Published online Nov 28, 2013. doi: 10.3748/wjg.v19.i44.8011
Revised: May 31, 2013
Accepted: June 19, 2013
Published online: November 28, 2013
Processing time: 280 Days and 22.8 Hours
AIM: To assess the acceptance, safety and efficacy of care and treatment for chronic hepatitis C (CHC) in drug addicts.
METHODS: We designed a multidisciplinary, phase IV prospective cohort study. All illicit drug users (IDUs) visited a Territorial Addiction Service (SerT) in the District of Brescia, and hepatitis C antibody (HCVAb) testing positive were offered as part of a standardised hepatologic visit in our Gastroenterology Unit. Patients with confirmed CHC and without medical contraindications were administered peginterferon alfa-2b 1.5 μg/kg per week plus ribavirin (800-1400 mg/d) for 16-48 wk. All IDUs were unselected because of ongoing addiction and read and signed an informed consent form. Virologic responses at weeks 4 and 12 of therapy, at the end of treatment and 24 wk after the end of treatment were the main measures of efficacy. Adherence was estimated according to the 80/80/80 criteria.
RESULTS: From November 2007 to December 2009, 162 HCVAb+ IDUs were identified. Sixty-seven patients (41% of the initial cohort) completed the diagnostic procedure, and CHC was diagnosed in 54 (33% of the total). Forty-nine patients were offered therapy, and 39 agreed (80% of acceptance rate). The prevalent HCV genotype was type 1, and the HCV RNA baseline level was over 5.6 log/mL in 61% of cases. Five patients dropped out, two because of severe adverse events (SAEs) and three without medical need. Twenty-three and 14 patients achieved end of treatment responses (ETRs; 59%) and sustained virologic responses (SVRs; 36%), respectively. Thirty-one patients were fully compliant with the study protocol (80% adherence). The prevalence of host and viral characteristics negatively affecting the treatment response was high: age over 40 years (54%), male gender (85%), overweight body type (36%), previous unsuccessful antiviral therapy (21%), HCV genotype and viral load (60% and 62%, respectively), earlier contact with HBV (40%) and steatosis and fibrosis (44% and 17%, respectively). In a univariate analysis, alcohol intake was associated with a non-response (P = 0.0018, 95%CI: 0.0058-0.4565).
CONCLUSION: Drug addicts with CHC can be successfully treated in a multidisciplinary setting using standard antiviral combination therapy, despite several “difficult to reach, manage and treat” characteristics.
Core tip: The paper reports results from a clinical trial on the management of chronic hepatitis C (CHC) in illicit drug users (IDUs). Two key elements characterise the trial: (1) the study was performed by a multidisciplinary team; and (2) the patients were unselected because of ongoing addiction. We assessed the acceptance of care and treatment for CHC among IDUs, who are classically considered to be a “difficult to reach and manage” group. For the IDUs accepting antiviral treatment, we analysed results on safety, efficacy and adherence and on the prevalence of negative prognostic factors affecting the virologic response to address whether IDUs are also “difficult to treat” patients.
- Citation: Zanini B, Benini F, Pigozzi MG, Furba P, Giacò E, Cinquegrana A, Fasoli M, Lanzini A. Addicts with chronic hepatitis C: Difficult to reach, manage or treat? World J Gastroenterol 2013; 19(44): 8011-8019
- URL: https://www.wjgnet.com/1007-9327/full/v19/i44/8011.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i44.8011
Hepatitis C virus (HCV) is estimated to chronically infect more than 180 million people worldwide, with approximately 4 million carriers in Europe alone[1]. The prognosis of chronic hepatitis C (CHC) is related to fibrosis progression, and the development of cirrhosis varies from 5%-25% over an average period of 30 years[2]. According to a recently validated mathematical model, morbidity and mortality from HCV are expected to rise in 2010 and to peak between 2030 and 2035[3]. The main reasons for this negative forecast are the low rates of screening for HCV and of treatment for CHC. The World Health Organization has defined chronic infection with HCV as a public health problem of primary importance, and during a consensus meeting in May 2010, all health authorities were invited to strive to prevent, identify and rapidly treat the infection[1].
In developed countries, HCV is mainly transmitted by needles during drug injections, and illicit drug users (IDUs) are considered to be the largest group affected by HCV, accounting for 20%-50% of cases of chronic infection[4]. A recent paper estimating viral transmission showed, for the first time, that HCV “super-spreading” is led by IDUs. According to this estimation model, each infected IDU is likely to infect approximately 20 other people, half of whom will be infected within 2 years of the initial infection[5].
International authorities on liver diseases (i.e., the National Institutes of Health since 2002, the American Gastroenterological Association since 2006 and the American Association for the Study of the Liver since 2009) recommend the treatment of CHC in IDUs and encourage clinical studies in chronically infected IDUs “to evaluate the safest and most effective treatment, factors favouring compliance, risk of relapse, side-effect profile and the impact of methadone maintenance treatment”[6-8].
Despite international recommendations, several barriers to treating IDUs persist not only among physicians but also among IDUs[9,10]. Physicians’ concerns mainly include IDUs’ chaotic lifestyle; IDUs’ possibly poor adherence to treatment; difficulties in the management of the psychiatric side effects of treatment, which are believed to be more frequent among IDUs; and the risk of re-infection after HCV eradication[10]. The risk of relapse into addiction due to interferon (IFN)-driven mood changes and the use of needles in CHC therapy, is also described as a relative contraindication to IFN treatment in IDUs, although the data from prospective trials on this risk are scarce[11,12]. Concerns about antiviral therapy for CHC are also present among IDUs: their conception of illness and death is often different, for cultural reasons, from the beliefs of the general population, and information on the natural history and treatment challenges of HCV is inexact or incomplete[10]. Moreover, factors including precarious working conditions, a lack of fixed adobe, undocumented migrant status and social isolation can affect access to care and treatment for liver diseases, and special efforts may be necessary to reach IDUs in their social environment[13]. In our recent review on the subject[14], we report that due to the barriers to treatment, most published prospective trials on the treatment of CHC in IDUs involve limited numbers of patients, ranging from 11-71, have no standardised intervention protocol and are mainly restricted to abstinent patients.
As an overview, IDUs are perceived as patients who are difficult to reach for social reasons and difficult to manage because of lifestyle. Moreover, no previous study has assessed whether IDUs are also difficult to treat because of the presence of negative prognostic factors, either viral or host-related, affecting the rate of success of antiviral therapy. For these difficulties to be addressed, and to successfully be able to contact, manage and treat these patients, a multidisciplinary approach is mandatory. This approach should involve health professionals engaged in the management of addiction and dedicated hepatologists with a highly personalised approach to patient care.
We performed a prospective clinical study designed to maximise IDUs’ access to treatment for HCV infection by involving both the physicians directly engaged in the management of addiction and the specialised hepatologist in our unit and by avoiding “a priori” exclusion criteria for antiviral therapy of active IDUs. The main objectives of the study were to specifically evaluate the rate of access to clinical care; the acceptance, safety and efficacy of antiviral treatment for CHC; and the prognostic factors for responses to standard antiviral therapy in a large cohort of IDUs, who were unselected because of ongoing addiction.
We designed a multidisciplinary, phase IV prospective cohort study. The multidisciplinary approach was ensured by close collaboration between six Territorial Addiction Services (SerT) of the Local Health Authority of the District of Brescia (ASL) and our Gastroenterology Unit (GU, Spedali Civili and University of Brescia). The physicians of the SerT were responsible for the identification of patients with hepatitis C antibody (HCVAb) positivity among those individuals visiting the SerT clinic. Based on the protocol definition, patients with “ongoing addiction problems”, actively using illicit drugs and/or alcohol or in a supportive/substitution treatment program, were all considered to be subjects. The SerT physicians were also responsible for collecting all demographic, social, psychological and addiction data in a standardised case report form. The patients selected by the SerT physicians were instructed to call a dedicated telephone number to make an appointment for an initial standard hepatologic evaluation in the GU, including a medical visit, laboratory tests and ultrasound evaluation. The aims of the hepatologic evaluation were to confirm HCV-related chronic hepatitis, to assess the severity of liver disease and to evaluate eligibility for antiviral treatment. A liver biopsy was not routinely performed. The patients with confirmed CHC and meeting standard criteria for HCV therapy[8] were offered antiviral treatment with pegylated interferon and ribavirin, according to the study protocol for treatment, and were asked to sign an informed consent form. To improve adherence, a mobile telephone number was activated for all patients on antiviral treatment, with a physician on call every day from 8 a.m. to 1 p.m.
The inclusion criteria were as follows: over 18 years of age, HCV RNA detected with a sensitive polymerase chain reaction (PCR) (cut-off of determination 50 IU/mL; COBAS Amplicor HCV test, Roche Diagnostics, Branchberg, NJ, United States) and confirmed on at least two occasions over a period of 6 mo, compensated liver disease (Child-Pugh score ≤ 5), absence of major medical contraindications to antiviral therapy (including malignancies, severe cardiac illness and uncontrolled psychiatric condition), willingness to avoid pregnancy during the entire treatment period and during the 6 mo after the last ribavirin dose intake, ability to read and sign a written informed consent form and willingness to adhere to the study protocol for treatment. Patients with suspected or confirmed idiosyncratic reactions to interferon or ribavirin were excluded.
The study protocol for treatment consisted of peginterferon alfa-2b (12 kDa) 1.5 μg/kg per week plus ribavirin 800-1400 mg according to body weight (800 mg for < 65 kg, 1000 mg for 65-80 kg, 1200 mg for 81-105 and 1400 mg for > 105 kg) divided into two daily administrations.
The duration of the treatment was 24 wk for HCV genotypes 2/3 and 48 wk for HCV genotypes 1/4. The achievement of a rapid virologic response (RVR; HCV RNA < 50 IU/mL at week 4 of therapy) was regarded as an indication for short-term therapy in all naïve patients fulfilling the criteria of no dose reduction during the first 4 wk of therapy, low baseline viral load (HCV RNA < 600000 IU/mL) and the absence of cirrhosis. The short-term scheme consisted of 16 wk for HCV genotypes 2/3 and 24 wk for HCV genotypes 1/4. Treatment was discontinued prematurely, according to international rules[8] (at week 12 if the HCV RNA level drops by < 2 Log and at week 24 if the HCV RNA level is > 50 IU/mL); in the case of virologic breakthrough; in the presence of severe adverse events (SAEs); or upon patients’ request, with no need for explanation. In the case of a virologic breakthrough, HCV genotyping [line probe assay (LIPA), Bayer HealthCare, Tarrytown, NY, United States] was performed to exclude mixed/new HCV infections.
Adverse reactions to interferon and/or ribavirin were managed according to international guidelines[8], and the use of erythropoietin and leucocyte growth factors was allowed, according to the current Italian Drug Agency (AIFA) recommendations.
The timetable of the study was as follows: a medical visit including a general physical examination, an assessment of body mass index, the administration of AUDIT-C for screening for at-risk alcohol-related behaviour and a Hamilton Test for scoring anxiety and depression. The measurement of the complete blood count and the alanine aminotransferase (ALT), aspartate aminotransferase (AST) and γ-glutamyl transferase (GGT) levels was requested every 4 wk during treatment and 24 wk after the end of therapy. Blood tests, including for thyroid function (FT4 and TSH), autoimmunity (ANA, AMA, LKM and ASMA) and liver function (albumin, PT and bilirubin), were mandatory at the beginning and every 12 wk of treatment. Quantitative and qualitative assays for HCV RNA were requested at week 0-4-12 and at week 4-12-24 of treatment, at the end of therapy and 24 wk after the end of therapy, respectively.
Access to liver care was calculated as the proportion of IDUs attending the first hepatologic visit and completing the diagnostic procedure among all HCVAb+ IDUs screened by the SerT and showing interest in this opportunity. Access to therapy was evaluated as the proportion of patients starting antiviral therapy among the eligible patients. The main measure of safety was the rate of withdrawal for SAEs, according to the Common Terminology Criteria for Adverse Events v3.0[15]. The main outcome measure of efficacy was the sustained virologic response (SVR; HCV RNA persistently < 50 IU/mL 24 wk after treatment discontinuation) among the treated patients. Secondary efficacy measures were the achievement of an RVR, an early virologic response (EVR; HCV RNA < 50 IU/mL at week 12 of therapy) and an end of treatment response (ETR; HCV RNA < 50 IU/mL at the end of therapy). Adherence was estimated according to the 80/80/80 criteria (80% of pegylated interferon, 80% ribavirin cumulative dosage and 80% of the duration of therapy[16]).
The entire study design was evaluated by the Ethics Committee of Spedali Civili of Brescia and fully approved on July 31st, 2007. The study was registered with EudraCT, number 2008-001283-37.
Treatment efficacy was measured according to the intention to treat (ITT) criteria, including all patients who had received at least one dose of interferon and one dose of ribavirin after signing the informed consent form. For statistical analyses, an unpaired t-test and Fisher’s exact test were used when appropriate using GraphPad Prism, version 5.0 (Graph Pad Software, Inc., San Diego, CA, United States). Logistic regression analysis was performed to assess the effect of baseline features on efficacy and was completed using Stata software (version 7, StataCorp LP, College Station, TX, United States). A P value < 0.05 was accepted to reject the null hypothesis.
From November 2007 to December 2009, a cohort of 162 HCVAb+ IDUs was identified by six SerT in the District of Brescia. Most patients were Italian males with a low level of education. At the time of recruitment, one third of the patients were unemployed, and one quarter had comorbidities (Table 1). The prevalent type of addiction was intravenous injection of heroin, with a mean duration of 13 years. Most patients were on opiate substitution treatment, with 66% on methadone and 12% on buprenorphine. All patients in methadone maintenance therapy received a dose lower than 100 mg/d (Table 2). Most IDUs who received information about HCV infection from health operators, the press or television were not confident about their knowledge and had moderate worries about the side effects of HCV therapy (Table 3).
| Patient characteristics | Selected by SerT (n = 162) | Accepting therapy (n = 39) | P value |
| Male gender | 135 (83) | 27 (69) | 0.8152 |
| Age, yr, mean ± SD | 38 ± 7 | 39 ± 6 | 0.8888 |
| Spoken language: Italian | 152 (94) | 33 (85) | 0.0912 |
| Place of birth | 0.1502 | ||
| Italy | 147 (91) | 32 (82) | |
| EU | 5 (3) | 2 (5) | |
| Non-EU | 10 (6) | 5 (13) | |
| Level of education | (n = 151) | 0.8320 | |
| ≤ 8 yr of school | 118 (79) | 30 (77) | |
| High school diploma | 32 (21) | 8 (21) | |
| University degree | 1 (0) | 1 (2) | |
| (n = 149) | 1.0000 | ||
| Unemployed | 49 (33) | 13 (33) | |
| Chronic associated conditions | 41 (25) | 11 (28) | 0.6888 |
| Cardiovascular | 5 (3) | 2 (5) | |
| Respiratory | 4 (2) | 1 (3) | |
| Allergic | 2 (1) | 1 (3) | |
| Psychiatric | 19 (12) | 3 (8) |
| Selected by SerT (n = 162) | Accepting therapy (n = 39) | P value | |
| Alcohol | |||
| Active | 11 (7) | 4 (10) | 0.4972 |
| Partial remission | 5 (3) | 0 (0) | 0.5867 |
| Total remission | 23 (14) | 10 (26) | 0.0944 |
| Cannabis | |||
| Active | 7 (11) | 2 (5) | 0.0590 |
| Partial remission | 3 (2) | 1 (3) | 0.5811 |
| Total remission | 4 (2) | 3 (8) | 0.1344 |
| Cocaine | |||
| Active | 33 (20) | 3 (8) | 0.0038 |
| Partial remission | 6 (4) | 1 (3) | 1.0000 |
| Total remission | 39 (24) | 12 (31) | 0.4150 |
| Heroin | |||
| Active | 48 (30) | 6 (15) | 0.1059 |
| Partial remission | 19 (12) | 5 (13) | 0.7885 |
| Total remission | 74 (46) | 23 (59) | 0.0647 |
| Duration of intravenous drug use, yr, mean ± SD (range) | (n = 98) | (n = 33) | |
| 13 ± 8 (7-34) | 13 ± 9 (6-32) | 0.8588 | |
| Opiate substitution treatment | 126 (78) | 28 (72) | 0.4089 |
| Methadone, mg, mean ± SD | 107 (66), 41 ± 22 | 19 (60), 46 ± 26 | 0.0642 |
| Buprenorphine, mg, mean ± SD | 19 (12), 5 ± 3 | 9 (23), 6 ± 4 | 0.0751 |
| Patient attitudes/knowledge | Selected by SerT (n = 162) | Accepting therapy (n = 39) | P value |
| Source of HCV information | (n = 150) | (n = 33) | NS |
| Other HCV patients | 44 (29) | 11 (33) | |
| Health operators | 72 (48) | 18 (55) | |
| Press | 54 (36) | 14 (42) | |
| Web | 15 (10) | 6 (18) | |
| Television | 62 (41) | 16 (48) | |
| None | 25 (17) | 6 (18) | |
| Feelings toward information | |||
| Complete | (n = 139) 72 (52) | (n = 32) 15 (47) | 0.6964 |
| Confident | (n = 131) 30 (23) | (n = 31) 16 (52) | 0.0033 |
| Reassuring | (n = 130) 68 (52) | (n = 28) 14 (50) | 0.8381 |
| Attitudes toward HCV therapy | |||
| Total fright | (n = 129) 3 (2) | (n = 29) 0 (0) | 1.0000 |
| Moderate worries | (n = 141) 102 (78) | (n = 32) 25 (78) | 0.5271 |
| Positive expectations | (n = 125) 70 (56) | (n = 28) 18 (64) | 0.5271 |
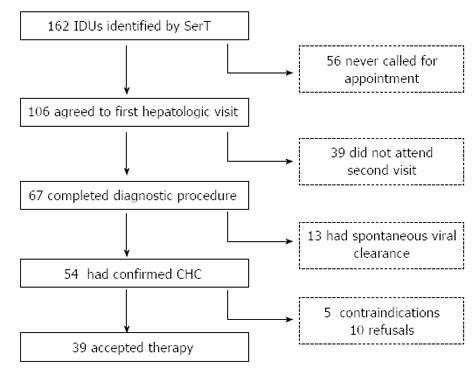
Patient disposition, according to the study protocol, is reported in Figure 1. Access to the first hepatologic work-up was observed in 106 patients, which was 65% of the initial cohort. Although 56 IDUs expressed interest in the opportunity for a dedicated medical examination to a SerT doctor, these individuals never called our clinic for an appointment. Sixty-seven patients completed the diagnostic procedure, or 41% of the initial cohort, corresponding to 63% of patients visiting our clinic for an initial evaluation. Patients who did not adhere to the diagnostic protocol (39 IDUs) were all contacted by telephone by a physician (BZ), and these patients all preferred to postpone the medical procedures because of other priorities.
CHC was confirmed in 54 IDUs (33% of the total), of which 13 patients had a confirmed HCV RNA-negative test, with an estimated rate of spontaneous clearance of 19%. Five patients had medical contraindications to specific antiviral therapy (two cases of decompensated cirrhosis, one case of hepatocellular carcinoma, one case of pregnancy and one case of uncontrolled severe psychiatric illness). The remaining 49 patients were offered specific treatment for CHC, and 39 accepted and signed the informed consent form (80% acceptance rate). Two patients never started treatment after signing the informed consent form and were thus excluded from the ITT analysis, and two patients, one with HCV genotype 1a and the other with HCV genotype 3a, relapsed after the end of therapy and asked for a second cycle of antiviral therapy (after a 6-mo wash-out period). We therefore report results for 39 treatments in 37 patients.
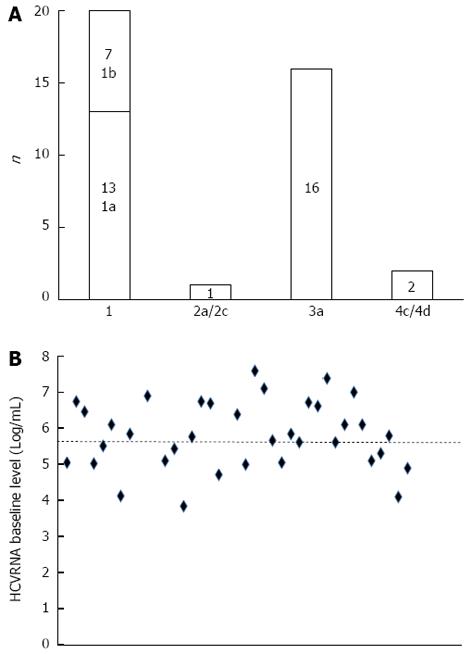
The virologic features of the treated patients are reported in Figure 2. The most represented HCV genotype was type 1 (13 patients with 1a and seven with 1b). The HCV RNA baseline level, available in 36 of 39 patients, was over 5.6 Log/mL in 22 cases (61%). In total, 36% of our patients were active illicit drug users, mainly using heroin; approximately one third had a history of depression; one quarter had a pathologic Hamilton score for anxiety or depression; four patients were addicted to alcohol; and seven patients had an AUDIT-C at-risk score (Table 4). As reported in Table 5, several prognostic factors negatively affecting the outcome of antiviral therapy for CHC were well represented among our treated IDUs. These factors included age over 40 years, male gender, overweight body type, previous unsuccessful HCV antiviral treatment, unfavourable HCV virologic genotype or viral load, earlier contact with HBV, steatosis and progression to cirrhosis.
| Characteristics | n = 39 |
| BMI (kg/m2), M (range) | 24.3 (17.6-34.6) |
| Duration of HCV infection (yr), M (range) | 5 (1-21) |
| Duration under 1 yr | 14 (36) |
| Duration of IDU status (yr), M (range) | 12 (1-32) |
| Active IDU | 14 (36) |
| History of depression | 11 (28) |
| Pathologic Hamilton score | |
| Anxiety | 10 (26) |
| Depression | 8 (21) |
| AUDIT-C at-risk score | 7 (18) |
| Leucocytes (n/mm3), M (range) | 6960 (3960-11960) |
| Haemoglobin (g/dL), M (range) | 15.5 (11.8-17.7) |
| Platelets | 224 (106-421) |
| ALT index (value/u.l.n.), M (range) | 2.5 (0.5-16.4) |
| AST index (value/u.l.n.), M (range) | 2.0 (06-6.6) |
| GGT index (value/u.l.n.), M (range) | 1.2 (0.3-13.9) |
| Features | Prevalence |
| Age over 40 yr | 54% |
| Males | 85% |
| BMI over 25 kg/m2 | 36% |
| Previous unsuccessful interferon treatment | 21% |
| Unfavourable HCV genotype (1 or 4) | 60% |
| HCV viral load > 5.6 Log (IU/mL) | 62% |
| HBcAb positivity | 40% |
| Ultrasonography suggestive of steatosis | 44% |
| Ultrasonography suggestive of cirrhosis | 17% |
Two SAEs occurred during the study period, leading to therapy discontinuation: a case of psychosis and a case of pneumonia with suspected tuberculosis at weeks 4 and 10, respectively. Three patients dropped out without medical need and were lost to follow-up; all dropouts occurred within the first 8 wk of antiviral treatment. Sixteen (41%) and 17 (44%) patients needed a dose adjustment of pegylated interferon and ribavirin, respectively. In six patients (15%), the use of erythropoietin was offered. The use of leucocyte grown factors was not necessary for any patient. One patient became pregnant during the 6 mo after the end of therapy and decided on an abortion for personal reasons.
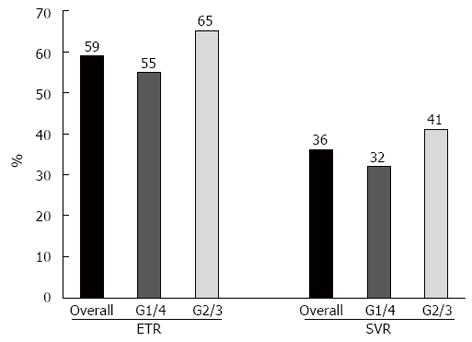
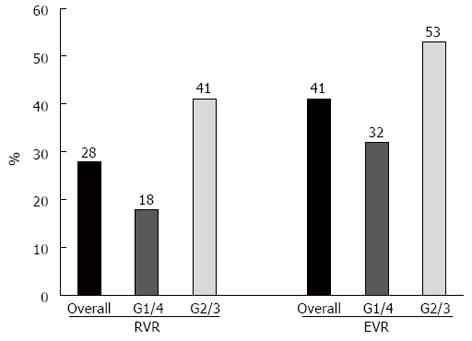
In the ITT analysis, 23 patients achieved an ETR (59%), and nine (23%) relapsed during the 6 mo after the end of therapy. Fourteen patients achieved an SVR (36%), seven of whom were infected with an unfavourable HCV genotype (Figure 3). The HCV RNA serologic clearance rates at weeks 4 and 12 are reported in Figure 4. Short-term therapy was offered to nine patients, according to the study protocol, and did not negatively affect the SVR rate based on univariate analysis.
According to our adherence definition, 31 patients (80%) were compliant with the study protocol.
In the univariate logistic regression analysis, the active use of cocaine and/or heroin, ongoing substitution treatment, the type of substitution treatment, the presence of comorbidity, spoken language and male gender did not affect the rate of the SVR, whereas alcohol intake was associated with a non-response (P = 0.0018, 95%CI: 0.0058-0.4565), independent of the dose and type of alcoholic beverage.
Our study indicates that antiviral treatment for CHC in IDUs is safe and effective and that a multidisciplinary approach is a key element of the care of such patients. We have considered three main aspects of this issue to understand whether these patients are, as generally perceived, difficult to reach, manage or treat.
IDUs, together with migrants and prison inmates, are regarded as special population groups. As recommended by an official position paper on behalf of several Italian scientific societies[13], in such vulnerable people, specific intervention is mandatory to identify, prevent and treat chronic viral infections of the liver. In our approach, collaboration with the territorial services involved in the care of addicts was the key means of reaching IDUs (of whom 6%-15% were migrants) who were at risk of exclusion from medical care for social reasons. In total, 65% of IDUs with HCVAb positivity and identified by physicians of the SerT agreed to and received a dedicated medical visit that, even in the case of patients not receiving treatment, provided an instructive opportunity for counselling on HCV transmission, the prevention of liver complications, healthy lifestyle and available therapeutic protocols. In contrast, 56 IDUs (35% of the total cohort identified by the SerT) never called for an appointment, despite an initial statement of interest in the project. Moreover, after the first medical evaluation, 39 patients (37% of the patients who agreed to the first visit) never completed the diagnostic procedure, even after encouragement by direct telephone contact with a physician. Because of incomplete procedures, clinical and laboratory data were not sufficient to confirm an active HCV infection and to stage liver disease in 95 patients (59% of the initial cohort). Such a finding indicates that difficulties in reaching and motivating this population of patients persist even in the context of a well-organised multidisciplinary approach.
Concerns about treating IDUs are mainly due to suspicion of low adherence, the risk of SAEs (typically psychiatric) and the inability to follow therapeutic prescriptions[10]. In our study, adherence was high and comparable to the adherence reported for clinical trials in the general population[17,18]. The use of a psychiatric questionnaire to monitor depression and anxiety was well accepted; one patient received antidepressant therapy before starting antiviral treatment, and paroxetine was offered to another patient after 12 wk of antiviral treatment. The patient with a psychotic reaction completely recovered after the withdrawal of antiviral treatment without consequences or the need for psychiatric drugs. These data on the psychiatric safety of CHC treatment in IDUs, as previously suggested by other studies[19-22], are encouraging.
An important feature of our study was the inclusion of people who were actively addicted, with no period of mandatory abstinence; 36% of our enrolled patients continued to use illicit drugs (mainly heroin and cocaine) during the study protocol. Despite this “difficult to manage” characteristic, the data on safety, efficacy and adherence are encouraging. Moreover, logistic regression failed to demonstrate a negative correlation with the viral response to therapy in patients who were actively addicted during antiviral treatment. Only alcohol consumption was related to a lower SVR rate, and this finding confirms the role of alcohol consumption in the impairment of antiviral treatment efficacy, which has already been demonstrated in the general population[23].
Although IDUs are considered to be poorly motivated to undergo medical care, the multidisciplinary setting and strict collaboration among the different physicians involved in the care of the IDUs led to a high rate of access to therapy; 76% of patients with confirmed CHC started treatment. Such a rate was markedly higher than the rate previously reported in studies in the general population[10].
Are IDUs difficult to treat?
Adherence to treatment was high (80%), and despite few withdrawals for safety reasons, the overall SVR of 36% was lower than expected for the general population. This “efficacy” goal must be observed in light of several “difficult to treat” characteristics in our study population[24]. Among our IDUs, viral features such as HCV genotype 1 and a high baseline level of viremia were prevalent. Moreover, 40% of our patients tested positive for HBcAb[25]. Male gender, an overweight body type and an age over 40 were frequent. Other unfavourable factors affecting the virologic response were a relatively high prevalence of steatosis and cirrhosis in 44% and 17%, respectively, of patients. Most patients had been addicted for over 10 years, and no patient was identified and treated during the acute phase of the infection. In total, 21% of patients experienced the failure of at least one antiviral treatment for CHC. A few of these features are not modifiable (HCV genotype, viral load and gender), whereas other features could be modified by a more prompt strategy of intervention (younger age, shorter duration of infection and lower score of fibrosis).
In conclusion, IDUs with HCV-related CHC, actively using illicit drugs and/or opioid substitution treatment, can be successfully treated in a multidisciplinary setting with a standard antiviral combination of ribavirin and pegylated interferon, with good adherence and a good safety profile. IDUs’“difficult to reach, manage and treat” characteristics should not be used to contraindicate antiviral therapy. An appropriate multidisciplinary setting is a key factor in overcoming the “difficult” characteristics of these patients, with a strategic aim of reducing HCV circulation in the largest reservoir of this viral infection. Whether treatment will benefit from upcoming new antiviral agents is currently under study in our unit.
ARNICA Study Group included the following: Cecilia Agnelli, Maurizio Cadoria, Piera Dettori, Anna Martinelli, Maurizio Parma, Fabio Roda, Marco Stilo, Alessandra Wuhrer, Elisabetta Secchi, Carmelo Scarcella, Territorial Addiction Service, Local Health Authority of Brescia, Brescia, Italy.
Hepatitis C virus (HCV) infection is a common condition worldwide with prevalence of 3%. Illicit drug users (IDUs) are regarded as an important reservoir of this infection and as “super-spreaders”. HCV infection is a progression disease possibly leading to chronic liver disease and ultimately to end stage liver disease. Is therefore important to identify strategy to eradicate infection particularly in the reservoir-population? Concerns about therapy of HCV infection in these populations are present in both physicians and IDUs.
The investigators report that within a multidisciplinary setting involving both liver and addiction specialists nearly half of identified HCV+ IDUs accept hepatologic counseling and nearly a quarter accept treatment. Eighty percent of treated patients are adherent to treatment according to 80/80/80 rule. Sustained virological response is achieved in a proportion similar of that reported in registration trials, is not influenced by ongoing addiction, but is negatively affected by alcohol consumption. Incidence of psychiatric and organic side effects is not different from that reported in the general population.
This article supports the concept that barriers to HCV therapy of IDUs can be overcome in the context of a multidisciplinary team, and that in this clinical context adherence and efficacy of therapy is similar as in the general population. The study highlights the point that the risk of HCV spreading by the super-spreaders IDUs can be reduced and that their habits can not be used as an argument to withhold antiviral therapy.
The study includes challenging for ethical difficulty of HCV treatment. It’s very interesting and authors may applaudable effort on this study. Of course, opposite opinions for HCV treatment on addicts may exist, nonetheless this study indicates possibility of HCV treatment for some addicts if patients can receive enough support from medical profession. Although this study may raise an ethical issue, it will give a strong impact to readers and make fascinating reading.
| 1. | World Health Organization. Global Alert and Response: Hepatitis C. http: //www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index1.html. Accessed January 14 2013; . |
| 2. | Vezali E, Aghemo A, Colombo M. A review of the treatment of chronic hepatitis C virus infection in cirrhosis. Clin Ther. 2010;32:2117-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Davis GL, Rodrigue JR. Treatment of chronic hepatitis C in active drug users. N Engl J Med. 2001;345:215-217. [PubMed] |
| 5. | Magiorkinis G, Sypsa V, Magiorkinis E, Paraskevis D, Katsoulidou A, Belshaw R, Fraser C, Pybus OG, Hatzakis A. Integrating phylodynamics and epidemiology to estimate transmission diversity in viral epidemics. PLoS Comput Biol. 2013;9:e1002876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C 2002 (June 10-12, 2002). Gastroenterology. 2002;123:2082-2099. [PubMed] |
| 7. | Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231-64; quiz 214-7. [PubMed] |
| 8. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2245] [Article Influence: 132.1] [Reference Citation Analysis (2)] |
| 9. | Edlin BR, Kresina TF, Raymond DB, Carden MR, Gourevitch MN, Rich JD, Cheever LW, Cargill VA. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin Infect Dis. 2005;40 Suppl 5:S276-S285. [PubMed] |
| 10. | Zanini B, Lanzini A. Antiviral treatment for chronic hepatitis C in illicit drug users: a systematic review. Antivir Ther. 2009;14:467-479. [PubMed] |
| 11. | Belfiori B, Chiodera A, Ciliegi P, Tosti A, Baldelli F, Stagni G, Francisci D. Treatment for hepatitis C virus in injection drug users on opioid replacement therapy: a prospective multicentre study. Eur J Gastroenterol Hepatol. 2007;19:731-732. [PubMed] |
| 12. | Guadagnino V, Trotta MP, Montesano F, Babudieri S, Caroleo B, Armignacco O, Carioti J, Maio G, Monarca R, Antinori A. Effectiveness of a multi-disciplinary standardized management model in the treatment of chronic hepatitis C in drug addicts engaged in detoxification programmes. Addiction. 2007;102:423-431. [PubMed] |
| 13. | Almasio PL, Babudieri S, Barbarini G, Brunetto M, Conte D, Dentico P, Gaeta GB, Leonardi C, Levrero M, Mazzotta F. Recommendations for the prevention, diagnosis, and treatment of chronic hepatitis B and C in special population groups (migrants, intravenous drug users and prison inmates). Dig Liver Dis. 2011;43:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 14. | Zanini B, Covolo L, Donato F, Lanzini A. Effectiveness and tolerability of combination treatment of chronic hepatitis C in illicit drug users: meta-analysis of prospective studies. Clin Ther. 2010;32:2139-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176-181. [PubMed] |
| 16. | McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, Dienstag J, Lee WM, Mak C, Garaud JJ. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061-1069. [PubMed] |
| 17. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [PubMed] |
| 18. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [PubMed] |
| 19. | Schaefer M, Schmidt F, Folwaczny C, Lorenz R, Martin G, Schindlbeck N, Heldwein W, Soyka M, Grunze H, Koenig A. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37:443-451. [PubMed] |
| 20. | Schaefer M, Hinzpeter A, Mohmand A, Janssen G, Pich M, Schwaiger M, Sarkar R, Friebe A, Heinz A, Kluschke M. Hepatitis C treatment in “difficult-to-treat” psychiatric patients with pegylated interferon-alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007;46:991-998. [PubMed] |
| 21. | Sylvestre DL, Clements BJ. Adherence to hepatitis C treatment in recovering heroin users maintained on methadone. Eur J Gastroenterol Hepatol. 2007;19:741-747. [PubMed] |
| 22. | Sasadeusz JJ, Dore G, Kronborg I, Barton D, Yoshihara M, Weltman M. Clinical experience with the treatment of hepatitis C infection in patients on opioid pharmacotherapy. Addiction. 2011;106:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Siu L, Foont J, Wands JR. Hepatitis C virus and alcohol. Semin Liver Dis. 2009;29:188-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Berg T, Andreone P, Pol S, Roberts S, Younossi Z, Diago M, Lawitz EJ, Focaccia R, Foster GR, Horban A. Predictors of virologic response with telaprevir-based combination treatment in HCV genotype 1-infected patients with prior peginterferon/ribavirin treatment failure: post-hoc analysis of the phase III realize study. Hepatology. 2011;54:375A-376A. |
| 25. | Sagnelli E, Coppola N, Scolastico C, Mogavero AR, Filippini P, Piccinino F. HCV genotype and “silent” HBV coinfection: two main risk factors for a more severe liver disease. J Med Virol. 2001;64:350-355. [PubMed] |
P- Reviewers: Yoshida S, Zhao HT S- Editor: Song XX L- Editor: A E- Editor: Zhang DN