Published online Nov 28, 2013. doi: 10.3748/wjg.v19.i44.7955
Revised: September 19, 2013
Accepted: October 19, 2013
Published online: November 28, 2013
Processing time: 134 Days and 12.3 Hours
Colorectal cancer (CRC) is the fourth most commonly diagnosed cancer and the second leading cause of cancer death in both men and women in the United States, with about 142820 new cases and 50830 deaths expected in 2013. Metastatic disease (mCRC) remains a challenge for oncologists worldwide due to its potential comorbidities. Recently, chemotherapy regimens containing 5-fluorouracil, leucovorin, oxaliplatin and irinotecan combinations are a standard of care in the metastatic disease. Currently, biological therapies involving vascular endothelial growth factor and epidermal growth factor receptor pathways, such as bevacizumab and cetuximab, have emerged as good option for improving mCRC patient survival. Now, aflibercept plus standard chemotherapy has also been approved in second line regimen for mCRC patients. Our review will discuss novel biological drugs and their indications for mCRC patients and will bring future perspectives in this regard.
Core tip: Metastatic colorectal cancer is a very aggressive disease. However, recently developed chemotherapeutic protocols and targeted drugs have emerged as a valuable tool for treating this set of patients. Our manuscript brings the readers current trends and future perspectives in this field.
- Citation: Marques I, Araújo A, Mello RA. Anti-angiogenic therapies for metastatic colorectal cancer: Current and future perspectives. World J Gastroenterol 2013; 19(44): 7955-7971
- URL: https://www.wjgnet.com/1007-9327/full/v19/i44/7955.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i44.7955
Colorectal cancer (CRC) is the fourth most commonly diagnosed cancer and the second leading cause of cancer death in both men and women in the United States, with about 142820 new cases and 50830 deaths expected in 2013[1]. In Europe, CRC represents the second most common cancer and leading cause of cancer death, in both genders combined[2]. Consequently, CRC is considered a prominent global health problem.
Usually, early CRC has no symptoms, and this is why screening is so important. Moreover, almost all symptoms (i.e., change in bowel habits, general abdominal discomfort, weight loss with no apparent cause, constant tiredness) are not well specific. Consequently, CRC might be diagnosed when a patient has symptoms or as a result of a screening program[3]. Colonoscopy is the main diagnostic tool for primary screening due to its great benefit on either flexible sigmoidoscopy or guaiac fecal occult blood test[4].
The 1- and 5-year relative survival rates for patients with CRC are respectively 83.2% and 64.3%, considering all stages. Additionally, ten years after diagnosis, survival continues to decline to 57.6%[5]. The most important problem remains disease relapse following surgery since, commonly, it is the cause of death in these patients[3]. This fact becomes relevant when we observe that when CRC are detected at a localized stage, the 5-year relative survival rate is 90.1% and, after disease involves adjacent organs or lymph nodes, the 5-year survival rate falls to 69.2%. Moreover, when cancer has spread to distant organs, the 5-year survival rate is 11.7%[5].
Many patients have metastatic disease (mCRC) initially not suitable for resection[6]. The majority of patients with mCRC cannot be cured, and the goals of chemotherapy for them are to prolong survival, improve quality of life and provide palliation, when applicable[7]. Over the past years, the outcome of these patients has been improved, with median survival reaching almost 24 mo[6,8].
The liver is the most common site of hematogenous metastasis in CRC, and its appearance is a frequent event for patients with CRC and remains a major cause of cancer-related death[9]. Approximately 25% of patients present synchronous liver metastasis at time of diagnosis, and another 25% of patients will develop liver metastases during the course of their disease, usually within a 2-year period after initial surgical treatment of their primary tumor[10]. The only potentially curative treatment for patients with liver metastasis is surgical resection, which results in a 5-year survival rate of 36%[11]. Nevertheless, 70% of these patients will suffer a relapse after resection of their hepatic metastasis, with the majority in the first 2 years after surgery and the remaining continuing to occur up to 10 years[12].
Over the past years, the development and incorporation of agents that target angiogenesis in clinical practice have led to improvements in the treatment of mCRC, with benefits in progression-free survival (PSF) and overall survival (OS) in these patients[13]. This paper aims to review the impact of known and new anti-angiogenic therapies for mCRC, especially those which target vascular endothelial growth factor (VEGF) pathways.
Blood vessel formation comprises two main types: vasculogenesis and angiogenesis. During early embryonic development, vasculogenesis is the process responsible for the formation of the primary vasculature of the body, which consists in the formation of blood vessels from endothelial cell progenitors (i.e., hemangioblasts)[14]. On the other hand, angiogenesis is a complex and highly regulated biological process that refers to the formation of new vascular segments. During this process, the combination of sprouting, splitting, and remodeling of the existing vessels occurs[15]. Physiologically angiogenesis occurs under tight regulation by a wide range of proangiogenic inducers, such as growth factors, chemokines, angiogenic enzymes, endothelial-specific receptors, and adhesion molecules as well as various antiangiogenic factors including angiostatin, endostatin, thrombospondin, canstatin, and pigment epithelium-derived factor[16]. As blood vessels are needed to supply nutrients and oxygen to tissues, angiogenesis plays an essential role in normal growth and development. Nevertheless, imbalances between the angiogenic mediators and inhibitors may result in the development of pathologies, as cancer[17].
In order to continue grow and metastasize, tumors need to continually acquire an adequate blood supply, which is accomplished by inducing angiogenesis[18]. Since Folkman recognized, in the early 1970s, the therapeutic potential for the inhibition of angiogenesis process in cancer, angiogenesis has been largely studied[19].
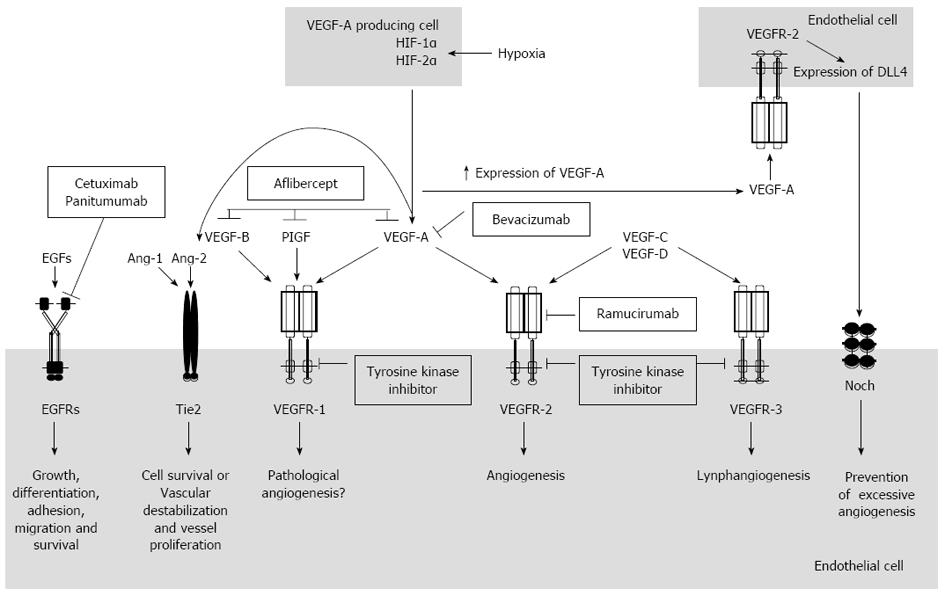
Figure 1 shows the main angiogenic mechanisms related to VEGF pathways. The VEGF family, which plays a critical role in tumor angiogenesis[20], includes six members: VEGF-A, -B, -C, -D, -E and placental growth factor (PIGF)[21]. VEGF-A, also known as VEGF, is the most important member and the major physiologic and pathologic mediator of angiogenic mechanism[20]. The VEGF-A gene, located on chromosome 6 (6p21.3), undergoes alternative splicing to yield mature isoforms of 121, 145, 165, 183, 189, and 206 amino acids[22-24]. In vivo, only three isoforms have been related to angiogenesis, VEGF121, VEGF145 and VEGF165. The latter has been demonstrated to be a predominant isoform secreted by malignant and benign cells[25]. VEGF signals, mainly through VEGF receptor 2 (VEGFR-2) which is tightly expressed by endothelial cells, are involved in angiogenesis. VEGF binds to VEGF receptor 1 (VEGFR-1), with approximately 10 times the affinity of VEGFR-2 binding. However, its signal-transducing properties are extremely weak[26].
Most solid tumors present hypoxic regions as they grow and, thus, outweigh their blood supply. The results of the cellular adaptation to hypoxic microenvironment are aggressive disease, resistance to therapy, and decreased patient survival[27]. The transcription factor hypoxia-inducible factor-1 (HIF-1 or HIF) is the most important regulator of the hypoxic response, which up-regulates expression of proteins involved in the regulation of several aspects of tumor biology, such as oxygen transport, iron metabolism, glycolysis, glucose transport, cell survival and proliferation, angiogenesis, invasion and metastasis[28,29]. VEGF is one of several proangiogenic factors directly activated by HIF-1 and acts to promote new blood vessel formation and thereby provide the reestablishment of oxygen and nutrient supply[27].
Paracrine mechanisms generated through VEGF production by tumor cells may also influence angiogenesis pathways. However, those cells cannot adequately respond to VEGF directly since they do not have enough cell surface VEGF receptors for that purpose. In contrast, endothelial cells recruited during angiogenesis express numerous VEGF receptors, but produce little or no detectable VEGF ligand. In this context, the amount of VEGF necessary to propel angiogenesis comes from several host cells in human body, such as platelets, smooth muscle cells, and tumor-associated stromal cells, which, together, produce the necessary amounts of VEGF for angiogenesis to begin[30-32].
The other VEGF family members are important for diverse mechanisms of new vessel creation. VEGF-B and PIGF bind to VEGFR-1 whereas VEGF-C and -D are specific ligands for VEGFR-2 VEGF receptor 3 (VEGFR-3), after proteolysis processing[26]. VEGF-B and PIGF act through VEGFR-1, which is capital for the organization of embryonic vasculature, but is not essential for endothelial cell differentiation[33,34]. VEGFR-1 is expressed in many non-endothelial cells such as monocyte/macrophages, dendritic cells, osteoclasts, pericytes and trophoblasts in the placenta. The value of VEGFR-1 expression in these cells remains unclear; however, this receptor could play a regulatory role in cell survival[26]. Furthermore, recent studies have shown that VEGFR-1 is present and functional on CRC cells and its activation, by VEGF family ligands, can result in activation of processes involved in tumor progression and metastasis[33]. VEGF-C and -D play an important role in lymphangiogenesis through VEGFR-2 and -3 binding[26]. Concerning VEGF-C, it is involved both in lymphangiogenesis and in promotion of metastasis to regional lymph nodes in multiple cancers, including CRC[35]. VEGF-D is also implicated in lymphangiogenesis and lymphatic metastasis[36].
Angiogenesis is regulated not only by VEGF pathways but also by other pathways including Notch, angiopoietins and integrins[20]. The Notch pathway, an intercellular signaling pathway, influences many biological processes, including cell fate determination, cellular differentiation, proliferation, survival and apoptosis[37,38]. There are four Notch cell-surface receptors (Notch-1, -2, -3 and -4) and five Notch membrane-anchored ligands [Jagged-1, Jagged-2, Delta-like (Dll)-1, -3, and -4], expressed by various cell types. Both ligand and receptor are transmembrane proteins with large extracellular domains that consist of epidermal growth factor (EGF)-like repeats. Notch is synthesized as a precursor protein that is processed in the Golgi before being transported to the cell surface, where it resides as a heterodimer. Interaction of Notch receptors with Notch ligands, between two bordering cells, initiates a series of successive proteolysis cleavages. The first cleavage, mediated by ADAM-family metalloproteases such as ADAM10 or tumor necrosis factor α-converting enzyme (TACE), generates a substrate for cleavage by the gamma-secretase complex. This cleavage leads to the release of Notch intracellular domain (NICD) from the cell membrane. This protein fragment, then, translocates into the nucleus and operates as a cofactor to regulate transcription of Notch target genes[39]. The induction of Dll4-Notch signaling acts as a mechanism intended to prevent excessive angiogenesis and to control the development of new blood vessels[40].
Vascular endothelial cells express Notch 1 and Notch 4 receptors and the Jagged-1, Dll1, and Dll4 ligands. Among these, Dll4 is expressed exclusively by endothelial cells[20]. Dll4 is usually induced by VEGF as a negative-feedback regulator of vascular growth. In contrast to VEGF blockade, which results in a loss of many tumor vessels and an apparent normalization of the remaining vessels of the tumor, DLL4 blockade results in a striking increase in these vessels. Paradoxically, this increased vascularity is associated with decreased tumor growth, even for tumors that are highly resistant to blockade of VEGF[41]. Since VEGF induces Dll4 and Dll4 induces vascular quiescence and differentiation, and down-regulates VEGFR-2[42], it is obvious that the balance of these two pathways may be important to the development and outcomes of therapeutic acting in these pathways[43].
Recently, the angiopoietins have emerged as important regulators of angiogenesis[16]. The human angiopoietin family comprises Ang-1, -2 and -3, all of which act as ligands for endothelial cell-specific tyrosine kinase receptor Tie2, expressed principally on the vascular endothelial cells[44-46].
Ang-1, which is predominantly expressed in perivascular cells such as pericytes, vascular smooth muscle cells, fibroblasts and tumor cells, binds to Tie2 receptor as an antagonist. Upon binding of Ang-1, Tie-2 receptor auto-phosphorylates, leading to stimulation of various intracellular signaling pathways which promote endothelial cell survival and the maintenance of an endothelial barrier and a quiescent vasculature. Mural cells, such as vascular smooth muscle cells and pericytes, constantly produce Ang-1 under physiological conditions, and maintain vascular stabilization and maturation[47]. On the other hand, Ang-2 produced by the endothelium, acts as an antagonist for Tie2 by competing with Ang-1[45]. It induces vascular destabilization and vessel proliferation. VEGF and angiopoietins have complementary roles in angiogenesis. In the presence of VEGF, Ang-2 stimulates tumor angiogenesis by promoting vessel destabilization, whereas in the absence of VEGF, Ang-2 promotes endothelial cell death and vessel regression[48].
Blockade of Tie-2 pathway has been more difficult than blockade of the VEGF pathway, due to the complexity of agonistic and antagonistic ligands for the same receptor. Moreover, it has been a challenge to find and design effective and specific drugs against Tie-2 or angiopoietins[20].
Fibroblast growth factor (FGF)/FGF receptor (FGFR) signaling is involved in multiple cellular processes, such as proliferation, anti-apoptosis, drug resistance, and angiogenesis[49]. FGFs are heparin-binding growth factors that are part of a family that comprises 23 members (FGF-1 to -23), of which only 18 are functional ligands for FGFR in humans. The members of the FGFR family (FGFR-1 to -4) share a common domain architecture consisting of extracellular immunoglobulin-like domains and a cytoplasmic tyrosine kinase domain[50]. Although FGF1 and FGF2 are among the first discovered molecules that contribute to angiogenesis, some members of the VEGF ligand family and VEGFR are now accepted to play a main role in driving embryonic vascularization, angiogenesis and lymphangiogenesis[51]. Nevertheless, both FGFs and VEGF cooperate to promote angiogenesis. FGF-2 induces the expression of VEGF in vascular endothelial cells, while the blockade of VEGF reduces the expression of endogenous FGF-2, suggesting a positive feedback mechanism. Furthermore, inhibition of FGFR-1 and FGFR-2 activity can reduce tumor vascularization as well as VEGF expression. Therefore, promotion of angiogenesis by FGFs may be dependent of crosstalk between FGF-VEGF signaling pathways[52].
EGF signaling is initiated by the binding of EGF family members to the extracellular domain of erythroblastic leukemia viral oncogene homologue (ErBb) receptors. The ErBb receptor tyrosine kinase family comprises 4 members, namely, EGF receptor (EGFR)/ERBB1/HER1, ERBB2/HER2, ERBB3/HER3 and ERBB4/HER4[53]. The major contributors of these receptors are a complex signaling cascade that modulates growth, signaling, differentiation, adhesion, migration and survival of cancer cells[54].
The EGF family members bind to the ErbB receptors and are classified into 3 groups based on their receptor affinities: in the first group, EGF, transforming growth factor-α, amphiregulin (AR), and epigen (EPG), specifically bind to EGFR; in the second group, betacellulin (BTC), heparin-binding EGF (HB-EGF), and epiregulin (EPR), which exhibit dual specificity, bind to both EGFR and ErbB4; and the third group, which includes neuregulins (NRGs), forms two subgroups on the basis of their capacity to bind ErbB3 and ErbB4 (NRG-1 and NRG-2) or only ErbB4 (NRG-3 and NRG-4)[53,55]. On binding, ErbBs form homo or heterodimers and initiate multiple pathways involving effectors including rat sarcoma viral oncogene homologue (RAS)/mitogen-activated protein kinase, phosphatidylinositol 3-kinase-AKT, mammalian target of rapamycin, signal transducer and activator of transcription, SRC tyrosine kinase, phospholipase C-γ1/protein kinase C (PKC) and p27. The activation of these pathways plays a relevant role in several aspects of development and tissue homoeostasis[54]. Increased EGFR signaling is particularly common in several cancers, including CRC, through one or more of the family members[56]. EGFR and its family members, due to their vast role in the progression of cancer, have emerged as attractive candidates for anti-cancer therapy.
Nowadays, there are many therapeutic strategies approved by the Food and Drug Administration (FDA) for the management of mCRC: 5-fluorouracil (5-FU), leucovorin (LV), irinotecan, capecitabine, oxaliplatin, regorafenib, ziv-aflibercept, and the monoclonal antibodies bevacizumab, cetuximab, and panitumumab. Of these drugs, only few have FDA-approved indications for use as monotherapies and reveal activity as a single agent against CRC, including fluoropyrimidines (5-FU and capecitabine), irinotecan, cetuximab, and panitumumab.
The combination chemotherapy is the only standard for first-line treatment of mCRC. Regardless of which regimen is used, outcome may be maximized in patients who receive, alone or in combination, 5-FU, LV, irinotecan, and oxaliplatin sometime during the course of treatment. These chemotherapy regimens have been extensively studied in phase II and III trials, both as first- and second-line therapies[57,58]. Tables 1 and 2 summarizes current and future trials on mCRC anti-angiogenic therapies.
| Clinical trial | Phase | Line | Regimen | Median PFS (mo) | Median OS (mo) | ORR (%) |
| Aflibercept | ||||||
| VELOUR NCT00561470[87] | III | 2nd | FOLFIRI + aflibercept vs FOLFIRI + placebo | 6.90 vs 4.67 HR = 0.758, P = 0.0001 | 13.50 vs 12.06 HR = 0.817, P = 0.0032 | 19.8 vs 11.1 P = 0.001 |
| AFFIRM NCT00851084[86] | II | 1st | mFOLFOX6 + aflibercept vs mFOLFOX6 | 8.48 vs 8.77 | 49.1 vs 45.9 | |
| Brivanib | ||||||
| NCT00640471[90] | III | 3rd | Cetuximab + brivanib vs cetuximab + placebo | 5.0 vs 3.4 HR = 0.72, P < 0.001 | 8.8 vs 8.1 HR = 0.88, P = 0.12 | 13.6 vs 7.2 P = 0.004 |
| Regorafenib | ||||||
| CORRECT NCT01103323[99] | III | 2nd | Regorafenib vs placebo | 1.9 vs 1.7 HR = 0.49, P < 0.000001 | 6.4 vs 5.0 HR = 0.77, P = 0.0052 | |
| Sorafenib | ||||||
| RESPECT NCT00865709[107] | II | 1st | mFOLFOX6 + sorafenib vs mFOLFOX6 + placebo | 9.1 vs 8.7 HR = 0.88, P = 0.46 | 17.6 vs 18.1 HR = 1.13, P = 0.51 | |
| Sunitinib | ||||||
| NCT00668863 | II | 1st | ||||
| NCT00457691[108] | III | 1st | FOLFIRI + sunitinib vs FOLFIRI + placebo | 7.8 vs 8.4 HR = 1.095, P = 0.807 | 20.3 vs 19.8 HR = 1.171, P = 0.916 | 32 vs 34 P = 0.683 |
| Valatanib | ||||||
| CONFIRM1 NCT00056459[110] | III | 1st | FOLFOX4 + vatalanib vs FOLFOX4 + placebo | 7.7 vs 7.6 HR = 0.88, P = 0.118 | 21.4 vs 20.5 HR = 1.08, P = 0.260 | P > 0.05 |
| CONFIRM 2 NCT00056446[111] | III | 2nd | FOLFOX4 + vatalanib vs FOLFOX4 + placebo | 5.6 vs 4.2HR = 0.83, P = 0.013 | 13.1 vs 11.9HR = 1.00, P = 0.957 | |
| Trial | Phase | Line | Therapy/arms | Status of trial | |
| Bevacizumab | NCT01321957 | II | 1st | FOLFOX + bevacizumab vs FOLFOX + bevacizumab + irinotecan | Currently recruiting participants |
| NCT00819780 | II | 1st | Panitumumab + mFOLFOX6 vs bevacizumab + mFOLFOX6 | Ongoing, but not recruiting participants | |
| NCT01531595 | II | 1st | 3 cycles of XELOX + bevacizumab alternating with 3 cycles of XELIRI + bevacizumab | Currently recruiting participants | |
| NCT01067053 | II | 1st | Bevacizumab + capecitabine + oxaliplatin - 6 cycles; after the first 6 cycles of treatment, continuing only with bevacizumab and capecitabine | Ongoing, but not recruiting participants | |
| NCT01765582 | II | 1st | FOLFOXIRI + bevacizumab vs Sequential FOLFOXIRI + bevacizumab vs FOLFOX + bevacizumab | Currently recruiting participants | |
| NCT01006369 | II | - | FOLFOX6 + bevacizumab + hydroxychloroquine vs XELOX + bevacizumab + hydroxychloroquine | Currently recruiting participants | |
| NCT01417494 | II | 1st | Chemotherapy (FOLFIRI, FOLFOX, LV5FU2) + bevacizumab vs Chemotherapy (FOLFIRI, FOLFOX, LV5FU2) | Currently recruiting participants | |
| NCT01532804 | II | 2nd | FOLFOX6 + bevacizumab (day 1 = day 15, 12 cycles) vs Raltitrexed + oxaliplatin + bevacizumab ( day 1 = day 21, 8 cycles) | Currently recruiting participants | |
| NCT00952029 | II/III | 1st | FOLFIRI + bevacizumab and during the chemotherapy-free interval maintenance with bevacizumab vs FOLFIRI + bevacizumab and during the chemotherapy-free interval NO maintenance | Currently recruiting participants | |
| Cetuximab | NCT01640405 | III | 1st | mFOLFOX6 + bevacizumab vs FOLFOXIRI + bevacizumab | Currently recruiting participants |
| NCT00444678 | II | - | Cetuximab + capecitabine + oxaliplatin | Ongoing, but not recruiting participants | |
| NCT01251536 | II | 1st | Cetuximab (standard dose: 250 mg/m2 weekly) vs Cetuximab (dose escalation: days 22 and 29-350 mg/m2, from day 36 onwards - 500 mg/m2 weekly) | Currently recruiting participants | |
| NCT01718808 | II | 1st | Cetuximab + capecitabine vs Cetuximab | Currently recruiting participants | |
| NCT01867697 | II | 1st | Cetuximab (biweekly) + FOLFIRI (continuously) vs Cetuximab (biweekly) + alternating FOLFIRI and mFOLFOX6 | Currently recruiting participants | |
| NCT00640081 | II | 1st | Intermittent chemotherapy plus intermittent cetuximab treatment (12 wk), plus cetuximab followed by a period off all therapy; reintroduction of the same chemotherapy and cetuximab regimen (12 wk after initial progression off treatment) vs Intermittent chemotherapy plus continuous cetuximab treatment (12 wk), plus cetuximab followed by a period of withdrawal of the chemotherapy, but continued weekly cetuximab monotherapy with reintroduction of the same chemotherapy regimen to the cetuximab (12 wk after initial progression off chemotherapy treatment) | Ongoing, but not recruiting participants | |
| NCT00479752 | II | 1st | FOLFOX4 + cetuximab (initial dose: 400 mg/m² in week 1, followed by weekly doses of 250 mg/m²) vs FOLFOX4 + cetuximab (500 mg/m² every 2 wk) | Ongoing, but not recruiting participants | |
| NCT00482222 | III | 1st | Oxaliplatin/fluoropyrimidine vs oxaliplatin/fluoropyrimidine + cetuximab pre and post surgery | Currently recruiting participants | |
| NCT00433927 | III | 1st | FOLFIRI + cetuximab vs FOLFIRI + bevacizumab | Ongoing, but not recruiting participants | |
| Panitumumab | NCT01228734 | III | 1st | Cetuximab + FOLFOX4 vs FOLFOX4 | Ongoing, but not recruiting participants |
| NCT01030042 | III | 2nd | FOLFOX4 followed, after progression, by irinotecan + cetuximab vs Cetuximab + irinotecan | Currently recruiting participants | |
| NCT00885885 | II | - | Panitumumab + FOLFOX4 vs Panitumumab + FOLFOX4 | Ongoing, but not recruiting participants | |
| NCT01215539 | II | 1st | Panitumumab + capecitabine + oxaliplatin | Currently recruiting participants | |
| NCT01126112 | II | 1st | Panitumumab (6 mg/kg every 2 wk ) | Ongoing, but not recruiting participants | |
| NCT00819780 | II | 1st | Panitumumab + mFOLFOX 6 vs bevacizumab + mFOLFOX 6 | Ongoing, but not recruiting participants | |
| NCT01328171 | II | 1st | FOLFOXIRI + panitumumab vs FOLFOXIRI | Currently recruiting participants | |
| NCT01508000 | II | 1st | mFOLFOX6 (6 cycles after and before surgery) + surgery vs mFOLFOX6 + bevacizumab (6 cycles after and before surgery) + surgery vs mFOLFOX6 + panitumumab (6 cycles after and before surgery) + surgery | Not yet open for participant recruitment | |
| NCT01814501 | II | 2nd | 5-FU + irinotecan + panitumumab | Currently recruiting participants | |
| NCT00940316 | II | 2nd | Erlotinib + panitumumab + irinotecan (treatment repeats every 2 wk) vs Erlotinib + panitumumab (treatment repeats every 2 wk) vs Erlotinib + panitumumab | Currently recruiting participants | |
| NCT00364013 | III | 1st | FOLFOX + panitumumab vs FOLFOX | Ongoing, but not recruiting participants | |
| NCT01910610 | III | 1st | FOLFIRI + cetuximab, followed by oxaliplatin-based chemotherapy + bevacizumab vs OPTIMOX + bevacizumab, followed by irinotecan-based chemotherapy + bevacizumab, followed by an anti-EGFR agent (cetuximab +/- irinotecan or panitumumab) with or without irinotecan | Not yet open for participant recruitment | |
| Aflibercept | NCT01669720 | II | 2nd | Aflibercept iv (4 mg/kg every 2 wk) | Currently recruiting participants |
| NCT01652196 | II | 1st | Aflibercept iv + mFOLFOX 6 iv (days 1 and 15; repeats every 28 d) | Currently recruiting participants | |
| NCT01802684 | II | 1st | Induction therapy (sequence #1)Regimen: Aflibercept + mFOLFOX7 - 6 cycles (3 mo) Maintenance after induction (sequence #2) First phase (sequence #2A); Regimen: Aflibercept + fluoropyrimidine (simplifed LV5FU2 or capecitabine) - 6 cycles (3 mo) Second phase (sequence #2B); Regimen: Aflibercept +/- fluoropyrimidine (simplifed LV5FU2 or capecitabine) - until PD or limiting toxicity Reintroduction (sequence #3); Regimen: Aflibercept + mFOLFOX7 - 6 cycles (3 mo) Maintenance after reintroduction (sequence #4); Regimen: Aflibercept + fluoropyrimidine - until PD or limiting toxicity | Not yet open for participant recruitment | |
| NCT01882868 | II | 2nd | Aflibercept iv + FOLFIRI Aflibercept + FOLFIRI (every 2 wk) | Currently recruiting participants | |
| NCT01889680 | II | 1st | mFOLFOX6 + aflibercept (every 14 d for 6 cycles) plus 5-FU/LV (every 14 d) vs mFOLFOX6 + aflibercept (every 14 d for 6 cycles) plus 5-FU/LV + aflibercept (every 14 d) | Not yet open for participant recruitment | |
| NCT01646554 | II/III | 1st | mFOLFOX6 and SURGERY 6 cycles before and 6 cycles after surgery consisting in: Hour 0: Oxaliplatin 85 mg/m²iv 2-h infusion; Hour 0: Folinic acid 400 mg/m² (DL form) or 200 mg/m2 (L form) iv 2-h infusion; Hour 2: 5-FU 400 mg/m²iv bolus over 2-4 min; Hour 2: 5-FU 2400 mg/m² given as a continuous infusion over 46 h; On day 1 of a 14 d cycle vs mFOLFOX6 + aflibercept and surgery; 6 cycles before and 6 cycles after surgery consisting in: Hour 0: Aflibercept 4 mg/kg intravenous infusion 1-h; Hour 1: Oxaliplatin 85 mg/m2 2-h infusion; Hour 1: Folinic acid 400 mg/m2 (DL form) or 200 mg/m2 (L form) 2-h infusion; Hour 3: 5-FU bolus 400 mg/m2iv bolus over 2-4 min; Hour 3: 5-FU 2400 mg/m² given as a continuous infusion over 46 h; Day 1 of a 14 day cycle | Not yet open for participant recruitment | |
| NCT01661270 | III | 2nd | Aflibercept iv (day 1 of each cycle, every 2 wk) + FOLFIRI vs Placebo iv (day 1 of each cycle, every 2 wk) + FOLFIRI | Currently recruiting participants | |
| NCT01571284 | III | 2nd | Aflibercept IV (every 2 wk ) + FOLFIRI | Currently recruiting participants | |
| NCT01670721 | III | 2nd | Aflibercept IV (on day 1) + FOLFIRI administered as follows: dI-leucovorin infusion over 2 h on day 1; Irinotecan: infusion over 90-min infusion, on day 1, followed by bolus 5-FU and 5-FU continuous infusion over 46 h or as individualized by physician's clinical judgment; Treatment cycle to be administered every 2 wk | Currently recruiting participants | |
| Brivanib | NCT01367275 | II | 2nd | Brivanib (800 mg orally daily days 1-14) + Irinotecan iv (180 mg/m2 on day 1) | Ongoing, but not recruiting participants |
| Cediranib | NCT00588900 | II | 2nd | Irinotecan iv (days 1 and 8) + Cediranib oral (days 1-21) | The recruitment status of this study is unknown because the information has not been verified recently |
| Ramucirumab | NCT01111604 | II | 2nd | mFOLFOX-6 vs mFOLFOX-6 + ramucirumab ( 8 mg/kg iv infusion, administered every 2 wk) vs mFOLFOX-6 + icrucumab ( 15 mg/kg iv infusion, administered every 2 wk) | Ongoing, but not recruiting participants |
| NCT01183780 | III | 2nd | FOLFIRI + ramucirumab ( 8 mg/kg administered intravenously every 2 wk) vs FOLFIRI + placebo | Currently recruiting participants | |
| Regorafenib | NCT01298570 | II | 2nd | Regorafenib (160 mg, po, daily, per 7 day cycle) + FOLFIRI (day 1 and day 15 of each 28 d cycle) vs Placebo (oral administration, days 4-10 and days 18-24 of 28 day cycle +)+ FOLFIRI (day 1 and day 15 of each 28 d cycle) | Currently recruiting participants |
| NCT01289821 | II | 1st | Day 1 and day 15 of each cycle: 85 mg/m² oxaliplatin + folinic acid (either 400 mg/m² D/L-folinic acid or 200 mg/m² L-folinic acid), iv + 400 mg/m² 5 FU iv + 2400 mg/m² 5 iv; Days 4 to 10 and days 18 to 24: regorafenib 160 mg (four 40 mg tablets) | Ongoing, but not recruiting participants | |
| NCT01875380 | II | 1st | Regorafenib (orally, 160 mg per day for 3 wk, followed by 1 wk of rest) | Not yet open for participant recruitment | |
| NCT01103323 | III | 2nd | Regorafenib (160 mg per oral once daily for 3 wk on 1 wk off of every 4 wk cycle) vs Placebo (per oral once daily for 3 wk on 1 wk off of every 4 wk cycle) | Ongoing, but not recruiting participants | |
| NCT01584830 | III | 2nd | Regorafenib [3 wk on/1 wk off (160 mg od po)]Placebo [3 wk on/1 wk off (160 mg od po)] | Ongoing, but not recruiting participants | |
| NCT01853319 | III | 2nd | Regorafenib (160 mg per oral every day for 3 wk of every 4 wk cycle) | Not yet open for participant recruitment | |
| NCT01538680 | III | 2nd | Regorafenib (160 mg po every day for 3 wk on, 1 wk off) | Expanded access is currently available for this treatment | |
| Semaxanib | NCT00021281 | III | 1st | Semaxanib iv (on days 1, 4, 8, 11, 15, 18, 22, 25, 29, 32, 36 and 39) + irinotecan iv, leucovorin calcium iv, fluorouracil iv (on days 1, 8, 15, and 22) (every 6 wk) vs Irinotecan iv, leucovorin calcium iv, fluorouracil iv (on days 1, 8, 15, and 22) (every 6 wk) vs Semaxanib iv (on days 1, 4, 8, 11, 15, 18, 22, 25, 29, 32, 36 and 39) + irinotecan iv (days 1, 15 and 29) + leucovorin calcium iv, fluorouracil iv (on days 1, 2, 15, 16, 29 and 30) vs Irinotecan iv, leucovorin calcium iv, fluorouracil iv (on days 1, 8, 15, and 22) (every 6 wk) | The recruitment status of this study is unknown because the information has not been verified recently |
| Sorafenib | NCT01715441NEXIRI 2 | II | 2nd | Irinotecan 180 mg/m2iv with cross over to irinotecan and sorafenib combination at progression vs Sorafenib 400 mg twice daily with cross over to irinotecan and sorafenib combination at progression vs Irinotecan 120 mg/m2iv at cycle 1, 150 mg/m² at cycle 2 and 180 mg/m² at cycle 3 + sorafenib 400 mg twice daily from cycle 1 | Currently recruiting participants |
| NCT01471353 | II | 2nd | Sorafenib 200-400 mg po twice daily on days 1-21 (dose escalation scheme) + capecitabine 1000 mg/m2po twice daily on days 1-14 repeated every 21 d | Currently recruiting participants | |
| NCT00826540 | II | 2nd | Sorafenib twice daily on days 1-5 and 8-12 + bevacizumab iv on day 1 | Ongoing, but not recruiting participants | |
| NCT00839111 | II | 2nd | Sorafenib (400 mg twice daily from day 3 to day 14, day 17-28) + FOLFIRI | The recruitment status of this study is unknown because the information has not been verified recently | |
| NCT01290926 | II | 2nd | Sorafenib (200 mg in the morning, 400 mg in the evening) + capecitabine (850 mg/m2 twice daily) | The recruitment status of this study is unknown because the information has not been verified recently | |
| NCT00326495 | II | 2nd | Oral sorafenib (400 mg by twice daily) plus cetuximab (400 mg/m2, week 1; 250 mg/m2iv, weekly) | Currently recruiting participants | |
| Sunitinib | NCT00936832 | II | 1st | FOLFIRI (on days 1, 15, and 29) + oral sunitinib (on days 1-28). (repeats every 6 wk) | The recruitment status of this study is unknown because the information has not been verified recently |
Bevacizumab is a humanized monoclonal antibody that binds and inactivates VEGF, preventing angiogenesis and, hence, tumor growth and proliferation. Bevacizumab contains human framework regions and the complementarity-determining regions of a murine antibody that inhibits all active isoforms of VEGF[59]. Currently, bevacizumab is the only agent specifically targeting the VEGF pathway for the treatment of CRC[60].
Over the past decades, many trials have investigated bevacizumab in mCRC. It has been studied with different active chemotherapy and biological agents, as well as in multiple treatment setting, sequencing and duration[61].
The phase II trial conducted by Kabbinavar et al[62] compared two doses of bevacizumab plus 5-FU/LV (low-dose bevacizumab: 5 mg/kg every 2 wk; high-dose bevacizumab: 10 mg/kg every 2 wk) with 5-FU/LV alone in 104 patients untreated. Compared with the 5-FU/LV control arm, treatment with bevacizumab (at both dose levels) plus 5-FU/LV resulted in a higher response rate (RR) (control arm: 17%; low-dose arm: 40%; high-dose arm: 24%), longer median time to disease progression (control arm: 5.2 mo; low-dose arm: 9.0 mo; high-dose arm: 7.2 mo), and longer median survival (control arm: 13.8 mo; low-dose arm: 21.5 mo; high-dose arm: 16.1 mo). Based on these results, in the most subsequent phase III trials on mCRC the 5 mg/kg bevacizumab dosing is chosen[61].
The phase III AVF 2107 trial (NCT00109070) randomized 813 patients to receive IFL plus either bevacizumab 5 mg/kg (n = 402) or placebo (n = 411), every 2 wk. The addition of bevacizumab compared with IFL alone provided significantly clinical and statistical improvement in median OS (20.3 mo vs 15.6 mo; HR = 0.66, P < 0.001), PFS (10.6 mo vs 6.2 mo, HR = 0.54, P < 0.001) and overall response rate (ORR) (44.8% vs 34.8%, P = 0.004)[13].
In the NO16966 phase III trial (NCT00069095), with 2 × 2 factorial design, 1401 patients with mCRC were randomized to receive FOLFOX or XELOX and then bevacizumab or placebo. Median PFS was significantly increased when bevacizumab was added (9.4 mo in bevacizumab group vs 8.0 mo in placebo group; HR = 0.83, P = 0.0023). Median OS was 21.3 mo in the bevacizumab group and 19.9 mo in the placebo group (HR = 0.89, P = 0.077), and RR was similar in both arms. A planned subset analysis demonstrated significant improvement of PFS with bevacizumab in the XELOX subgroup (P = 0.0026), as opposed when FOLFOX4 (P = 0.187) was added. Safety results showed that grade 3 or higher adverse events were slightly higher in the bevacizumab group (30% vs 21%)[63].
In the phase III MAX study, 471 patients with previously untreated and unresectable mCRC were randomly assigned to the following arms: capecitabine alone, capecitabine plus bevacizumab, or capecitabine, bevacizumab, and mitomycin. Median PFS was 5.7 mo for the capecitabine arm, 8.5 mo for the capecitabine-bevacizumab arm, and 8.4 mo for the capecitabine-bevacizumab-mitomycin arm. Thus, there was statistical improvement in PFS between the capecitabine arm and the other two arms (capecitabine vs capecitabine-bevacizumab: HR = 0.63, P < 0.001; capecitabine vs capecitabine-bevacizumab-mitomycin: HR = 0.59, P < 0.001)[64]. Based on these results, in United States and Europe, bevacizumab in association with standard chemotherapy has been approved for first-line treatment of KRAS-mutant mCRC or for second-line treatment of KRAS-wild type patients previously treated with anti-EGFR drugs.
Despite those interesting benefits reported in previous trials, researchers and clinicians should be knowledgeable about toxicities, such as for hypertension and bleeding.
Cetuximab and panitumumab are two EGFR inhibitors currently indicated as monotherapy in patients with wild-type KRAS tumors as a first or second-line treatment[65]. Only cetuximab is indicated in combination with irinotecan, and has been approved for use in first-line in Europe as mono-therapy or in combination with chemotherapy[66].
Cetuximab is a recombinant human-murine chimeric IgG1 monoclonal antibody that binds to the extracellular region of the EGFR with high specificity and with higher affinity than EGF on normal and tumor cells[67].
A phase II clinical trial conducted by Tabernero et al[68] assessed 43 patients who received cetuximab and FOLFOX4 as first-line chemotherapy. RR was 72%; median PFS was 12.3 mo and median OS was 30 mo. Cetuximab did not increase the characteristic toxicity of FOLFOX4 and was collectively well tolerated. The most commonly reported grade 3 or higher adverse events were diarrhea, neutropenia, and paresthesia.
The OPUS study, also a phase II trial (NCT00125034), included 337 patients who were randomized to receive FOLFOX4 with cetuximab (n = 169) or alone (n = 168) in first-line chemotherapy[69]. In 93% of measured KRAS patient samples, 57% were KRAS-wild type. Patients whose tumors were KRAS-wild type who received cetuximab plus FOLFOX4 had a 2.6-fold increased odds ratio of response (ORR: 57% vs 34%, OR = 2.551, P = 0.0027) and a 43% decrease in the risk of disease progression (median PFS 8.3 mo vs 7.2 mo, HR = 0.567, P = 0.0064) compared with those who received FOLFOX4 alone. Also, median OS was improved by the addition of cetuximab to FOLFOX4 for patients in that group (22.8 mo vs 18.5 mo, HR = 0.855, P = 0.39). On the other hand, patients whose tumors carried KRAS mutations who received cetuximab plus FOLFOX4 had a decreased odd of response (34% vs 53%, OR = 0.459, P = 0.0290) and a higher risk of disease progression (median PFS 5.5 mo vs 8.6 mo, HR = 1.720, P = 0.0153) compared with those who received FOLFOX4 alone[70].
In the phase III CRYSTAL study (NCT00154102), 1198 patients who received cetuximab plus FOLFIRI (n = 599) or FOLFIRI alone (n = 599) were included. The addition of cetuximab to chemotherapy significantly reduced the risk of progression by 15% (8.9 mo vs 8.0 mo, HR = 0.85, P = 0.048) and improved ORR (46.9% vs 38.7%, OR = 1.40, P = 0.048). On the other hand, no significant difference in median OS between the two treatment groups was observed (19.9 mo vs 18.6 mo, HR = 0.93, P = 0.31)[71]. In that study, KRAS and BRAF mutations were detected in 37% and 6% of patients, respectively. The addition of cetuximab to FOLFIRI in patients with wild-type KRAS resulted in significant improvement in median OS (23.5 mo vs 20.0 mo, HR = 0.796, P = 0.0093), median PSF (9.9 mo vs 8.4 mo, HR = 0.696, P =0.0012), and RR (57.3% vs 39.7%, OR = 2.069, P < 0.001) compared with FOLFIRI alone. These results showed the role of KRAS mutation status as a powerful predictive biomarker for the efficacy of cetuximab plus FOLFIRI. Concerning grade 3 or 4 adverse events, they were more common with use of regimen with cetuximab and included skin reactions, infusion reactions and diarrhea[72].
In the phase III study NORDIC VII (NCT00145314), 571 patients with mCRC were randomized to one of the following three arms: continuous FLOX alone or with cetuximab or intermittent FLOX with continuous weekly cetuximab. No differences were found in RR, median PFS or OS in patients receiving cetuximab, either in KRAS-mutant or -wild-type[73].
In the phase III trial MRC COIN, 1630 patients were randomized to receive oxaliplatin-based chemotherapy (FOLFOX or XELOX) with or without cetuximab. The determination of KRAS mutation was performed in 1316 (81%) patients and it was identified in 729 (55%) patients[74]. Patients with wild-type KRAS tumors showed no improvements in median OS for cetuximab combined with chemotherapy when compared with chemotherapy alone (17.0 mo vs 17.9 mo, HR = 1.038, P = 0.68) or PFS (8.6 mo vs 8.6 mo, HR = 0.96, P = 0.60); however, there was an increase in ORR (57% vs 64%, P = 0.049). Furthermore, there was a potential benefit with improvement in PFS for wild-type KRAS patients who received cetuximab plus infused 5-FU (HR = 0.72, P = 0.037) but not cetuximab plus capecitabine (HR = 1.02, 95%CI: 0.82-1.26, P = 0.88)[74].
Based on those trials, cetuximab in addition with standard chemotherapy has been approved in United States and Europe for wild-type KRAS mCRC patients in first-line regimen. It is important to monitor toxicity profile such as skin rash, diarrhea, nausea and mucositis in order to provide a good tolerability for patients. Regular medical visits before each cycle and support medication could help address this concern.
Panitumumab is a recombinant human IgG2к monoclonal antibody that binds EGFR and prevents receptor dimerization, tyrosine autophosphorylation of EGFR, and the activation of downstream signaling molecules[75].
The phase III trial PRIME (NCT00364013) included 1183 patients without prior chemotherapy for mCRC, who were randomly assigned to receive FOLFOX4 with or without panitumumab therapy. In the wild-type KRAS subgroup, panitumumab plus FOLFOX4 produced a significantly improved median PFS compared with FOLFOX4 alone (9.6 mo vs 8.0 mo, respectively; HR = 0.80, P = 0.02). Nevertheless, a non-significant increase in median OS was found for panitumumab plus FOLFOX4 versus FOLFOX4 alone (23.9 mo vs 19.7 mo, respectively, HR = 0.83, P = 0.072). In the mutant KRAS subgroup PFS was significantly reduced in the panitumumab plus FOLFOX4 arm when compared with the FOLFOX4 alone arm (HR = 1.29, P = 0.02), and median OS was 15.5 mo vs 19.3 mo, respectively (HR = 1.24, P = 0.068)[76].
As a conclusion, the use of cetuximab or panitumumab for wild-type KRAS mCRC patients will depend on the patient fitness, toxicity profile and drug wiliness in each circumstance. Both drugs are safe and prove to improve OS in the metastatic setting.
The efficacy of bevacizumab and anti-EGFR agents in first-line treatment of mCRC encouraged two clinical trials of double monoclonal antibody therapy[77].
In the phase III PACCE (NCT00115765) study, a total of 1053 patients were randomized to receive first-line chemotherapy [oxaliplatin/5-FU/LV (n = 823 patients) or irinotecan/5-FU/LV (n = 230 patients)] and bevacizumab with or without panitumumab. The study was discontinued early after a planned interim analysis showed reduced PFS and increased toxicity in the panitumumab arm. In the final analysis, median PFS (10.0 mo vs 11.4 mo for the panitumumab and control arms, respectively, HR = 1.27) and OS (19.4 mo vs 24.5 mo for the panitumumab and control arms, respectively) were shorter in the panitumumab arm in the entire study cohort as well as in the subset with wild-type KRAS. Grade 3/4 adverse events in the oxaliplatin (panitumumab vs control) cohort included skin toxicity (36% vs 1%), diarrhea (24% vs 13%), infections (19% vs 10%), and pulmonary embolism (6% vs 4%)[78].
Similarly, in the phase III CAIRO2 trial, 755 patients with previously untreated mCRC were randomly assigned to receive capecitabine, oxaliplatin, and bevacizumab (CB regimen, n = 378 patients) or the same regimen plus weekly cetuximab (CBC regimen, n = 377 patients). The addition of cetuximab to XELOX plus bevacizumab resulted in shorter PFS in the entire study cohort (10.7 mo in the CB group vs 9.4 mo in the CBC group, P = 0.01) and in the wild-type KRAS subset compared with XELOX plus bevacizumab. No difference in OS (20.3 mo in the CB group vs 19.4 mo in the CBC group, P = 0.16) or ORR (50.0% in the CB group vs 52.7% in the CBC group, P = 0.49) was verified between treatment arms. Patients treated with cetuximab who had tumors bearing a mutated KRAS gene had significantly decreased PFS as compared with cetuximab-treated patients with wild-type KRAS tumors (8.1 mo vs 10.5 mo, P = 0.04) or patients with mutated KRAS tumors in the CB group (8.1 mo vs 12.5 mo, P = 0.003). Grade 3 or 4 adverse events were more frequent in the CBC group, which were attributed to cetuximab-related adverse cutaneous effects[79].
On the basis of these studies, double monoclonal antibody therapy with bevacizumab and an anti-EGFR agent is not recommended[77].
In order to determine the treatment strategy for hepatic metastases of CRC, it is important to verify the presence of one of three situations: metastases are readily resectable; metastatic disease is initially considered to be unresectable, principally due to location; or liver metastases are unlikely ever to become resectable[80]. Surgical resection undoubtedly remains the gold standard for the treatment of resectable colorectal liver metastases because it improves patient’s prognosis if the metastases are resectable. When surgery is not indicated for hepatic metastases, chemotherapy is the first-choice treatment. In cases where surgical resection becomes possible and chemotherapy is effective, the long-term prognosis may be good[81].
For patients with initially resectable disease, with good prognostic factors, one approach is immediate surgical resection and another is perioperative chemotherapy such as FOLFOX4[82,83]. Today, chemotherapy before surgery, even in patients with resectable metastases, can increase the complete resection rate, facilitate limited hepatectomies, improve postoperative recovery, treat micrometastases, provide a test of chemoresponsiveness, identify aggressive disease, spare ineffective therapy and prolong relapse-free survival[80].
In potentially resectable colorectal liver metastases, neoadjuvant chemotherapy, infused 5-FU/LV, in combination with either irinotecan or oxaliplatin, as well as triple cytotoxic drug therapy, e.g., FOLFOXIRI, and more recent combination chemotherapy regimens with targeted agents cetuximab and bevacizumab, should be considered to enhance the chance of cure of patient with initially unresectable liver metastases[80,82].
In liver metastases that are unlikely to ever become resectable, palliative chemotherapy based on FOLFOX4/XELOX, FOLFIRI, with or without biological therapies, should be considered. In this setting, the possibility of doing a resection should not be excluded[82].
Others drugs are also under investigation or have been recently approved for the use in the metastatic setting, as showed in Tables 1 and 2. Further down, we will discuss the main trials in each field.
Aflibercept (Ziv-aflibercept, VEGF-Trap) is a recombinant VEGFR-antibody protein generated by the fusion of second immunoglobulin (Ig) domain of the VEGFR-1 and the third Ig domain of the VEGFR2 to the Fc domain of human IgG1[84]. In contrast to bevacizumab, which only binds to VEGF-A and forms multimeric complexes, aflibercept traps the different isoforms of VEGF-A, with approximately 1000-fold higher affinity than bevacizumab. In addition, aflibercept binds to VEGF-B and PIGF[85]. This VEGF-Trap effectively suppresses tumor growth and vascularization in vivo, resulting in stunted and almost completely avascular tumors[84].
To investigate the potential role of aflibercept in the first-line treatment of mCRC with chemotherapy, the phase II AFFIRM trial (NCT00851084) recruited 236 patients who had never received therapy for mCRC or angiogenesis inhibitors. A total of 117 patients received mFOLFOX6 alone and 119 received mFOLFOX6 plus aflibercept (4 mg/kg iv every 2 wk). This study showed similar efficacy of FOLFOX plus aflibercept vs FOLFOX alone with respect to ORR (49.1% vs 45.9%, respectively) and median PFS (8.48 mo vs 8.77 mo, respectively)[86].
The purpose of the phase III randomized, placebo-controlled clinical trial VELOUR (NCT00561470) was to investigate the efficacy and safety of aflibercept plus FOLFIRI in the second-line treatment of mCRC after oxaliplatin failure. 614 participants were randomly assigned to receive aflibercept (4 mg/kg intravenously; 612 patients) or placebo (614 patients) every 2 wk in combination with FOLFIRI. Median OS was 13.50 mo for aflibercept and 12.06 mo for placebo (HR = 0.817, P = 0.0032). Adding aflibercept to FOLFIRI also increased PFS relative to placebo plus FOLFIRI (HR = 0.758, P = 0.0001), with median PFS times of 6.90 mo vs 4.67 mo, respectively. The ORR in the aflibercept group was 19.8% compared with 11.1% in the placebo group (P = 0.001). Grade 3/4 adverse events with an at least 2% higher incidence with aflibercept versus placebo were diarrhea, asthenia/fatigue, stomatitis/ulceration, infections, hypertension, gastrointestinal/abdominal pain, neutropenia/neutropenic complications and proteinuria[87]. Approximately one third of study participants had previously been treated with bevacizumab (187 in the placebo and 186 in the aflibercept group). Aflibercept produced a consistent trend towards prolonged OS (P = 0.7231) and PFS (P = 0.6954), regardless of prior use of bevacizumab. The incidence of adverse events in the aflibercept arm was similar in patients with prior bevacizumab (100%) to those without (98.9%), with a similar incidence of grade 3/4 events (82.5% and 83.9%, respectively). Results of this subgroup analysis showed that the addition of aflibercept to FOLFIRI leads to a consistent trend of increased OS and PFS, regardless of prior bevacizumab use[88].
Brivanib alaninate (BMS582664) is an oral, potent selective inhibitor of both the FGF and VEGF family of receptors[89]. Besides its antiangiogenic activity from blocking VEGFR-2 and -3, its ability to disrupt FGF receptors (FGFRs) -1, -2 and -3 has been suggested to circumvent primary and/or acquired resistance to VEGF blockade, and block FGF-dependent tumor proliferation[90]. In preclinical studies using in vivo tumor xenograft models of CRC resistant to bevacizumab, the strong antiangiogenic effects and antitumor activity of brivanib[91] were established. Phase I studies evaluated brivanib in combination with cetuximab in advanced gastrointestinal malignancies, including CRC, and demonstrated good tolerability and some evidence of clinical activity[92,93].
A phase III study (NCT00640471) was carried out to evaluate combined use of brivanib and cetuximab without chemotherapy in third-line therapy for mCRC. A total of 750 patients were randomly assigned to treatment: 376 on brivanib plus cetuximab arm and 374 on placebo plus cetuximab arm. Patients included in this trial had wild-type K-RAS, had received prior fluoropyrimidine, and had been treated with irinotecan and oxaliplatin. Despite positive effects on PFS (5.0 mo in brivanib arm and 3.4 mo in placebo arm-HR = 0.72, P < 0.001) and objective response, cetuximab plus brivanib increased toxicity and did not significantly improve OS in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal cancer (8.8 mo in brivanib arm and 8.1 mo in placebo arm-HR = 0.88, P = 0.12). A total of 51 patients in brivanib arm and 27 patients in placebo arm had complete or partial response, yielding ORR of 13.6% and 7.2% for brivanib and placebo arms, respectively. The difference in ORR was statistically significant, supporting the brivanib plus cetuximab combination (P = 0.004). The median duration of response was 5.8 mo in brivanib arm and 5.4 mo in placebo arm (P = 0.04). Incidence of grade 3 or higher adverse events was 78% in brivanib arm and 53% in placebo arm, particularly fatigue, hypertension, rash, abdominal pain, diarrhea, dehydration, and anorexia. Hematologic adverse events were uncommon in both arms[90].
Cediranib (AZD2171) is a highly potent and selective inhibitor of the three VEGFRs and has a half-life suitable for once-daily oral dosing[94]. Cediranib is currently in phase III development for the first-line treatment of mCRC. The clinical development program includes two global phase II/III studies (HORIZON II and HORIZON III) in the first-line treatment setting, and a phase II study in second-line treatment.
HORIZON II (NCT00399035) is a randomized phase II/III trial aimed to compare chemotherapy (FOLFOX or XELOX) with cediranib or placebo as first-line therapy in patients with mCRC. In this study, cediranib plus chemotherapy significantly improved PFS (HR = 0.84) but not OS (HR = 0.94) or ORR, compared with placebo plus chemotherapy.
HORIZON III (NCT00384176) incorporated a phase II/III study design. An end-of-phase-II analysis of efficacy and safety was undertaken to determine whether the study should continue into the phase III part. In this study, a randomized comparison of mFOLFOX6 in combination with cediranib versus mFOLFOX6 in combination with bevacizumab as first-line chemotherapy was made in patients with mCRC.
Ramucirumab (IMC-1121B) is a fully humanized IgG1 monoclonal antibody that binds with high affinity to the extracellular VEGF-binding domain of VEGFR-2. Ramucirumab binds to a VEGFR-2 epitope involved in ligand binding and blocks VEGF ligands from binding this site and activating the receptor[95]. The inhibition of VEGF-stimulated VEGFR-2 activation provides ramucirumab significant antitumor activity in a range of malignancies in animal models as a single agent or in combination with other therapeutics[96].
Several studies assessing ramucirumab in mCRC are currently underway (Table 2), without reported results. In a phase II study (NCT00862784) participants were treated with ramucirumab (8 mg/kg infusions every 2 wk) in combination with mFOLFOX6 as first-line therapy. In another phase II study (NCT01111604), patients with disease progression on an irinotecan-based, first-line chemotherapy regimen (FOLFIRI or CAPIRI) received mFOLFOX-6 alone or in combination with ramucirumab (8 mg/kg infusions every 2 wk). The phase II study NCT01079780 evaluated the combination of ramucirumab, cetuximab, and irinotecan versus cetuximab and irinotecan in patients with mCRC and progression following a bevacizumab-based regimen. A phase III study (NCT01183780) evaluates the role of ramucirumab, in combination with FOLFIRI chemotherapy, in patients with progression following first-line combination therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine. Soon, ramucirumab may show its place in the current clinical practice scenario.
Regorafenib (BAY 73-4506) is an oral multikinase inhibitor that blocks the activity of multiple protein kinases, including kinases involved in the regulation of tumor angiogenesis (VEGFR-1, -2, and -3, and angiopoietin-1 receptor), oncogenesis (KIT, RET, RAF1, BRAF, and BRAFV600E), and the tumor microenvironment (PDGFR and FGFR)[97]. Preclinical studies (both in vitro and in vivo) showed a broad spectrum of antitumor activity of Regorafenib as a result of its ability to block several angiogenic, stromal and oncogenic kinases[98].
The phase III trial CORRECT (NCT01103323) investigated the use of regorafenib in 760 patients who had received all locally approved standard therapies and had progressed during or within 3 mo after the last standard therapy. Patients were randomized in a 2:1 ratio to receive regorafenib (160 mg orally daily for 3 out of 4 k; n = 500) versus placebo (3 wk on and 1 wk off; n = 253), respectively. Randomization was based on pre-allocated block sizes and patients were stratified by previous treatment with VEGF-targeting drugs, time from diagnosis of metastatic disease (≥ 18 or < 18 mo), and geographical region. This study reported an increase in OS for regorafenib-treated patients against best supportive care, after progression on standard therapy (6.4 mo vs 5.0 mo, respectively, HR = 0.77, one-sided P = 0.0052). Also, median PFS was 1.9 mo vs 1.7 mo when compared with placebo (HR = 0.49, one-sided P < 0.000001). After the interim analysis, the study was unblinded and patients were allowed to cross over to the regorafenib arm. Treatment-related adverse events occurred in 93%of patients in the regorafenib arm and in 61% of those in the placebo arm. The most common grade 3 or higher side effects related to regorafenib were hand-foot skin reaction (17%), fatigue (10%), diarrhoea (7%), hypertension (7%), and rash or desquamation (6%)[99]. Based on the CORRECT study, regorafenib received approval from the FDA in October 2012 for the treatment of chemorefractory mCRC patients. However, we believe that this drug should be provided only in a specific context due to the modest results reported on OS benefit and pharmaco-economic evaluation.
Semaxanib (SU5416) is a potent, specific and competitive (with respect to ATP) inhibitor of the tyrosine kinase activity of Flk-1/KDR. Semaxanib was shown to inhibit VEGF-dependent mitogenesis of human endothelial cells, without inhibiting the growth of a variety of tumor cells in vitro[100,101].
A clinical phase III study (NCT00004252) studied the combination of 5-FU/LV with semaxanib or alone, as a first-line therapy for mCRC patients. Although the study had already been completed, its results are not yet known.
Sorafenib is an oral multikinase inhibitor with anti-proliferative and anti-angiogenic effects. It inhibits the activity of the serine/threonine kinases c-Raf and B-Raf; the mitogen-activated protein kinases MEK and ERK; VEG; PDGFR; the cytokine receptor c-KIT; the receptor tyrosine kinases Flt-3 and RET; and the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway[102]. In vivo and in vitro studies showed that sorafenib inhibits tumor growth and disrupts tumor microvasculature through antiproliferative, antiangiogenic, and/or proapoptotic effects[103].
In the double-blind placebo-controlled phase II study RESPECT (NCT00865709), the addiction of sorafenib to mFOLFOX6 was evaluated. 198 patients were randomized to receive sorafenib (400 mg bid) (n = 97) or placebo (n = 101), combined with mFOLFOX6 every 14 d. Median PFS was 9.1 mo for the sorafenib arm and 8.7 mo for the placebo arm (HR = 0.88, P = 0.46). Similar results were observed in the subgroup analyses: in patients with wild-type KRAS, the median PFS was 9.5 mo vs 9.2 mo, respectively (HR = 0.84), with corresponding medians of 7.8 mo vs 7.6 mo, respectively, in the mutant KRAS subgroup (HR = 0.96). In patients with wild-type BRAF, the median PFS was 9.2 mo vs 9.0 mo, respectively (HR = 0.91), and the median PFS for patients with mutant BRAF was 8.6 mo vs 7.3 mo, respectively (HR = 0.89). There was no difference between treatment arms for median OS (17.6 mo in the sorafenib arm vs 18.1 mo in the placebo arm-HR = 1.13, P = 0.51). In patients with wild-type KRAS, median overall survival was 19.9 mo vs 16.8 mo, respectively (HR = 0.89), and 17.0 mo vs 19.4 mo, respectively, in patients with mutant KRAS (HR = 1.29). In patients with wild-type BRAF, median overall survival was 18.8 mo vs 18.3 mo, respectively (HR = 1.09), and 13.9 mo vs 11.9 mo, respectively, in patients with mutant BRAF (HR = 0.46). The most common grade 3/4 adverse events in the sorafenib and placebo arms were neutropenia (48% vs 22%), peripheral neuropathy (16% vs 21%), and grade 3 hand-foot skin reaction (20% vs 0%). Treatment discontinuation because of adverse events was 9% and 6%, respectively. Generally, dose intensity (duration and cumulative doses) was lower in the sorafenib arm than in the placebo arm. This study did not detect a PFS benefit with the addition of sorafenib to first-line FOLFOX6 for mCRC, and KRAS and BRAF status did not seem to impact treatment outcomes. These results do not support further development of sorafenib in combination with mFOLFOX6 in molecularly unselected patients with mCRC[104].
The clinical phase II study FOSCO (NCT00889343) studied the combination of FOLFOX6 or FOLFIRI with sorafenib or alone, as a second-line therapy in mCRC patients. Although the study had already been completed, its results are not yet known.
Sunitinib malate (SUTENT) is an oral, multitargeted tyrosine kinase inhibitor that selectively inhibits the VEGFR and PDGFR family members, as well as stem-cell factor receptor (KIT), glial cell line-derived neurotrophic factor receptor (rearranged during transfection; RET), colonystimulating factor receptor (CSF-1R), and FMS-like tyrosine kinase-3[105-107].
In a phase III trial (NCT00457691), 768 patients with mCRC were randomly assigned to receive intravenous FOLFIRI (every 2 wk) plus sunitinib (37.5 mg/d, 4 wk on, 2 wk off) (n = 386) or placebo (n = 382). Median PFS was 7.8 mo in the sunitinib plus FOLFIRI arm and 8.4 mo in the placebo plus FOLFIRI arm (HR = 1.095; P = 0.807), indicating a lack of superiority for sunitinib plus FOLFIRI. Median OS was 20.3 mo in the sunitinib arm and 19.8 mo in the placebo arm (HR = 1.171, one-sided stratified Log-rank P = 0.916). In addition, the ORR in the sunitinib arm failed to be significantly better than that in the placebo arm (32% vs 34%; P = 0.683). The study failed to demonstrate superiority for FOLFIRI plus sunitinib. Sunitinib plus FOLFIRI was associated with more grade ≥ 3 adverse events and laboratory abnormalities when compared to FOLFIRI plus placebo [neutropenia (68% s 30%), diarrhea (16% vs 8%), thrombocytopenia (11% vs 1%), anemia, stomatitis, fatigue, hand-foot syndrome and febrile neutropenia)]. In addition, more deaths as a result of toxicity (12 vs 4) and significantly more dose delays, dose reductions and treatment discontinuations occurred in the sunitinib arm[108].
A phase II, open-label, single-arm study (NCT00668863) investigated oral sunitinib (37.5 mg/d 4 wk on, 2 wk off) combined with intravenous FOLFIRI (every 2 wk) for the first-line treatment of Japanese patients with unresectable or metastatic CRC. Median PFS was 6.7 mo by independent review and 7.2 mo by investigator assessment. ORR was 36.6% by independent review and 42.3% by investigator assessment. There was a high incidence of adverse events such as neutropenia (97.2%), leukopenia (97.2%); thrombocytopenia (84.5%), diarrhea (78.9%), nausea (78.9%), decreased appetite (74.6%) and fatigue (66.2%). Furthermore, almost 20% of patients discontinued study treatment permanently, due to adverse events and over 90% of required temporary interruptions of study treatment to perform treatment for related toxicities. The study was closed early when the concurrent phase III study of first-line sunitinib plus FOLFIRI in non-Japanese patients with mCRC was stopped due to futility, as discussed previously.
Vatalanib (PTK 787/ZK 222584; PTK/ZK) is a potent, orally active angiogenesis inhibitor that interferes with the kinase activity of all three VEGF receptors, acting as a competitive inhibitor at the ATP-binding site of the receptor kinase. This inhibition is reversible, highly selective for VEGFRs and translates to growth inhibition in a variety of different experimental tumor models. Although tumor regression did not occur, an attenuation of tumor growth was observed[109].
In the clinical phase III trial CONFIRM1 (NCT00056459), 1168 patients with untreated mCRC were randomly assigned 1:1 to receive FOLFOX4 plus vatalanib or placebo. This study showed that the addition of vatalanib to FOLFOX4 did not improve PFS (7.7 mo in vatalanib arm and 7.6 mo in placebo arm: HR = 0.88, P = 0.118) or OS (21.4 mo in vatalanib arm and 20.5 mo in placebo arm: HR = 1.08, P = 0.260) and no statistically significant differences between the two treatment groups were observed in ORR (42% in vatalanib arm and 46% in placebo arm). Furthermore, vatalanib increased toxicity and more patients withdrew from treatment because of events other than disease progression in the vatalanib arm. Incidence of adverse event was 85.3% in vatalanib group and 77.5% in placebo group, particularly neutropenia, hypertension, and diarrhea. Concerning grade 3 or higher adverse events, the most notable differences were noted for hypertension (23.0% vs 6.8%, respectively), diarrhea (15.4% vs 11.1%, respectively), dizziness (7.4% vs 2.3%, respectively), and pulmonary embolism (5.7% vs 1.7%, respectively)[110].
The CONFIRM 2 (NCT00056446) was a phase III trial aimed to compare treatment with vatalanib plus FOLFOX4 versus placebo plus FOLFOX4 in patients with previously treated mCRC, whose disease had recurred or progressed during or within 6 mo of treatment with irinotecan in combination with a fluoropyrimidine. The median OS was 13.1 and 11.9 mo (HR = 1.00, P = 0.957). Median PFS was longer with vatalanib than with placebo (5.6 and 4.2 mo, respectively; HR = 0.83, P = 0.013). Treatment-related adverse events occurred in 81.4% patients in vatalanib arm and in 71% of those in placebo arm. The most common grade 3 or higher side effects related to vatalanib were neutropenia, hypertension, diarrhea, fatigue and nausea[111].
Nowadays, mCRC treatment remains a challenge for oncologists worldwide. Over last three decades, mCRC treatment has came from fluropirimidine based chemotherapy to the addition of innovative chemotherapies regimen combination, such as FOLFOX, FOLFIRI, XELOX, XELIRI, 5-FU + LV, and innovative biologic therapies, such as bevacizumab, cetuximab and panitumumab. More recently, Aflibercept was approved for combination with standard chemotherapy in second line regimens for mCRC patients. Therefore, many options are now available with a powerful capacity to improve survival for metastatic patients. Thus, we should be aware for those previous mentioned innovative opportunities to fit them for each patient according to the adequate indication and tolerability. Also, pharmaco-economic studies are warranted to provide useful tools for public health entities, which might allow better clinical decisions, especially when willing those advances in research.
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9875] [Article Influence: 759.6] [Reference Citation Analysis (5)] |
| 2. | Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3526] [Cited by in RCA: 3674] [Article Influence: 282.6] [Reference Citation Analysis (2)] |
| 3. | Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v70-v77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Lieberman D. Colorectal cancer screening: practice guidelines. Dig Dis. 2012;30 Suppl 2:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1973] [Cited by in RCA: 2085] [Article Influence: 148.9] [Reference Citation Analysis (3)] |
| 6. | Van Cutsem E, Nordlinger B, Cervantes A. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol. 2010;21 Suppl 5:v93-v97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 7. | Cersosimo RJ. Management of advanced colorectal cancer, part 2. Am J Health Syst Pharm. 2013;70:491-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Cersosimo RJ. Management of advanced colorectal cancer, Part 1. Am J Health Syst Pharm. 2013;70:395-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Bird NC, Mangnall D, Majeed AW. Biology of colorectal liver metastases: A review. J Surg Oncol. 2006;94:68-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Millikan KW, Staren ED, Doolas A. Invasive therapy of metastatic colorectal cancer to the liver. Surg Clin North Am. 1997;77:27-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 818] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 12. | Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 13. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7792] [Article Influence: 354.2] [Reference Citation Analysis (8)] |
| 14. | Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neurooncol. 2000;50:1-15. [PubMed] |
| 15. | Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687-698. [PubMed] |
| 16. | Bisht M, Dhasmana DC, Bist SS. Angiogenesis: Future of pharmacological modulation. Indian J Pharmacol. 2010;42:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Bhadada SV, Goyal BR, Patel MM. Angiogenic targets for potential disorders. Fundam Clin Pharmacol. 2011;25:29-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 2907] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 19. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5115] [Cited by in RCA: 5981] [Article Influence: 108.7] [Reference Citation Analysis (1)] |
| 20. | Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1666] [Cited by in RCA: 1773] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 21. | Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2218] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 22. | Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 897] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 23. | Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9-22. [PubMed] |
| 24. | Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947-11954. [PubMed] |
| 25. | Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1494] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 26. | Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 788] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 27. | Koh MY, Spivak-Kroizman TR, Powis G. HIF-1alpha and cancer therapy. Recent Results Cancer Res. 2010;180:15-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Poon E, Harris AL, Ashcroft M. Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev Mol Med. 2009;11:e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4764] [Cited by in RCA: 5119] [Article Influence: 222.6] [Reference Citation Analysis (0)] |
| 30. | Jüttner S, Wissmann C, Jöns T, Vieth M, Hertel J, Gretschel S, Schlag PM, Kemmner W, Höcker M. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006;24:228-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 840] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 32. | González RP, Leyva A, Melo RAB, Moreira RDM, Pessoa C, Farias RF, Moraes MO. Método para o estudo in vivo da angiogênese: indução de neovascularização na córnea de coelho. Acta cir bras. 2000;15:168-173. |
| 33. | Fan F, Wey JS, McCarty MF, Belcheva A, Liu W, Bauer TW, Somcio RJ, Wu Y, Hooper A, Hicklin DJ. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24:2647-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 261] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 34. | Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 1807] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 35. | Wang TB, Chen ZG, Wei XQ, Wei B, Dong WG. Serum vascular endothelial growth factor-C and lymphoangiogenesis are associated with the lymph node metastasis and prognosis of patients with colorectal cancer. ANZ J Surg. 2011;81:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, Zhang YF, Williams SP, Farnsworth RH, Chai MG. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012;21:181-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 37. | Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4480] [Cited by in RCA: 4604] [Article Influence: 170.5] [Reference Citation Analysis (0)] |
| 38. | Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2119] [Cited by in RCA: 2051] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 39. | Li JL, Harris AL. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Front Biosci (Landmark Ed). 2009;14:3094-3110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Yan M, Plowman GD. Delta-like 4/Notch signaling and its therapeutic implications. Clin Cancer Res. 2007;13:7243-7246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 224] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 42. | Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 297] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 43. | Oon CE, Harris AL. New pathways and mechanisms regulating and responding to Delta-like ligand 4-Notch signalling in tumour angiogenesis. Biochem Soc Trans. 2011;39:1612-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1417] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 45. | Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2548] [Cited by in RCA: 2555] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 46. | Valenzuela DM, Griffiths JA, Rojas J, Aldrich TH, Jones PF, Zhou H, McClain J, Copeland NG, Gilbert DJ, Jenkins NA. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci USA. 1999;96:1904-1909. [PubMed] |
| 47. | Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 300] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 48. | Ichihara E, Kiura K, Tanimoto M. Targeting angiogenesis in cancer therapy. Acta Med Okayama. 2011;65:353-362. [PubMed] |
| 49. | Katoh M, Nakagama H. FGF Receptors: Cancer Biology and Therapeutics. Med Res Rev. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 468] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 50. | Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1344] [Cited by in RCA: 1470] [Article Influence: 70.0] [Reference Citation Analysis (7)] |
| 51. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 7049] [Article Influence: 306.5] [Reference Citation Analysis (0)] |
| 52. | Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell Cycle. 2009;8:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 53. | Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 744] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 54. | Lu X, Kang Y. Epidermal growth factor signalling and bone metastasis. Br J Cancer. 2010;102:457-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 585] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 56. | Joudeh J, Allen JE, Das A, Prabhu V, Farbaniec M, Adler J, El-Deiry WS. Novel antineoplastics targeting genetic changes in colorectal cancer. Adv Exp Med Biol. 2013;779:1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 799] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 58. | Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol. 2005;23:9441-9442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 59. | Gordon MS, Margolin K, Talpaz M, Sledge GW, Holmgren E, Benjamin R, Stalter S, Shak S, Adelman D. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843-850. [PubMed] |
| 60. | Braghiroli MI, Sabbaga J, Hoff PM. Bevacizumab: overview of the literature. Expert Rev Anticancer Ther. 2012;12:567-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 61. | Yeung Y, Tebbutt NC. Bevacizumab in colorectal cancer: current and future directions. Expert Rev Anticancer Ther. 2012;12:1263-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1209] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 63. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2298] [Article Influence: 127.7] [Reference Citation Analysis (0)] |
| 64. | Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, van Hazel GA, Robinson B, Broad A, Ganju V, Ackland SP. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28:3191-3198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 327] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 65. | Peeters M, Price T, Van Laethem JL. Anti-epidermal growth factor receptor monotherapy in the treatment of metastatic colorectal cancer: where are we today? Oncologist. 2009;14:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (4)] |
| 66. | Broadbridge VT, Karapetis CS, Price TJ. Cetuximab in metastatic colorectal cancer. Expert Rev Anticancer Ther. 2012;12:555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 67. | Baselga J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist. 2002;7 Suppl 4:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 348] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 68. | Tabernero J, Van Cutsem E, Díaz-Rubio E, Cervantes A, Humblet Y, André T, Van Laethem JL, Soulié P, Casado E, Verslype C. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2007;25:5225-5232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 246] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 69. | Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1250] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 70. | Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 586] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 71. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2901] [Cited by in RCA: 3159] [Article Influence: 185.8] [Reference Citation Analysis (6)] |
| 72. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1452] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 73. | Tveit K, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, Kure E, Ikdahl T, Skovlund E, Christoffersen T. Randomized phase III study of 5-fluorouracil/folinate/oxaliplatin given continuously or intermittently with or without cetuximab, as first-line treatment of metastatic colorectal cancer: the NORDIC VII Study (NCT00145314). Ann Oncol. 2010;21 Suppl 8:Abstr LBA20. |
| 74. | Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 766] [Cited by in RCA: 768] [Article Influence: 51.2] [Reference Citation Analysis (2)] |
| 75. | Foon KA, Yang XD, Weiner LM, Belldegrun AS, Figlin RA, Crawford J, Rowinsky EK, Dutcher JP, Vogelzang NJ, Gollub J. Preclinical and clinical evaluations of ABX-EGF, a fully human anti-epidermal growth factor receptor antibody. Int J Radiat Oncol Biol Phys. 2004;58:984-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 76. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1413] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 77. | Cartwright TH. Treatment decisions after diagnosis of metastatic colorectal cancer. Clin Colorectal Cancer. 2012;11:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 78. | Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 640] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 79. | Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 1009] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 80. | Nordlinger B, Van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P, Sobrero A, Ychou M. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 271] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 81. | Heinrich S, Lang H. Liver metastases from colorectal cancer: technique of liver resection. J Surg Oncol. 2013;107:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Ismaili N. Treatment of colorectal liver metastases. World J Surg Oncol. 2011;9:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 83. | Juez I, Rubio C, Figueras J. Multidisciplinary approach of colorectal liver metastases. Clin Transl Oncol. 2011;13:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 84. | Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393-11398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1269] [Cited by in RCA: 1289] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 85. | Rudge JS, Holash J, Hylton D, Russell M, Jiang S, Leidich R, Papadopoulos N, Pyles EA, Torri A, Wiegand SJ. VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci USA. 2007;104:18363-18370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 86. | Pericay C, Folprecht G. Phase 2 randomized, noncomparative open-label study of aflibercept and Modified FOLFOX6 in the first line treatment of metastatic colorectal cancer (AFFIRM). Ann Oncol. 2012;23:O-0024. |
| 87. | Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 1054] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 88. | Allegra CJ, Lakomy R, Tabernero J, Prausová J, Ruff P, Van Hazel G, Moiseyenko VM, Ferry DR, McKendrick JJ, Van Cutsem E. Effects of prior bevacizumab (B) use on outcomes from the VELOUR study: A phase III study of aflibercept (Afl) and FOLFIRI in patients (pts) with metastatic colorectal cancer (mCRC) after failure of an oxaliplatin regimen. J Clin Oncol. 2012;30 (Suppl): 3505. |
| 89. | Bhide RS, Cai ZW, Zhang YZ, Qian L, Wei D, Barbosa S, Lombardo LJ, Borzilleri RM, Zheng X, Wu LI. Discovery and preclinical studies of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5- methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan- 2-ol (BMS-540215), an in vivo active potent VEGFR-2 inhibitor. J Med Chem. 2006;49:2143-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 90. | Siu LL, Shapiro JD, Jonker DJ, Karapetis CS, Zalcberg JR, Simes J, Couture F, Moore MJ, Price TJ, Siddiqui J. Phase III randomized, placebo-controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal carcinoma: the NCIC Clinical Trials Group and AGITG CO.20 Trial. J Clin Oncol. 2013;31:2477-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 91. | Ayers M, Dito G, Henley B, Jeyaseelan R, Yoganthan S, Han X, Wu Q, Platero S, Wu S, Feltquate D. Comparison of a dual inhibitor of VEGF and FGF signaling, BMS-582664, to the activity of bevacizumab, an inhibitor exclusively of VEGF signaling, in xenograft models of colon carcinoma. Proc Am Assoc Cancer Res. 2007;48:Abstract 1618. |
| 92. | Chou T, Finn RS. Brivanib: a review of development. Future Oncol. 2012;8:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | Garrett CR, Siu LL, El-Khoueiry A, Buter J, Rocha-Lima CM, Marshall J, LoRusso P, Major P, Chemidlin J, Mokliatchouk O. Phase I dose-escalation study to determine the safety, pharmacokinetics and pharmacodynamics of brivanib alaninate in combination with full-dose cetuximab in patients with advanced gastrointestinal malignancies who have failed prior therapy. Br J Cancer. 2011;105:44-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389-4400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 576] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 95. | Lu D, Jimenez X, Zhang H, Bohlen P, Witte L, Zhu Z. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer. 2002;97:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 96. | Spratlin J. Ramucirumab (IMC-1121B): Monoclonal antibody inhibition of vascular endothelial growth factor receptor-2. Curr Oncol Rep. 2011;13:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 97. | Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 1049] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 98. | Strumberg D, Schultheis B. Regorafenib for cancer. Expert Opin Investig Drugs. 2012;21:879-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 99. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2213] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 100. | Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99-106. [PubMed] |
| 101. | Mendel DB, Laird AD, Smolich BD, Blake RA, Liang C, Hannah AL, Shaheen RM, Ellis LM, Weitman S, Shawver LK. Development of SU5416, a selective small molecule inhibitor of VEGF receptor tyrosine kinase activity, as an anti-angiogenesis agent. Anticancer Drug Des. 2000;15:29-41. [PubMed] |
| 102. | Strumberg D. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Drugs Today (Barc). 2005;41:773-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 103. | Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, Wilhelm SM, Santoro M. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 390] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 104. | Tabernero J, Garcia-Carbonero R, Cassidy J, Sobrero A, Van Cutsem E, Köhne CH, Tejpar S, Gladkov O, Davidenko I, Salazar R. Sorafenib in combination with oxaliplatin, leucovorin, and fluorouracil (modified FOLFOX6) as first-line treatment of metastatic colorectal cancer: the RESPECT trial. Clin Cancer Res. 2013;19:2541-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 105. | Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327-337. [PubMed] |
| 106. | O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597-3605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 673] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 107. | Murray LJ, Abrams TJ, Long KR, Ngai TJ, Olson LM, Hong W, Keast PK, Brassard JA, O’Farrell AM, Cherrington JM. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis. 2003;20:757-766. [PubMed] |
| 108. | Carrato A, Swieboda-Sadlej A, Staszewska-Skurczynska M, Lim R, Roman L, Shparyk Y, Bondarenko I, Jonker DJ, Sun Y, De la Cruz JA. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: a randomized, phase III trial. J Clin Oncol. 2013;31:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 109. | Hess-Stumpp H, Haberey M, Thierauch KH. PTK 787/ZK 222584, a tyrosine kinase inhibitor of all known VEGF receptors, represses tumor growth with high efficacy. Chembiochem. 2005;6:550-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 110. | Hecht JR, Trarbach T, Hainsworth JD, Major P, Jäger E, Wolff RA, Lloyd-Salvant K, Bodoky G, Pendergrass K, Berg W. Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol. 2011;29:1997-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 111. | Van Cutsem E, Bajetta E, Valle J, Köhne CH, Hecht JR, Moore M, Germond C, Berg W, Chen BL, Jalava T. Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol. 2011;29:2004-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
P- Reviewers: Cejas P, Nayak TK S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Zhang DN