Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7751
Revised: May 13, 2013
Accepted: July 17, 2013
Published online: November 21, 2013
Processing time: 244 Days and 10 Hours
AIM: To investigate whether transanal natural orifice specimen extraction (NOSE) is a better technique for rectal cancer resection.
METHODS: A prospectively designed database of a consecutive series of patients undergoing laparoscopic low anterior resection for rectal cancer with various tumor-node-metastasis classifications from March 2011 to February 2012 at the First Affiliated Hospital of Sun Yat-Sen University was analyzed. Patient selection for transanal specimen extraction and intracorporeal anastomosis was made on the basis of tumor size and distance of rectal lesions from the anal verge. Demographic data, operative parameters, and postoperative outcomes were assessed.
RESULTS: None of the patients was converted to laparotomy. Respectively, there were 16 cases in the low anastomosis and five in the ultralow anastomosis groups. Mean age of the patients was 45.4 years, and mean body mass index was 23.1 kg/m2. Mean distance of the lower edge of the lesion from the anal verge was 8.3 cm. Mean operating time was 132 min, and mean intraoperative blood loss was 84 mL. According to the principle of rectal cancer surgery, we performed D2 lymph node dissection in 13 cases and D3 in eight. Mean lymph nodes harvest was 17.8, and the number of positive lymph nodes was 3.4. Median hospital stay was 6.7 d. No serious postoperative complication occurred except for one anastomotic leakage. All patients remained disease free. Mean Wexner score was 3.7 at 11 mo after the operation.
CONCLUSION: Transanal NOSE for total laparoscopic low/ultralow anterior resection is feasible, safe and oncologically sound. Further studies with long-term outcomes are needed to explore its potential advantages.
Core tip: Natural orifice specimen extraction (NOSE) is an emerging technique that has been recently applied to the field of rectal cancer resection. However, which is the better approach for rectal cancer remains controversial. In this paper, we present our surgical technique and short-term outcomes of transanal NOSE in total laparoscopic low/ultralow anterior resection (L-AR) for patients with rectal cancer. Based on our limited experience, transanal NOSE in L-AR for rectal cancer is feasible, safe and oncologically sound.
- Citation: Han FH, Hua LX, Zhao Z, Wu JH, Zhan WH. Transanal natural orifice specimen extraction for laparoscopic anterior resection in rectal cancer. World J Gastroenterol 2013; 19(43): 7751-7757
- URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7751.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7751
The incidence of rectal cancer is higher in Asia compared with western countries[1]. Technically, resection of low rectal cancer may be the most difficult among all colorectal operations.
At present, traditional colorectal surgery has increasingly given way to laparoscopic anterior resection with total mesorectal excision (L-AR/TME). Evidence-based medicine has established that L-AR/TME is a feasible surgical approach for managing rectal cancer. There were similar results in recent short-term therapeutic effects, local recurrence rate and postoperative survival rate between laparoscopic surgery (LS) and traditional open surgery for radical colon cancer[2]. Meanwhile, the mean estimated blood loss, discharge time after operation, and postoperative hospital stay were significantly reduced in the LS[3]. However, incision of the abdomen is still necessary in order to remove the specimens in LS, which could cause incision infection and increase the incidence of incisional hernia[4].
Natural orifice specimen extraction (NOSE) may be an effective way to address the challenge. NOSE is feasible and safe technically for radical colorectal cancer surgery by traditional laparoscopic techniques, and for removal of the specimens through a natural orifice[5,6]
Total laparoscopic hemicolectomy has been performed successfully by transvaginal NOSE[7]. However, due to its innate limitations, transvaginal NOSE is difficult for radical rectal cancer surgery, especially in low rectal cancer. Here, we introduce a technique used for the laparoscopic radical rectal surgery with TME, and the specimen was removed and anastomosis was accomplished through the anus.
This study was approved by the ethics committee of the hospital and written informed consent was obtained from the patients. Twenty-one patients with rectal adenocarcinoma underwent the procedure from March 2011 to February 2012 (Table 1). All patients with preoperative diagnosis of rectal cancer were confirmed by endoscopic colonoscopy, pathology, endosonography, and staged by specialized oncologists at our hospital, and preoperatively managed following the guidelines of the National Comprehensive Cancer Network (NCCN). All operations were performed by a single surgeon who was proficient in various laparoscopic colorectal procedures and laparotomy at our hospital.
| Patient demographic data | Value |
| Age (yr) | 45.4 ± 3.6 |
| Body mass index | 23.1 ± 2.8 |
| Sex (male/female ratio) | 12/9 |
| Mean wexner score | 3.7 ± 1.6 |
These patients with tumor stage T4, tumor covering over half of the circumference of the rectum, metastasis in the liver or lungs on preoperative imaging assessment, or body mass index > 28 were excluded.
Three patients whose tumor-node-metastasis classification of T3 was confirmed by endosonography, magnetic resonance imaging (MRI) and computed tomography (CT) received three cycles of chemotherapy prior to surgery. Radiotherapy followed by resection was conducted based on the national guidelines. The feasibility of the surgery was reappraised at 2 wk after the treatment. All three patients had symptom relief, the tumor was reduced in size, and there were limited side effects of neoadjuvant chemotherapy.
The day before the operation, all patients underwent systemic bowel preparation, and used prophylactic antibiotics.
Laparoscopic phase. The patient was positioned in a modified lithotomy position, and the abdomen was then insufflated with 10-12 mmHg CO2. Four ports were used in the following procedure. The first port was a 12-mm blunt-tip for the laparoscope, which was placed in the umbilicus using the minilaparotomy technique. The second to fourth ports were 10-mm operating ports in the right lower quadrant, and two 5-mm ports in the right middle abdomen and left lower quadrant, respectively.
Colon mobilization, lymph node dissection, and mesenteric excision were performed laparoscopically in the usual manner. First, the sacral promontory was separated by ultrasonic scalpel (Harmonic ACE; Ethicon Endo-Surgery, OH, United States) from the right side of the rectum. Second, before the tumor was mobilized, the inferior mesenteric artery was ligated at its point of pedicle from the aorta with a large or oversized Hem-o-lock. The I-III branches of artery and vein of sigmoid were cut off while the marginal artery of the proximal colon was preserved. Next, the inferior mesenteric vein was ligated at the corresponding height to the artery. We mobilized the splenic flexure in two patients because there were existing tensions in the anastomosis. Third, the posterior mesorectal fascia was identified and the dissection was extended to the level of the sacral promontory in the avascular plane. The rectum was fully dissociated to the levator ani muscle plane as far as possible along the Denonvillier’s fascia. The fragment of the distal rectum that was located 2 cm above the tumor was clamped with a detachable clip.
We preserved the inferior hypogastric nerves as far as possible during the procedure.
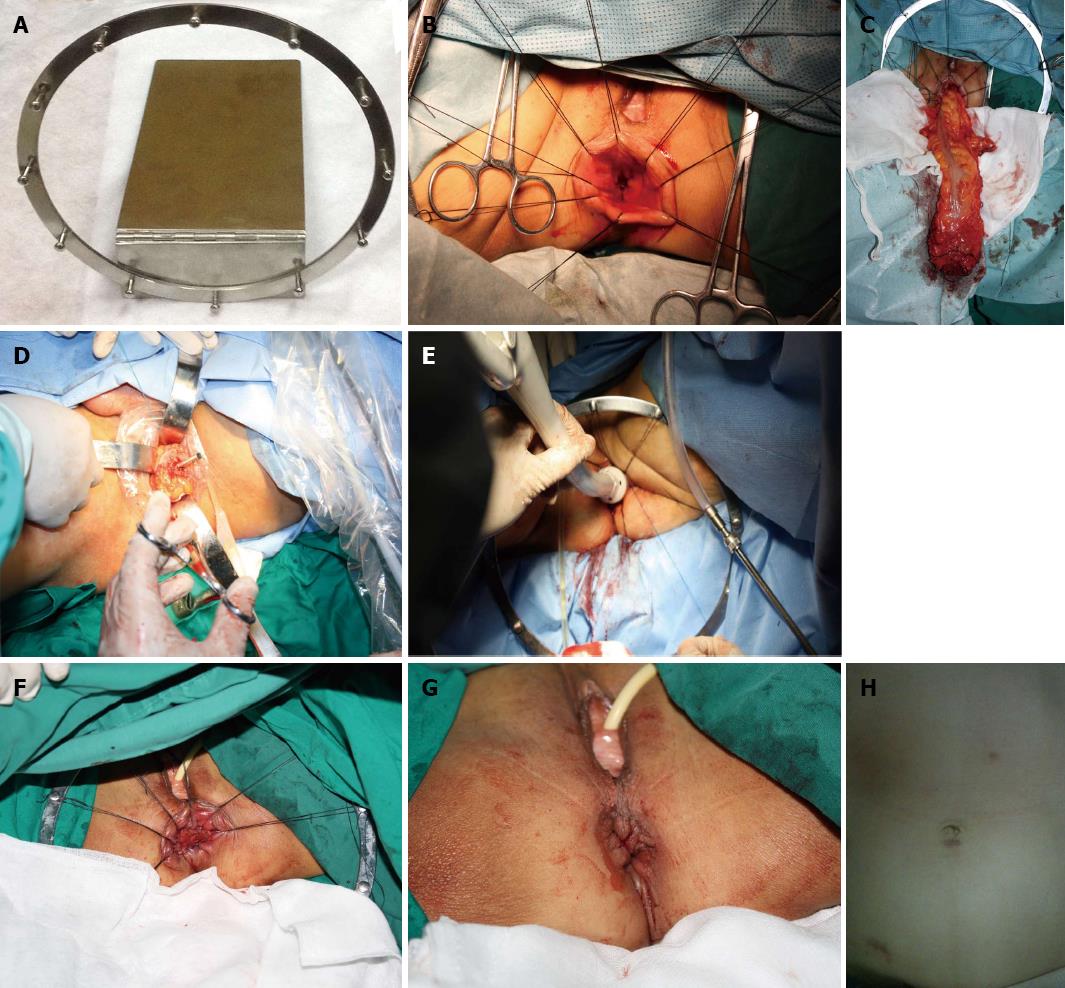
Perineal phase. The anus was dilated gently until it could accommodate four fingers. A home-made anus dilator and fine silk traction sutures were placed into the proximal lip of the exposed mucosal edge at a vertical orientation, in order to expand the anus and expose the rectum (Figure 1A and B). The level of intended transection had to maintain a margin of 2 cm distal from the tumor[8]. After irrigating the rectum with 1 L diluted povidone-iodine solution, we sutured two parallel circle-purse-strings in the distal rectal wall with 2-0 prolene lines through the dilated anus. The upper one maintained a minimal margin of 1-1.5 cm distal from the tumor, while the lower was located in the rectal mucosa at 1 cm above the dentate line. Between the two circle-purse-strings, full-thickness rectal circumferential dissection was extended by ultrasonic scalpel. At this point, the peritoneal cavity was extended circumferentially cephalad as far as possible, and then joined the perineal and laparoscopic dissection planes.
The stump of the proximal rectosigmoid was exteriorized through the dilated anus and opened stump of the distal rectum (Figure 1C). After clamping with purse string forceps, the section of proximal colon, which had to be maintained at a minimal margin of 10 cm above the tumor, was transected under direct vision. After purse-string suturing, an anvil shaft was placed into the stump of the proximal colon, then it was pushed gently back into the peritoneal cavity (Figure 1D). The purse-string suture was tied to the anvil shaft before connecting it to the central shaft of the circular stapler (CDH 29; Ethicon Endo-Surgery, OH, United States). After tightening the lower circle-purse string, the anastomosis was placed into the anus (Figure 1E). Then the anastomat was fired to create an end-to-end coloanal anastomosis in the usual manner[9]. An air test was conducted through the anus. The stitching was reinforced by bioabsorbable suture if necessary. A pelvic drain was inserted.
We performed the procedure successfully in 16 patients. Due to the low position of the stump of the distal rectum, we performed manual anastomosis and protective loop ileostomy in 5 patients (Figure 1F).
The negative margins were confirmed in all patients by intraoperative frozen biopsy. The mesorectal integrity[10] and circumferential situation[11] of the resected specimens were evaluated by a senior surgeon and qualified pathologist macroscopically and microscopically, in order to ensure that the tumor had been resected completely. The status of the mesorectal specimens was graded into three categories. We differentiated them as complete (intact mesorectum > 5 cm, while defect of mesentery < 5 mm); nearly complete (intact mesorectum > 5 cm, while defect of mesentery > 5 mm); and incomplete (incomplete mesorectum). We defined a positive margin if the circumferential margin from the tumor was < 2 mm under microscopy.
We successfully performed the procedure in all 21 patients, and none of them was converted to laparotomy (Table 2). There were 16 and five patients in the low or ultralow anastomosis groups, respectively. According to macroscopic specimen assessment of TME, the status was complete for 18 patients, while nearly complete for three patients. In addition, the circumferential resection margin was negative in all patients (Table 3).
| Intraoperative information | Value |
| Mean operation time (min) | 132 ± 85 |
| Mean intraoperative blood loss( mL) | 84 ± 15 |
| Mean tumor diameter (cm) | 4.6 ± 1.7 |
| Distance of lesion from anal verge (cm) | 8.3 ± 3.5 |
| Protective ileostomy | 5 (23.8) |
| Defecation time after operation (d) | 2.5 ± 1.4 |
| Items | Number of cases |
| Pathological diagnoses | |
| Well differentiated | 10 |
| Poorly differentiated | 7 |
| Myxoadenocarcinoma | 4 |
| Specimen macro-assessment of TME | 21 (radical resection) |
| Circumferential resection margin | 21 (Negative) |
| Postoperative pathology staging (TNM) | |
| T1-4N0M0 | 7 |
| T1-2N1M0 | 5 |
| T3-4N1M0 | 6 |
| T3-4N2M0 | 3 |
| Lymph nodes harvest (mean) | 17.8 ± 4.6 |
| Metastatic lymph nodes (mean) | 3.4 ± 1.8 |
The mean maximum tumor diameter was 4.6 ± 1.7 cm. According to the principle of rectal cancer surgery and no-touch isolation technique, we performed D2 lymph node dissection in 13 patients and D3 dissection in eight patients. The postoperative course was unremarkable in most patients, with prompt return of bowel activity and short postoperative stay, except for one patient who was complicated by anastomotic leakage (Table 4). Anastomotic leakage was confirmed by stools leaking from a drain. He was treated with nil by mouth, decompression of the rectum by transanal drainage, and antibiotic infusion until the leak healed spontaneously. He was discharged on the postoperative day 15.
| Length of hospitalization (d) | 6.1 ± 2.7 |
| Postoperative complications | |
| UTI | 2 (9.5) |
| Anastomotic leakage | 1 (4.7) |
| Anastomotic bleeding | 0 |
| Incision infection | 0 |
| Intestinal obstruction | 0 |
| Impotence | 1 (4.7) |
| Fecal incontinence | 0 |
| Anal stenosis | 0 |
| Total | 4 (18.9) |
According to the guidelines of NCCN, all patients with T3/T4 or postoperative node-positive tumors underwent postoperative chemotherapy for 6-9 cycles. The follow-up period ranged from 11 to 23 mo. Follow-up examinations were scheduled at 2 wk and 1, 2, 3, 6, 9 and 12 mo, and every 6 mo thereafter until 5 years. All patients underwent CT of the chest, abdomen, and pelvis every 6 mo and colonoscopy at 12 mo, but remained disease free. All five patients who had handsewn coloanal anastomosis with a diverting ileostomy had their ileostomies reversed at 3-6 mo after the operation, based on the diagnosis of free from tumor recurrence and anastomotic stenosis, which were confirmed by endoscopic colonoscopy, barium enema, MRI, and CT. Anal continence was measured with the validated Wexner fecal incontinence scoring system (0 = perfect continence, 20 = complete incontinence). The mean Wexner score was 3.7 (range 0-5) at > 11 mo after the operation.
In the past 10 years, L-AR has been performed at our hospital according to the principle of TME for patients with low rectal cancer. Traditional large abdominal incision has been replaced gradually by small abdominal incision. L-AR benefits patients not only in terms of cosmetics and postoperative rehabilitation, but also in reducing surgical interference, maintaining immune function and homeostasis, rapid recovery, and relieving psychological stress after surgery. However, L-AR is still considered imperfect due to the requirement for abdominal incision of 5-7 cm at minimum to remove the specimen completely. There are still some complications, such as abdominal incision infection, postoperative somatic pain, and incisional hernia[12]. According to bulk analysis of cases, wound infections occurred in 13.5% of patients after L-AR (2.7% trocar and 10.8% extraction site), and incisional hernias developed in 24.3%, and extraction sites accounted for 85.7% of all wound complications[13].
In order to reduce the impact of L-AR incision and eliminate abdominal incision completely, natural orifice transluminal endoscopic surgery (NOTES) has increased in recent years, which can avoid incisional infection and hernia, and achieve better cosmetic results[14,15].
Recently, transvaginal (posterior fornix incision) has been the main approach of NOTES in most colectomy procedures[16-18]. However, there are still some negative factors in low/ultralow rectal cancer which hinder the application of transvaginal approach NOTES. First, there are technical shortcomings, such as lack of experience and technical complexity, additional adjacent organ injury, extended operation time, and specialized equipment requirement, which account for the increased cost of the operation. Second, it is sometimes difficult to remove a larger tumor specimen through the posterior vaginal fornix incision. Third, there are many technical difficulties in achieving sphincter preservation for low/ultralow rectal cancer by the transvaginal approach. Finally, the transvaginal approach is obviously limited to female patients, which is a major hindrance for widespread use of the technique in clinical practice.
As a result, more surgeons have been trying to find new approaches for NOTES in low rectal cancer. With regard to the applicability of NOTES in colorectal surgery, the transanal access route of NOTES is intuitively the optimal one. First, rather than creating an opening through an otherwise healthy organ to perform the rectal anterior resection, enterotomy is carried out on the diseased organ itself. Second, the enterotomy is ultimately closed by incorporating it into a standard colorectal anastomosis, which is the requirement of surgery regardless of whether it is achieved via NOTES or standard surgery. Finally, transanal NOTES could have substantial benefits over standard transabdominal approaches[19].
At present, transanal access NOTES in radical colorectal cancer surgery has been completed successfully in animal models, but few surgeons have put it into clinical practice due to potential technical difficulties, such as intra-abdominal intestinal fecal contamination, or increased possibility of infection through the colon lumen. All of these factors may affect the safety of the procedure. For example, clinical reports have confirmed that common complications included wound infection (56.7%), septicemia (31.7%), and enterocutaneous fistula (16.7%) in patients who sustained penetrating colon injuries[20]. However, with the improvement in anatomical techniques and equipment, transanal NOTES has been performed for resection of the rectum in pig models in vivo or fresh cadavers[21], as well as laparoscopy-assisted transanal NOTES for left-sided colorectal resection[22] and sigmoidectomy[23]. Unfortunately, these techniques require expensive equipment, which limits the clinical application of NOTES, especially in developing countries.
As a development of NOTES, transanal NOSE is an emerging technique that has been recently applied to the field of rectal excision. Darzi et al[24] have described a technique of total laparoscopic left-sided colonic resection and transanal specimen delivery. Franklin et al[25] have reported that laparoscopic colectomy in patients with stage III colorectal cancer is oncologically adequate. Fukunaga et al[26] have performed radical rectal cancer surgery with removal of the specimens through the anus, thus avoiding abdominal incision. Transanal specimen extraction can also resolve the problems found in obese patients with short or hypertrophic mesentery, or deep abdominal wall, which have been challenges for transabdominal specimen removal[27]. It has been confirmed that transanal NOSE is technically feasible. It may be a bridge between NOTES and the conventional laparoscopic approach for radical colorectal cancer surgery.
Our current experience showed that transanal NOSE, combined with TME and L-AR techniques for rectal cancer, could be adapted for radical tumor resection and minimally invasive surgery. Its technical feasibility and oncological principles have been demonstrated by many surgeons[28]. The rectal stump is a “necessary” trauma. We can accurately determine the distal cutting edge of the rectum through the full use the rectal stump. Combination of traditional laparoscopic techniques and removal of specimens through a natural orifice can minimize surgical injury[29]. Traditional laparoscopic surgical techniques provide a large operating space, mature technology and broad vision, which allows one to dissect accurately the mesorectal, pelvic visceral and parietal fascia. We can ensure that the inferior mesenteric artery is ligated at the root, in order to block the tumor blood supply and venous drainage, and minimize the chance of metastasis. Care is required to avoid any injury to the mesenteric arcades so as to guarantee an adequate blood supply to the descending colon. The operation was carried out following the “holy plane”, which is placed between the pelvic visceral fascia and rectal fascia propria, and then to the anterior Denonvillier’s fascia. The mesorectum should be completely mobilized while the pelvic autonomic nerve is preserved.
After the anus was fully dilated, we used a home-made anal dilator and fine silk traction sutures to evert the anus and expose the rectum, then placed a protective bag into the anus. In the premise of protecting blood supply of the residual colon, the pre-cut specimen was fully freed in the peritoneal cavity, then gently pulled out through the anus.
We paid attention to protecting the functions of anal sphincter while performing a standard radical resection of rectal cancer. Even if the specimen is a relatively large one, for example, the hypertrophic mesorectum, it can be removed smoothly from the fully dilated anus routinely without tearing the rectum or damaging the anal sphincter. The anus and rectum can be returned to their normal diameter after the operation (Figure 1H).
When the stump of the proximal rectum was exteriorized through the dilated anus and opened stump of the distal rectum, we transected the proximal colorectum under direct vision[30]. After intracorporeal purse-string sutures with 2/0 prolene, we used an anastomat to create an end-to-end coloanal anastomosis in the usual manner. Although some studies have found that the J-pouch is superior to end-to-end reconstruction for low rectal cancer[9,31], the latter resulted in acceptable anal function at 6 mo follow-up in our study, due to the careful protection of the anal sphincter, with no tension and a good blood supply in the anastomotic stoma.
In order to prevent peritoneal seeding and trocar-site metastasis, we implemented the general rules for laparoscopic surgery, such as the no-touch technique, appropriate resection margins, early bagging of the resected specimen, and wound protection into our laparoscopic colorectal procedures. Compared to the traditional laparoscopic techniques, our technique had good cosmetic results and reduced the chance of metastasis in the abdominal wall, without increasing complications[32].
Laurent et al[33] reported that the conversion rate of laparoscopic radical resection for low rectal cancer was 15.5%. The conversion rate was higher due to the difficulties experienced in fixing colorectal, separating in the pelvic, unexpected intraoperative bleeding, and failure of the closure device, or anastomosis. However, such difficulties did not jeopardize our treatment due to the elasticity and compliance of the tissue while we used mature laparoscopic techniques to remove the specimens through the anus. With full use of the natural orifice of the anus and rectum, total laparoscopic rectal resection is feasible and safe. Such a technique decreases the abdominal surgery complications, and maintains the operation time and the cost of surgery to those of standard L-AR. It also provides significant improvement of the traditional laparoscopic techniques.
However, the present surgical indications are limited to patients with early cancer. Mesorectal invasion and tumor diameter > 6 cm are not included here due to the lack of a large randomized controlled study for this procedure. The operation field is narrowed and the vision is not clear through the anal approach in some conditions, such as a narrow pelvis or large tumor. Although there are reports of microsurgical resection through the anus[34], there is no specialized surgical instrument to complete the procedures for anus dilation, specimen removal, and distal suturing. There is urgency to develop better-adapted tools such as a modified flexible transanal endoscopic platform, longer and more flexible dissecting instruments, staplers and hemostatic devices to permit safe completion of these procedures without any transabdominal assistance. This technique requires further regulation and improvement.
In our limited experience, transanal specimen extraction in total laparoscopic low/ultralow anterior resection is feasible, safe, and oncologically sound for selected cases. The majority of patients have an acceptable functional outcome. Further studies with long-term outcomes are needed to explore the potential advantages of this technique.
The incidence of rectal cancer is higher in Asia compared with western countries. Technically, the resection of low rectal cancer may be one of the most difficult among all colorectal surgery procedures.
At present, traditional colorectal surgery has increasingly given way to laparoscopic anterior resection with total mesorectal excision (L-AR/TME). Evidence-based medicine has established that L-AR/TME is a feasible surgical approach for managing rectal cancer. There have been similar results recently for short-term therapeutic effects, local recurrence rate, and postoperative survival rate between laparoscopic surgery and traditional open surgery for radical colon cancer.
This study showed that transanal specimen extraction by total laparoscopic low/ultralow anterior resection is feasible, safe, and oncologically sound for selected cases. The majority of patients had an acceptable functional outcome.
There is an urgency to develop better adapted tools such as a modified flexible transanal endoscopic platform, longer and more flexible dissecting instruments, and staplers and hemostatic devices to permit safe completion of these procedures without any transabdominal assistance. This technique needs further standardization and improvement.
This study was interesting and highly innovative in terms of colorectal surgical technique, especially for a surgical rather than gastroenterological audience.
| 1. | Kim DH, Shin MH, Ahn YO. Incidence pattern of colorectal cancer in Korea by subsite of origin. J Korean Med Sci. 2000;15:675-681. [PubMed] |
| 2. | Lee JE, Joh YG, Yoo SH, Jeong GY, Kim SH, Chung CS, Lee DG, Kim SH. Long-term Outcomes of Laparoscopic Surgery for Colorectal Cancer. J Korean Soc Coloproctol. 2011;27:64-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Liberman MA, Phillips EH, Carroll BJ, Fallas M, Rosenthal R. Laparoscopic colectomy vs traditional colectomy for diverticulitis. Outcome and costs. Surg Endosc. 1996;10:15-18. [PubMed] |
| 4. | Vestweber B, Galetin T, Lammerting K, Paul C, Giehl J, Straub E, Kaldowski B, Alfes A, Vestweber KH. Single-incision laparoscopic surgery: outcomes from 224 colonic resections performed at a single center using SILS. Surg Endosc. 2013;27:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Whiteford MH, Spaun GO. A colorectal surgeons viewpoint on natural orifice translumenal endoscopic surgery. Minerva Chir. 2008;63:385-388. [PubMed] |
| 6. | Takayama S, Hara M, Sato M, Takeyama H. Hybrid natural orifice transluminal endoscopic surgery for ileocecal resection. World J Gastrointest Surg. 2012;4:41-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Diana M, Perretta S, Wall J, Costantino FA, Leroy J, Demartines N, Marescaux J. Transvaginal specimen extraction in colorectal surgery: current state of the art. Colorectal Dis. 2011;13:e104-e111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Ueno H, Mochizuki H, Hashiguchi Y, Ishikawa K, Fujimoto H, Shinto E, Hase K. Preoperative parameters expanding the indication of sphincter preserving surgery in patients with advanced low rectal cancer. Ann Surg. 2004;239:34-42. [PubMed] |
| 9. | Wexner SD. Restorative proctectomy with colon pouch-anal anastomosis by laparoscopic transanal pull-through: an available option for low rectal cancer? Surg Endosc. 2007;21:1679. [PubMed] |
| 10. | García-Granero E, Faiz O, Muñoz E, Flor B, Navarro S, Faus C, García-Botello SA, Lledó S, Cervantes A. Macroscopic assessment of mesorectal excision in rectal cancer: a useful tool for improving quality control in a multidisciplinary team. Cancer. 2009;115:3400-3411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Wiggers T, van de Velde CJ. The circumferential margin in rectal cancer. Recommendations based on the Dutch Total Mesorectal Excision Study. Eur J Cancer. 2002;38:973-976. [PubMed] |
| 12. | Ihedioha U, Mackay G, Leung E, Molloy RG, O’Dwyer PJ. Laparoscopic colorectal resection does not reduce incisional hernia rates when compared with open colorectal resection. Surg Endosc. 2008;22:689-692. [PubMed] |
| 13. | Winslow ER, Fleshman JW, Birnbaum EH, Brunt LM. Wound complications of laparoscopic vs open colectomy. Surg Endosc. 2002;16:1420-1425. [PubMed] |
| 14. | Gettman MT, Lotan Y, Napper CA, Cadeddu JA. Transvaginal laparoscopic nephrectomy: development and feasibility in the porcine model. Urology. 2002;59:446-450. [PubMed] |
| 15. | Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117. [PubMed] |
| 16. | Gettman MT, Blute ML. Transvesical peritoneoscopy: initial clinical evaluation of the bladder as a portal for natural orifice translumenal endoscopic surgery. Mayo Clin Proc. 2007;82:843-845. [PubMed] |
| 17. | Lacy AM, Delgado S, Rojas OA, Almenara R, Blasi A, Llach J. MA-NOS radical sigmoidectomy: report of a transvaginal resection in the human. Surg Endosc. 2008;22:1717-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Wilson JI, Dogiparthi KK, Hebblethwaite N, Clarke MD. Laparoscopic right hemicolectomy with posterior colpotomy for transvaginal specimen retrieval. Colorectal Dis. 2007;9:662. [PubMed] |
| 19. | Dozois EJ, Larson DW, Dowdy SC, Poola VP, Holubar SD, Cima RR. Transvaginal colonic extraction following combined hysterectomy and laparoscopic total colectomy: a natural orifice approach. Tech Coloproctol. 2008;12:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Adesanya AA, Ekanem EE. A ten-year study of penetrating injuries of the colon. Dis Colon Rectum. 2004;47:2169-2177. [PubMed] |
| 21. | Bhattacharjee HK, Buess GF, Becerra Garcia FC, Storz P, Sharma M, Susanu S, Kirschniak A, Misra MC. A novel single-port technique for transanal rectosigmoid resection and colorectal anastomosis on an ex vivo experimental model. Surg Endosc. 2011;25:1844-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Costantino FA, Diana M, Wall J, Leroy J, Mutter D, Marescaux J. Prospective evaluation of peritoneal fluid contamination following transabdominal vs. transanal specimen extraction in laparoscopic left-sided colorectal resections. Surg Endosc. 2012;26:1495-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Leroy J, Diana M, Wall J, Costantino F, D’Agostino J, Marescaux J. Laparo-endoscopic single-site (LESS) with transanal natural orifice specimen extraction (NOSE) sigmoidectomy: a new step before pure colorectal natural orifices transluminal endoscopic surgery (NOTES®). J Gastrointest Surg. 2011;15:1488-1492. [PubMed] |
| 24. | Darzi A, Super P, Guillou PJ, Monson JR. Laparoscopic sigmoid colectomy: total laparoscopic approach. Dis Colon Rectum. 1994;37:268-271. [PubMed] |
| 25. | Franklin ME, Kazantsev GB, Abrego D, Diaz-E JA, Balli J, Glass JL. Laparoscopic surgery for stage III colon cancer: long-term follow-up. Surg Endosc. 2000;14:612-616. [PubMed] |
| 26. | Fukunaga M, Kidokoro A, Iba T, Sugiyama K, Fukunaga T, Nagakari K, Suda M, Yoshikawa S. Laparoscopy-assisted low anterior resection with a prolapsing technique for low rectal cancer. Surg Today. 2005;35:598-602. [PubMed] |
| 27. | Raftopoulos I, Courcoulas AP, Blumberg D. Should completely intracorporeal anastomosis be considered in obese patients who undergo laparoscopic colectomy for benign or malignant disease of the colon? Surgery. 2006;140:675-682; discussion 682-683. [PubMed] |
| 28. | Ohtani H, Tamamori Y, Azuma T, Mori Y, Nishiguchi Y, Maeda K, Hirakawa K. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and conventional open surgery for rectal cancer. J Gastrointest Surg. 2011;15:1375-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Sylla P, Willingham FF, Sohn DK, Gee D, Brugge WR, Rattner DW. NOTES rectosigmoid resection using transanal endoscopic microsurgery (TEM) with transgastric endoscopic assistance: a pilot study in swine. J Gastrointest Surg. 2008;12:1717-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303-312. [PubMed] |
| 31. | Steffen T, Tarantino I, Hetzer FH, Warschkow R, Lange J, Zünd M. Safety and morbidity after ultra-low coloanal anastomoses: J-pouch vs end-to-end reconstruction. Int J Colorectal Dis. 2008;23:277-281. [PubMed] |
| 32. | Laurent C, Leblanc F, Wütrich P, Scheffler M, Rullier E. Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg. 2009;250:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 33. | Laurent C, Leblanc F, Gineste C, Saric J, Rullier E. Laparoscopic approach in surgical treatment of rectal cancer. Br J Surg. 2007;94:1555-1561. [PubMed] |
| 34. | Darwood RJ, Wheeler JM, Borley NR. Transanal endoscopic microsurgery is a safe and reliable technique even for complex rectal lesions. Br J Surg. 2008;95:915-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
P- Reviewers: Kirshtein B, Kim YJ, Leitman M, M’Koma A S- Editor: Gou SX L- Editor: A E- Editor: Ma S