INTRODUCTION
In the past decade, several advanced endoscopic imaging technologies that enable clinicians to examine the luminal gastrointestinal tract at a microscopic level were introduced. These techniques are called optical biopsies, as they are real-time histologic biopsies of the tissue. These include confocal laser endomicroscopy (CLE) and endocytoscopy (EC). The CLE and EC systems come as either probe-based (pCLE and pEC) or endoscope-based (iCLE and iEC). In this review, we will describe the technical performance of the procedure, and discuss the clinical indications for optical biopsy in relation to inflammatory bowel diseases (IBD). We will also highlight some active research applications for optical biopsy in our understanding of the pathogenesis of IBD.
TECHNICAL ASPECTS OF OPTICAL BIOPSY
CLE was introduced in 2003 and allows in vivo microscopic imaging of cellular and subcellular structures at approximately 1000-fold magnification[1]. The technique is based on tissue illumination with a low power laser after application of fluorescence agents, which can either be applied systemically (i.e., fluorescein sodium) or topically (e.g., acriflavine hydrochloride, cresyl violet). The laser light is reflected from the tissue and then refocused onto the detection system by the same lens, so that only returning light refocused through the pinhole is detected. Therefore, this process decreases the effect of scattered light resulting in the construction of two-dimensional grey-scale images.
Currently, two CE certified and Food and Drug Administration approved CLE-devices are available[1]. One is integrated into the distal tip of a standard, high-resolution endoscope (iCLE, Pentax, Tokyo, Japan). The other one is probe based, capable of passage through the accessory channel of any standard endoscope (pCLE, Cellvizio, Mauna Kea Technologies, Paris, France). Both systems use an incident 488 nm wavelength laser (blue laser light) enabling the detection of fluorescence between 205 nm and 585 nm wavelengths.
The iCLE-system collects images at a manually adjustable scan rate of 1.6 frames per second with a resolution of 1024 × 512 pixels, or at 0.8 frames per second with a resolution of 1024 × 1024 pixels with dynamically adjustable depth of scanning ranging from 0 to 250 μm. The examiner can manually adjust the laser power between 0 and 1000 μW and the optical slice thickness is 7 μm, with lateral and axial resolution of 0.7 μm and a confocal image field of view of 475 × 475 μm. The pCLE-system is a stand-alone confocal probe which is advanced through the working channel of any endoscope and could thereby also being used with high-definition video endoscopes in combination with dye-less chromoendoscopy (e.g., Narrow Band Imaging, Fuji Intelligent Chromo Endoscopy, i-scan) as red-flag techniques.
pCLE-systems are available for different indications throughout the entire gastrointestinal tract and use a fixed laser power and a fixed image plane depth which is dependent on the probe type used. Confocal images are streamed at a frame rate of 12 frames per second. CLE in IBD is mostly being performed by using the ColoFlex Ultra-High Definition probe which requires a 2.8 mm working channel to be advanced through the scope. Lateral resolution is 1 μm and field of view is 240 μm with an imaging plane depth of 65 μm. In addition, a special computer algorithm (“mosaicing”) allows reconstruction of single video frames with an increased field of view (4 mm × 2 mm). Costs for single probes vary and are approximately 100-200 Euro per procedure. Like any other endoscopic technique CLE requires special training in performing the procedure and interpretation of images. Therefore, especially at the beginning, extensive close collaboration with an expert histopathologist is strictly recommended. In addition, when starting with CLE optical biopsies should always be compared with physical biopsies. After an appropriate learning phase, CLE interpretation shows high inter- and intraobserver variabilities as compared to standard histology. Both CLE-systems have unique advantages. Advantages of the integrated system are its higher resolution and the possibility to alter the laser power and imaging plane depth. Advantages of the probe-based system include the possibility of an ad hoc usage and a greater versatility of the pCLE probes, which can be used with nearly any endoscopes.
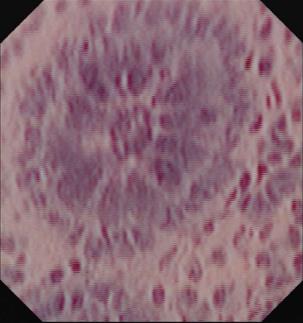
In contrast to CLE, endocytoscopy (EC; Olympus, Tokyo, Japan) is based on the principle of contact light microscopy[2]. EC-systems are either integrated into the distal tip of a standard endoscope (iEC) or probe-based (pEC). Through-the-scope pEC-systems require a working channel of at least 3.2 mm. Similar to contact light microscopy, EC requires thorough mucolysis with N-acetyl cysteine followed by staining of the mucosa with absorptive staining agents, like methylene blue, toluidine blue, or cresyl violet. In fact, a combination of different dye agents is often used to acquire optimal tissue contrast[3]. After an appropriate time of exposure to the dye (approximately 60 s), repeat washing of the mucosa is necessary to remove the excess contrast dye before endocytoscopic imaging. Repeat staining is mostly necessary while using absorptive contrast agents. Depending on the system used (iEC or pEC), EC visualizes architectural details (e.g., epithelial structure), cellular features (e.g., size and arrangement of cells), and vascular pattern morphology (e.g., size and tortuosity) at a magnification of up to 1390-fold[2,3]. Representative image of normal colon mucosa is shown in Figure 1.
Figure 1 Endocytoscopic image at magnification × 1390 showing one single colonic crypt.
Goblet and epithelial cells are clearly evident.
CLINICAL APPLICATIONS: ASSESSMENT OF INFLAMMATION IN IBD
Endomicroscopy
Accurate assessment of mucosal inflammation in patients with IBD is of crucial importance as mucosal healing has emerged as an important treatment goal and appears to be of paramount importance for optimized medical therapy[4]. Various studies and one recent systematic review has shown that mucosal healing as assessed by endoscopy is predictive of reduced disease activity, a decreased need for active treatment, reduced rates of hospitalizations/surgical resections, and is associated with sustained clinical remission[4-7]. While standard white-light endoscopy is likely an insensitive test for assessment of mucosal healing, being false negative in up to fifty percent of patients, there is an urgent need for new endoscopic imaging techniques allowing assessment of microscopic inflammation even in case of macroscopic non-inflamed mucosa[8,9]. In this context CLE was proven to be efficient for real-time in vivo assessment of mucosal inflammation by requiring only a short learning curve[10].
One early study investigated the features of CLE in inflamed and non-inflamed rectal mucosa and compared these results to standard histology[11]. On CLE, colonic crypts of normal colonic mucosa were small, round and regularly arranged, and the crypt lumens of the colonic glands were small and round. In contrast colonic crypts in non-active ulcerative colitis were small, round and slightly irregular in arrangement and the crypt lumens of the colonic glands were small and round. Inflammatory cells and capillaries were visible in the lamina propria. The colonic crypts in active ulcerative colitis were large, variously shaped and irregular in arrangement and in addition numerous inflammatory cells and capillaries were visible in the lamina propria. Li et al[12] confirmed these early results in a study including 73 consecutive patients. CLE-assessment of crypt architecture and fluorescein leakage showed good correlation with the corresponding histology results. Of note, more than half of the patients with normal mucosa seen on conventional white-light endoscopy revealed acute inflammation on histology, whereas no patients with normal mucosa or with chronic inflammation seen on CLE showed acute inflammation on histology. Therefore, CLE appears to be a sensitive tool for real-time assessment of inflammatory activity in patients with ulcerative colitis.
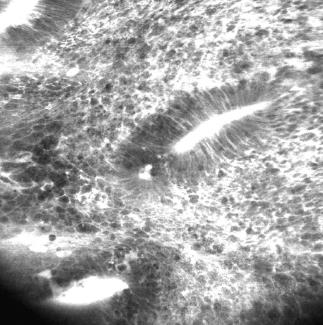
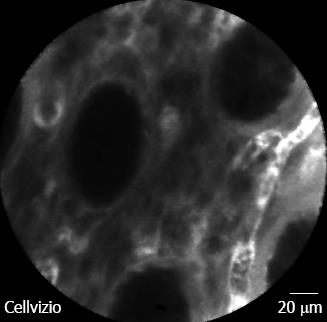
For Crohn’s disease, our group evaluated in a case-control study whether CLE is feasible for in vivo diagnosis of Crohn’s disease associated histological changes[13]. It was shown that a significantly higher proportion of patients with active Crohn’s disease had increased colonic crypt tortuosity, enlarged crypt lumen, microerosions, augmented vascularization, and increased cellular infiltrates within the lamina propria. In quiescent Crohn’s disease, a significant increase in crypt and goblet cell number was detected compared with controls. Based on these findings, the Crohn’s Disease Endomicroscopic Activity Score was proposed, allowing the assessment of Crohn’s disease activity in vivo, even in macroscopically non-inflamed mucosa. Representative iCLE and pCLE images of Crohn’s disease are shown in Figures 2 and 3. Taken these and the above mentioned results into account, CLE is reliable for real time in vivo assessment of microscopic inflammation in patients with IBD and macroscopically non-inflamed mucosa.
Figure 2 Endoscope-based confocal laser endomicroscopy of Crohn’s disease shows remarkable cell infiltration, disturbed colonic architecture and leakage indicating severe inflammation.
Figure 3 Probe-based confocal laser endomicroscopy in patient with Crohn’s disease without histologic activity.
Colonic crypts are regularly arranged with normal shape and size. Micro-vessels within the lamina propria are easily visible. Bar = 20 μm.
As most commonly used drugs for treatment of IBD are systemically bioavailable, they cover a potential risk of severe side effects. Therefore, a targeted, individualized approach to inflamed areas of the intestine with specific drugs is highly desirable. In this context, one recent study evaluated the potential of nanoparticle and microparticle uptake into the rectal mucosa of human IBD patients[14]. CLE was performed two hours after rectal application of fluorescent-labeled placebo nanoparticles and microparticles to 33 patients with IBD and healthy controls in order to visualize the particles in inflamed mucosal areas. A significantly enhanced accumulation of microparticles was observed in ulcerated areas, whereas nanoparticles were only visible in trace amount on mucosal surfaces of normal patients. Therefore, nanoparticles may enable local drug delivery to intestinal lesions in humans, thereby minimizing the risk of unintended translocation into the blood system. Very recently, our group created a fluorescent labeled antibody for molecular membrane-bound tumor necrosis factor (mTNF) imaging in Crohn’s disease patients[15]. Topical antibody administration led to detection of intestinal mTNF positive immune cells during CLE. Interestingly, patients with high amounts of mTNF positive cells showed significantly higher short-term response rates at week 12 (92%) upon subsequent anti-TNF therapy as compared to patients with low amounts of mTNF positive cells (15%). This clinical response in the former patients was sustained over a follow-up period of one year and associated with mucosal healing on follow-up endoscopy. These results are promising and offer the exciting potential for individualized therapy for IBD patients using molecular imaging with fluorescent labeled antibodies.
Endocytoscopy
Only limited data are currently available on the potential of EC in patients with IBD. One recent article correlated the efficacy between endocytoscopy and conventional histopathology for assessment of microscopic features in patients with ulcerative colitis[16]. Fifty-five patients were included and mucosal patterns were evaluated by using EC with × 450 magnification. Based on EC-findings a scoring system was developed that showed a strong correlation with Matts’ histopathological grades. In addition, there was a strong correlation between the conventional Matts’ endoscopic grade and Matts’ histopathological grade. Furthermore, the newly developed EC-score showed high reproducibility among investigators with a κ value of 0.79. Another recent study determined the reliability of EC for the discrimination of mucosal inflammatory cells and intestinal inflammatory disease activity in patients with IBD[17]. In total, 40 patients were included and EC was reliable to distinguish single inflammatory cells, including neutrophilic, basophilic, and eosinophilic granulocytes and lymphocytes. Sensitivity and specificity ranges among different cell types between 60% and 89% and 90% and 95%, respectively. Interobserver agreement among investigators was substantial whereas intraobserver agreement was almost perfect. Moreover, concordance between endocytoscopy and histopathology for grading of intestinal disease activity was 100%.
In conclusion, EC holds significant potential in identifying early signs of mucosal inflammation in real-time by identifying single mucosal inflammatory cells in conjunction with architectural details. Large, prospective multicenter trials evaluating EC for prediction of disease course in IBD are thus highly warranted and anticipated.
CLINICAL APPLICATIONS: DYSPLASIA DETECTION IN IBD
Patients with IBD are at an increased risk for the development of dysplasia (also known as intraepithelial neoplasia) and colitis associated cancer (CAC). The risk of developing CAC is comparable for Crohn’s colitis and ulcerative colitis patients. In both cases the risk is increased with: duration of colitis, early age of IBD onset, extent of colonic involvement, severity of inflammation, family history of colorectal cancer and particularly the presence of primary sclerosing cholangitis[18]. The cumulative risk for developing CAC in primary sclerosing cholangitis associated IBD (PSC-IBD) patients is 33% at 20 years and 40% at 30 years compared to 8% and 18% in IBD without concomitant liver disease[19]. Although IBDs contribute only 1%-2% to all cases of colorectal cancer, the cancer-related mortality rate in IBD patients is approximately 15%[20,21].
Colonoscopy with random biopsies, which is the gold standard for CAC screening in long standing IBD, can significantly reduce CAC-related mortality[22] and IBD patients undergoing surveillance colonoscopy had detection of neoplasia at an earlier stage, resulting in a better corresponding prognosis[23]. The principal limitation with this modality is that neoplasia may not be appreciated in up to a third of colonoscopies[24,25]. Taking four-quadrant biopsies is time-consuming and has only moderate sensitivity for neoplasia detection. The efficacy of surveillance for detection of early malignancies could also be questioned from the standpoint of cost and adherence. A population-based analysis of the cost and practice of colonic surveillance of patients with PSC-IBD in Alberta, Canada revealed that only 1/3 of the colonoscopies expected were actually performed, but despite suboptimal surveillance, the incidence of colorectal neoplasia was high. The study also found that the cost of finding one additional case of dysplasia was substantial[26]. Moreover, dysplasia in IBD can be found at distant sites from the cancer itself or before the cancer develops and is difficult to recognize on colonoscopy, as it often arises from flat, normal-appearing mucosa[20]. Dysplasia can also occur within or near plaque-like lesions or raised polypoid masses, defined as dysplasia-associated lesion or mass (DALM).
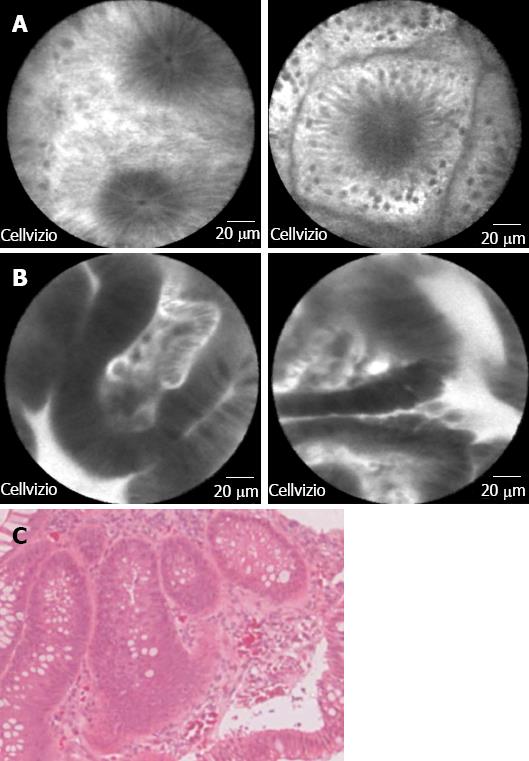
Targeted biopsies are an attractive alternative to random biopsies to increase the yield of dysplasia detection. CLE allows evaluation of suspicious lesions at the subcellular level with great detail prior to performing targeted biopsies, thus facilitating earlier diagnosis of CAC. While CLE only covers a limited field of view within the mucosa, pan-CLE of the whole colon is not feasible. Therefore, macroscopic visualization of suspected areas with chromoendoscopy (red flag technique) is useful before performing targeted endomicroscopy[27,28]. Prospective evaluation of CLE with concurrent chromoendoscopy predicted neoplasia in a randomized controlled trial of ulcerative colitis patients (n = 153) with great accuracy (98%), sensitivity (95%) and specificity (98%). By using methylene-blue aided chromoendoscopy with CLE, the diagnostic yield of neoplasia was increased by 4.75-fold compared with conventional colonoscopy with random biopsies (P = 0.005), though 50% fewer biopsy specimens were required[29]. Hurlstone et al[30] demonstrated remarkable results in a smaller ulcerative colitis cohort (n = 36), where CLE enabled differentiation of DALM and adenoma-like mass with 97% accuracy and an excellent agreement between endomicroscopy and histopathological diagnosis was found (P = 0.91). CLE based, targeted biopsies increased the diagnostic yield of intraepithelial neoplasia by 2.5-fold compared with chromoendoscopy-guided biopsies alone. One recent pilot study of pCLE during colonoscopic surveillance of patients with longstanding ulcerative colitis (n = 22) demonstrated that the method is feasible with reasonable diagnostic accuracy[31]. A recent meta-analysis of 15 studies of confocal endomicroscopy for dysplasia detection[32] showed CLE could distinguish neoplasms from non-neoplasms in IBD patients for surveillance with a sensitivity of 0.83 (95%CI: 0.70-0.92) and specificity of 0.90 (95%CI: 0.87-0.93). Representative pCLE images of adenomatous polyp with low grade dysplasia and corresponding histologic images are shown in Figure 4.
Figure 4 Probe-based confocal laser endomicroscopy images of (A) normal colonic mucosa; B: Adenomatous polyp with low grade dysplasia.
Bar = 20 mm; and C: The corresponding histologic image for the adenomatous polyp with dysplasia (magnification × 40).
CLE seems to be a particularly promising method for dysplasia detection in PSC-IBD patients. PSC-IBD is recently suggested to represent a specific IBD phenotype characterized by extensive colitis, low inflammatory activity, right-sided colonic inflammation and a high risk of CAC. Jørgensen et al[33] showed a difference between the macroscopic and microscopic picture in PSC-IBD: in general the inflammatory activity in these patients was low and was not always visible endoscopically, though it could be seen histologically. Since proximal cancers are more common in PSC-IBD patients[34,35] and inflammatory activity is not always visible endoscopically the use of CLE may increase surveillance efficiency particularly in the right colon. One ongoing study evaluates the efficacy of pCLE as a complementary tool to high definition white-light endoscopy (HD-WLE) for the detection of colonic dysplasia in patients with PSC-IBD. Preliminary results (n = 25) showed excellent accuracy (99%), sensitivity (93%) and specificity (100%) of pCLE in dysplasia detection that was superior to HD-WLE alone[36]. Low-grade intraepithelial neoplasia was found in 20% of patients and 60% of confirmed dysplastic lesions were localized in the right colon. These preliminary results suggest that careful CLE examination of at least the right colon in PSC-IBD patients may be warranted.
CLINICAL APPLICATIONS: ASSESSMENT OF CELL EXTRUSION AND BARRIER FUNCTION FOR DISEASE RELAPSE
Mucosal healing has emerged as the most important endoscopic predictor of disease relapse in IBD patients. In the pre-biologic era, mucosal healing was associated with lower rate of relapse in ulcerative colitis but not Crohn’s disease patients[6]. In the biologic era, mucosal healing is predictive of clinical and endoscopic remission for both Crohn’s[7,37] and ulcerative colitis patients[38,39]. The use of optical biopsy in the small bowel in IBD patients have been studied by several groups[1,40,41]. The principal finding on CLE that appears to predict disease relapse is epithelial cell extrusion and associated barrier dysfunction[42,43]. Using CLE, Kiesslich et al[40] first reported unambiguous identification of epithelial gaps or extrusion zones in the intestine of patients. Epithelial cell extrusion occurs as part of a normal physiological renewal process of the intestine. Therefore, qualitative description of the presence or absence of epithelial gaps is not sufficient to discern a diseased from a healthy state. We have therefore developed a quantitative measure called epithelial gap density, defined as the total number of epithelial gaps counted normalized to the total number of epithelial cells counted on pCLE images[41]. Gap density was validated against conventional multi-photon confocal microscopy and conventional white-light microscopy[44]. The gap density was found to be increased in IBD patient[45]. More recently, increased gap density and certain types of epithelial gaps were found to be predictive of aggressive disease[43] and relapse in IBD patients[42]. Thus, qualitative and quantitative studies of epithelial gaps in the small intestine may have a role in defining new management algorithms for IBD patients.
The clinical applications of CLE (both pCLE and iCLE) in the small intestine of IBD patients have been largely in the evaluation of the terminal ileum[40,42,43,45]. The terminal ileum is the preferred site of intestinal evaluation in IBD for a number of reasons: (1) it is within the reach of a standard colonoscope; (2) it is an active site of immunologic activity; (3) it is often the first site of disease in Crohn’s patients; and (4) it is usually possible to find endoscopically normal appearing areas, even in patients with active Crohn’s ileitis. We have devised a quantitative determination of the density of epithelial gaps or extrusion zone called the epithelial gap density in the terminal ileum[41,45]. The epithelial gap density derived using CLE could be validated against multi-photon confocal microscopy and light microscopy[46]. Increased epithelial gap density could identify patients at high risk for major events such as hospitalization or surgery in follow up[43]. In another recent study, certain types of epithelial gaps were found to be predictive of disease relapse in IBD patients[42]. The current evidence seems to suggest that quantification and evaluation of extrusion zones in the terminal ileum of IBD patients have predictive values for disease relapse. However, these are single-centered studies, and there are no multicenter trials to determine the role of CLE in the management of IBD patients.
RESEARCH APPLICATIONS: FUNCTIONAL MUCOSAL ASSESSMENT IN IBD
The intestinal epithelium functions as a selective barrier to the luminal contents[47,48]. The epithelium separates the immune and neural networks of the human intestine from the intestinal microbiota, which comprises an estimated 1013 to 1014 microorganisms[49]. Compromised epithelial barrier expose the subepithelial immune system to resident microbes which induces secretion of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α[50], which in turn induces shedding of epithelial cells from the intestine which then further contributes to barrier dysfunction and promotes inflammation[51]. Other proinflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-18 and TNF-α have been reported to be increased in active IBD and correlate with endoscopic severity of inflammation[52,53].
Until advances of optical biopsy, either via CLE or EC, it was not possible to investigate the interactions between the intestinal epithelium and resident microbiota in vivo. Histologic evaluation of the intestinal mucosa is limited by fixation and processing artefacts, including contamination with luminal microbes. CLE was used to identify intramucoal bacteria, and IBD patients were found to have significantly higher distribution of involvement with intramucosal bacteria in the colon and terminal ileum[54]. CLE was recently used for molecular imaging to identify single bacteria species and in vivo diagnosis of bacteria associated colitis[55].
Barrier dysfunction is another area of research interest for the past few years. Restoration of normal epithelial barrier function which prevent the translocation of commensal bacteria is the structural basis of mucosal healing[4]. Intestinal permeability function as assessed with disaccharide solutions[56,57] has not been widely adopted for clinical use. Optical biopsy permits assessment of mucosal barrier function in the appropriate structural context. Studies have shown increased epithelial cell shedding may contribute to barrier dysfunction in the intestine[42,58]. The clinical relevance of cell shedding and barrier dysfunction is reflected in the ability of these measures to predict disease relapse and major events in follow up[42,43].
CONCLUSION
Optical biopsy of the intestine in IBD have been used for a variety of clinical and research indications, and appears to hold significant promise to improve the diagnosis and management of IBD patients. Future large, multi-centered studies are needed to validate these early study findings to facilitate clinical adaptation of this group of new advanced imaging technique.
P- Reviewers: Akyuz F, Afzal NA, Ciacci C, Ciccone MM S- Editor: Gou SX L- Editor: A E- Editor: Wu HL