroblast involvement; i.e., post-burn cutaneous hypertrophic scars, liver fibrosis and cirrhosis, and hepatocellular carcinomas.
MATERIALS AND METHODS
Human liver samples and tissue processing
Human tissue samples used in this study were selected from the files of the tissue bank of the Department of Pathology (CHU Bordeaux, Pellegrin Hospital, Bordeaux, France). Normal liver tissues (n = 3) were obtained from macroscopically normal parts of livers after hepatectomy, taken at a distance from hemangiomas. Pathological specimens included post-burn cutaneous hypertrophic scars (n = 3); fibrotic liver tissue (n = 5), ranging from F1 to F3 stage according to the Metavir score[17]; cirrhotic tissues (viral and alcoholic hepatitis) (n = 5); and hepatocellular carcinomas (n = 5). Tissue samples were fixed in 10% buffered formalin, embedded in paraffin and processed for diagnostic purposes and immunohistochemistry, or were immediately frozen in liquid nitrogen-cooled isopentane for confocal microscopy analysis. The procedures were carried out in accordance with the European Guidelines for the use of human tissues.
Immunohistochemistry and confocal microscopy
Mouse monoclonal antibodies against smoothelin (immunoglobulin G, IgG1 clone R4A, which reacts with smoothelin A and B, MUbio Products, Maastricht, The Netherlands), and α-smooth muscle actin (IgG2a clone 1A4, Dako SA, Trappes, France) were used. These two antibodies have been extensively used and their specificity has been clearly documented[1,18]. Immunohistochemistry was essentially performed as previously described[19]. Briefly, after incubation with the first antibody, the epitopes were detected using the Vectastain® ABC system (Vector Laboratories, Peterborough, United Kingdom) or the Envision™ system (DakoCytomation, Trappes, France), with diaminobenzidine as the color substrate. Slides were then counterstained with hematoxylin.
For double immunofluorescence, cryostat sections were first incubated with the antibodies against smoothelin and α-smooth muscle actin, then with a TRITC-conjugated goat anti-mouse IgG1 (Southern Biotech, Birmingham, AL, United States) and an Alexa Fluor® 488 goat anti-mouse IgG2a (Molecular Probes, Eugene, OR, United States). Nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI).
The specificity of staining was confirmed by incubation in non-immune serum and in the absence of the primary antibody. For immunohistochemistry, sections were examined with a Zeiss Axioplan 2 microscope (Carl Zeiss Microscopy, Jena, Germany). Images were acquired with an AxioCam camera (Carl Zeiss Vision, Hallbergmoos, Germany) by means of the AxioVision image processing and analysis system (Carl Zeiss Vision). For double immunofluorescence, sections were analyzed with a confocal microscopy (LSM Meta 510, Carl Zeiss Microscopy).
For quantitative evaluation of staining, cell counting was performed in the septa of fibrotic and cirrhotic livers, and in the stroma reaction of hepatocellular carcinoma. Vessels were not included in this evaluation. For each field, the ratio of the number of smoothelin-positive and α-smooth muscle actin-positive cells over the number of cells only expressing α-smooth muscle actin was calculated. The analysis was performed on an average of 10 fields/zone using the × 40 objective. Only positive cells containing a nucleus were counted.
RESULTS
α-smooth muscle actin and smoothelin expression in hypertrophic scars
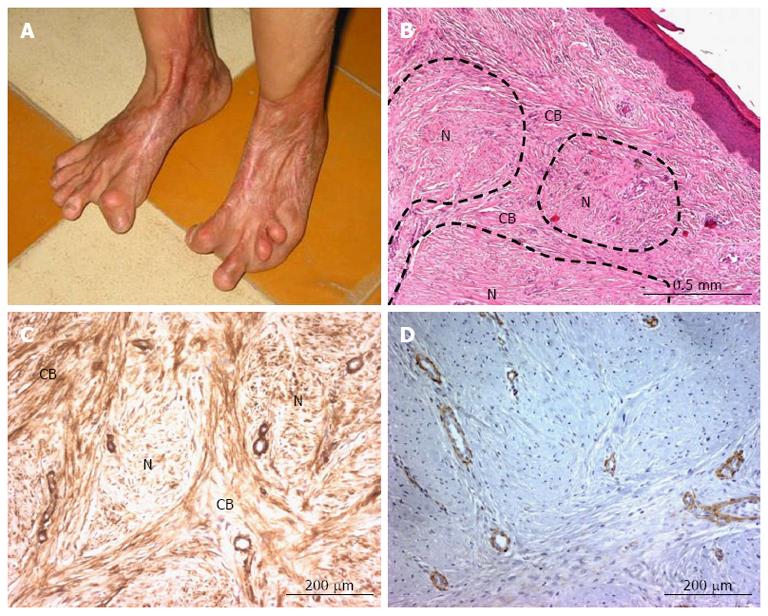
In many situations, hypertrophic scars develop significant contractile activity (because of the presence of myofibroblasts) (Figure 1A), and are usually organized in a nodular pattern (Figure 1B). By immunohistochemistry, α-smooth muscle actin was detected in blood vessel walls and in numerous myofibroblasts present in and around the nodules (Figure 1C), whereas smoothelin was exclusively shown in the vessel walls (Figure 1D). Our data showed that myofibroblasts present in hypertrophic scars do not express smoothelin.
Figure 1 α-smooth muscle actin and smoothelin expression in a cutaneous hypertrophic scar.
A: Following a burn injury, a retractile hypertrophic scar was observed (from Vincent Casoli, Plastic Surgery and Burns Unit, University Hospital of Bordeaux, France); B: Hematoxylin and eosin staining shows typical hypertrophic scar architecture with nodules (N) surrounded by cell bundles (CB); C: α-smooth muscle actin is expressed in vascular smooth muscle cells and myofibroblasts both in and around the nodules; D: Smoothelin is only expressed by vascular tunica media smooth muscle cells.
α-smooth muscle actin and smoothelin expression in normal and pathological livers
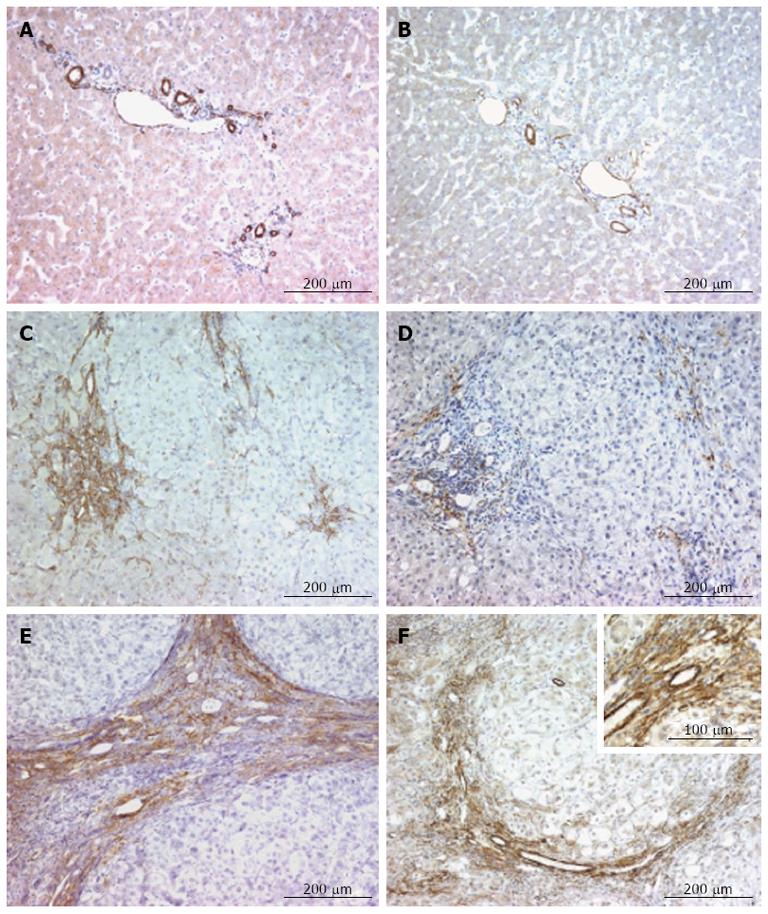
In the normal liver, α-smooth muscle actin was exclusively expressed by the smooth muscle cells within the tunica media of portal arteries and veins, and of the centrilobular veins (Figure 2A). The expression of smoothelin in the normal liver was also detected in the smooth muscle cells of these vessels (Figure 2B). Smoothelin and α-smooth muscle actin showed a similar distribution.
Figure 2 α-smooth muscle actin and smoothelin expression in normal, fibrotic and cirrhotic livers.
A and B: In the normal liver, α-smooth muscle actin (A) and smoothelin (B) show similar expression in tunica media smooth muscle cells of the portal arteries and veins and in the centrilobular veins; C and D: In severe fibrosis, myofibroblasts express high amounts of α-smooth muscle actin (C), while smoothelin is only expressed at low levels (D) (α-smooth muscle actin and smoothelin are coexpressed in vascular smooth muscle cells); E and F: In the cirrhotic liver, myofibroblasts express α-smooth muscle actin in fibrotic septae (E) and numerous myofibroblasts also express smoothelin (F); however, smoothelin expression is lower compared with α-smooth muscle actin expression (insert). α-smooth muscle actin and smoothelin are co-expressed in vascular tunica media smooth muscle cells (F).
In livers with mild or moderate fibrosis (F1-F2), myofibroblasts present in fibrotic areas clearly expressed α-smooth muscle actin, but did not express smoothelin (data not shown). In livers showing severe fibrosis (F3), strong staining for α-smooth muscle actin was detected in myofibroblasts present in fibrotic areas (Figure 2C). Weak expression of smoothelin was present in a few myofibroblasts (Figure 2D). In cirrhotic livers (F4), myofibroblasts present in cirrhotic septa expressed high amounts of α-smooth muscle actin (Figure 2E). Numerous myofibroblasts also expressed smoothelin (Figure 2F). In all stages of liver fibrosis, α-smooth muscle actin and smoothelin were co-expressed in vascular smooth muscle cells (Figure 2C-F).
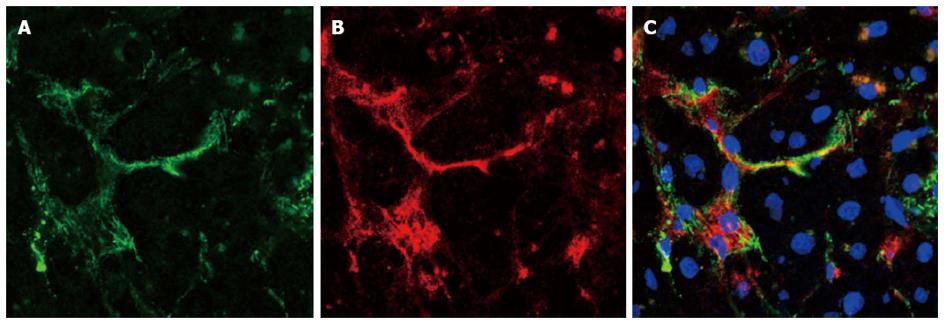
The co-localization of α-smooth muscle actin and of smoothelin in the same cells was confirmed by double immunofluorescence (Figure 3). All cells expressing smoothelin also expressed α-smooth muscle actin, including vascular smooth muscle cells and myofibroblasts. Moreover, a quantitative evaluation revealed that in livers showing severe fibrosis (F3), 5% to 10% of myofibroblasts co-expressed α-smooth muscle actin and smoothelin. In cirrhotic septa, more than 50% of myofibroblasts co-expressed α-smooth muscle actin and smoothelin.
Figure 3 Cellular co-localization of α-smooth muscle actin and smoothelin in a fibrotic liver.
A: α-smooth muscle actin expression (green); B: Smoothelin expression (red); C: Merged (nuclei are stained with DAPI). Smoothelin-expressing cells also express α-smooth muscle actin.
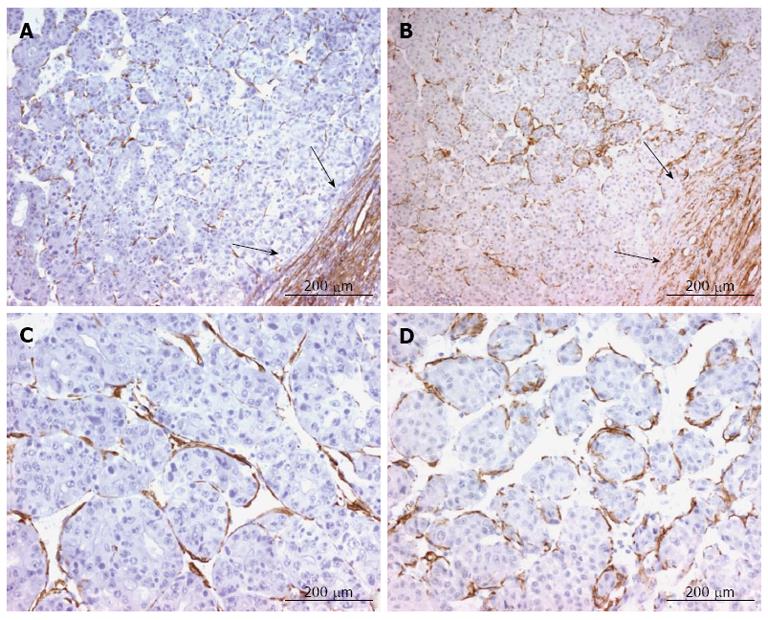
In hepatocellular carcinomas, we observed the same expression pattern of α-smooth muscle actin and smoothelin in the stroma reaction surrounding the tumor (Figure 4A and B). These two proteins were also expressed by fusiform pericyte-like cells between endothelial cells of the sinusoidal capillaries and tumoral cell plates (Figure 4C and D).
Figure 4 α-Smooth muscle actin and smoothelin expression in hepatocellular carcinoma.
A and B: α-smooth muscle actin (A) and smoothelin (B) are expressed similarly in the stroma reaction surrounding the tumor (arrows) and around tumoral hepatocytes; C and D: α-smooth muscle actin (C) and smoothelin (D) are expressed by pericyte-like cells underlying capillaries between tumoral hepatocytes.
DISCUSSION
In normal conditions, several cell types that have contractile properties express α-smooth muscle actin, such as smooth muscle cells and, to a lesser extent, pericytes. Smooth muscle cells display a large variation in phenotype among, and even within, tissues and organs. Different patterns of marker expression reflect, in part, the heterogeneity of smooth muscle cell subpopulations and their phenotypic modulation[20]. Smoothelin, a recently described smooth muscle cell marker, illustrates these phenotypic features. Firstly, smoothelin expression varies, with isoform A being expressed in visceral smooth muscle cells, and isoform B being expressed in vascular smooth muscle cells[4]. Secondly, smoothelin is expressed only by fully differentiated smooth muscle cells and not by proliferative or non-contractile smooth muscle cells[1]. Thirdly, visceral smoothelin expression is different according to the location of the smooth muscle cells within the organ. For example, in the digestive tract and urinary tract, most of the smooth muscle cells of the muscularis propia express smoothelin, but the smooth muscle cells of the muscularis mucosae do not[21,22]. During fibrotic processes, it is assumed that it is mainly fibroblasts that are recruited and acquire a myofibroblastic phenotype, i.e., a smooth muscle cell-like phenotype. However, it has been suggested that smooth muscle cells can also contribute to the appearance of myofibroblasts. Similarly to smooth muscle cells, fully differentiated myofibroblasts express α-smooth muscle actin and are contractile cells; in addition, myofibroblasts are responsible for extracellular matrix deposition. Like myofibroblasts observed within bladder carcinoma[21], blood vessel adventitia[23] or the airway wall of patients with asthma[24], our study shows that myofibroblasts in cutaneous hypertrophic scars do not express smoothelin. In the skin, myofibroblasts are mostly derived from locally recruited connective tissue fibroblasts, which, prior to injury, do not express α-smooth muscle actin or smoothelin[8]. These fibroblasts, when transformed into myofibroblasts, express α-smooth muscle actin but not smoothelin. Additionally, it has been shown that, in hypertrophic scars, a population of fibrogenic circulating cells, called fibrocytes, is recruited and participates in scar formation[25]. Here, we showed that, in hypertrophic scars, myofibroblasts do not express smoothelin, which suggests that these myofibroblasts probably do not derive from local smooth muscle cells. However, our study showed that, in fibrotic liver diseases, myofibroblasts can express smoothelin. Double immunofluorescence and confocal analysis confirmed the co-expression of α-smooth muscle actin and smoothelin in the same cells. This expression is stronger in parallel with the progression and the chronicity of the fibrotic lesion: i.e., there are more smoothelin-expressing myofibroblasts within advanced stages of fibrosis or cirrhosis than in the first stages of fibrosis. Smoothelin expression may thus appear gradually during fibrosis development; therefore, smoothelin could represent a good marker for evaluating the prognosis of liver fibrosis. In the liver, numerous cell types can be recruited and acquire a myofibroblastic phenotype; generally these are fibroblasts within the portal tract and hepatic stellate cells in the space of Disse[11]. As shown here, these cells do not express smoothelin. However, myofibroblasts can also originate from the smooth muscle cells present in portal vessels and centrilobular veins[11], which, as described here, do express smoothelin. In chronic hepatic schistosomiasis, it has been suggested that smooth muscle cells from the artery and vein tunica media are involved in the portal fibrogenesis that occurs in this pathology[12]. In the experimental model of porcine serum-induced fibrosis in rats, mesenchymal cells, located around the centrilobular vein, called second layer cells, participate in the formation of fibrotic septa[26]. The expression of smoothelin within smooth muscle cells can also vary. In early atherosclerotic lesions, smoothelin expression decreases in the vicinity of the affected area[27,28]; however, when the plaques become “quiescent”, smoothelin expression can be detected again[2]. Finally, we cannot exclude the possibility that the expression of smoothelin within septal myofibroblastic cells is a residual smooth muscle feature, underlining the possible smooth muscle cell origin of a subpopulation of myofibroblasts. Lastly, the tumoral stroma is the connective tissue surrounding tumoral cells[29]. No smoothelin-expressing stromal cells were described in published studies of bladder carcinoma[21,30]. In hepatocellular carcinoma, we show that numerous α-smooth muscle actin expressing myofibroblasts in the stroma reaction also express smoothelin, underlining the possible smooth muscle origin of these myofibroblasts. Surprisingly, we also showed that, in hepatocellular carcinomas, cells located between endothelial cells and tumoral hepatocytes express α-smooth muscle actin and smoothelin. Pericytes and hepatic stellate cells, also termed liver-specific pericytes[31,32], can express α-smooth muscle actin, but not smoothelin[2]. This pattern of smoothelin expression, which was not found in the fibrotic liver, illustrates the complete reorganization of the capillarized liver sinusoids during tumor development, with the appearance of specific cells, and may represent an additional feature for diagnosing hepatocellular carcinomas. In summary, we hypothesize that during liver fibrosis, a subpopulation of myofibroblasts express smoothelin. These myofibroblasts may be derived from smooth muscle cells coming from vascular walls, which are recruited to participate in the fibrotic process. These cells expressing smoothelin are recruited in advanced stages of cirrhosis, when a significant vascular reorganization occurs, including portovenous and arteriovenous shunting; therefore, smoothelin could be a useful marker to distinguish advanced stages of cirrhosis. In addition, in hepatocellular carcinomas, smoothelin expression shows a very specific pattern. These data again illustrate the different cellular origins of the so-called myofibroblastic cells involved in liver fibrogenesis.
COMMENTS
Background
Myofibroblasts are fibrogenic and contractile cells involved in remodeling and healing processes. In most organs, myofibroblasts are rare in normal physiological conditions. After tissue injury, myofibroblasts appear, which are derived from local stromal cells, such as resident fibroblasts or organ specific stromal cells, such as hepatic stellate cells in the liver.
Research frontiers
To explore the origin of myofibroblasts, the authors studied the expression of smoothelin, a constituent of the smooth muscle cell cytoskeleton, in normal and fibrotic livers, in hepatocellular carcinoma and in a cutaneous hypertrophic scar. Immunohistochemistry was used to identify smoothelin-expressing cells.
Innovations and breakthroughs
This is the first study exploring smoothelin expression in the normal liver, fibrotic liver and in hepatocellular carcinoma. The results show an increase in expression that correlated with the degree of fibrosis, suggesting the progressive involvement of resident smooth muscle cells in myofibroblast recruitment. In hepatocellular carcinoma, smoothelin is expressed by pericyte-like cells between the endothelial cells of the capillaries and the tumoral cell plates.
Applications
This preliminary study underlines that tracing the origin of myofibroblasts may be useful to evaluate the degree of fibrosis in the liver and the level of reorganization in hepatocellular carcinomas.
Terminology
Smoothelin is a constituent of the smooth muscle cell cytoskeleton. It has been described as a marker of end-stage differentiation of smooth muscle cells because its expression has been found only in contractile smooth muscle cells.
Peer review
This is a good descriptive study in which the authors explore the expression pattern of a smooth muscle marker, smoothelin, within normal, fibrotic and tumoral livers, as well as in a cutaneous hypertrophic scar. The results are interesting and suggest that smoothelin expression increases with the degree of fibrosis, allowing the evaluation of the transition between fibrosis and cirrhosis. Moreover, in hepatocellular carcinoma, smoothelin is expressed by some stromal cells present beneath endothelial cells of the capillary surrounding the tumoral cell plates and this distinctive feature could be useful for hepatocellular carcinoma diagnosis.