Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7500
Revised: September 29, 2013
Accepted: October 17, 2013
Published online: November 21, 2013
Processing time: 112 Days and 21 Hours
Hepatocellular carcinoma (HCC) is the most common form of liver cancer worldwide. It is caused by a variety of risk factors, most common ones being infection with hepatitis viruses, alcohol, and obesity. HCC often develops in the background of underlying cirrhosis, and even though a number of interventional treatment methods are currently in use, recurrence is fairly common among patients who have had a resection. Therefore, whole liver transplantation remains the most practical treatment option for HCC. Due to the growing incidence of HCC, intense research efforts are being made to understand cellular and molecular mechanisms of the disease so that novel therapeutic strategies can be developed to combat liver cancer. In recent years, it has become clear that innate immunity plays a critical role in the development of a number of liver diseases, including HCC. In particular, the activation of Toll-like receptor signaling results in the generation of immune responses that often results in the production of pro-inflammatory cytokines and chemokines, and could cause acute inflammation in the liver. In this review, the current knowledge on the role of innate immune responses in the development and progression of HCC is examined, and emerging therapeutic strategies based on molecular mechanisms of HCC are discussed.
Core tip: Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide. Growing incidence of HCC has generated immense interest to understand the mechanisms of disease at the physiological, cellular and molecular levels with the hope of developing novel therapeutics for the treatment of HCC. In the past few years, it has become clear that innate immunity plays a critical role in the development and progression of HCC. In this review, these new developments and possibilities of developing novel therapeutic options based on this newly gained knowledge are discussed.
- Citation: Aravalli RN. Role of innate immunity in the development of hepatocellular carcinoma. World J Gastroenterol 2013; 19(43): 7500-7514
- URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7500.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7500
Hepatocellular carcinoma (HCC) is the most common form of liver cancer, and is the third leading cause of cancer-related deaths worldwide. It accounts for approximately 70%-80% of all primary liver cancer cases[1]. A variety of risk factors such as hepatitis viruses, vinyl chloride, tobacco, foodstuffs contaminated with aflatoxin B1 toxin, heavy alcohol intake, nonalcoholic fatty liver disease (NAFLD), diabetes, obesity, oral contraceptives, and hemochromatosis cause HCC[2]. Recurrence is quite common in patients who have had a resection, and survival rate is 30%-40% at five years post-surgery[2]. As a recent surveillance, epidemiology, and end results study using the Medicare dataset of elderly patients in the United States has shown, in addition to the human loss, there is a substantial burden of health care expense of illness associated with HCC[3]. To underscore this point, the Centers for Disease Control has recently recommended one-time health screening for the entire generation born between 1945 and 1965. The dramatic rise in the incidence of HCC in Western countries in recent years has generated intense efforts to understand the mechanisms of disease at the physiological, cellular and molecular levels with the hope of developing novel therapeutics for the treatment of HCC.
The normal liver lobule is formed by hepatocytes, cholangiocytes and various non-parenchymal cells [Kupffer cells (KCs), liver sinusoidal endothelial cells (LSECs), and hepatic stellate cells (HSCs)]. Intrahepatic lymphocytes and liver-specific natural killer (NK) cells are also present in the sinusoidal lumen and perisinusoidal space of Disse[4]. Exposure to toxic substances and induction of immune responses in the liver can result in inflammation through the activation of KCs and HSCs, and can cause necrosis. In this process, liver fibrosis and cirrhosis may also occur. Even though the molecular basis for cancer-promoting effect of cirrhosis is unknown, the process of recurrent liver cell necrosis and regeneration with increased cell turn-over renders liver cells more sensitive to the adverse effects of other mutagenic agents[4]. Cirrhosis is responsible for significant morbidity and mortality, and is one of the most important risk factors for the development of HCC.
Carcinogenesis is a process that involves the transition of a normal cell into a preneoplastic lesion that develops into malignant tumor[4]. Growing evidence suggests that gradual accumulation of mutations and genetic changes in preoneoplastic hepatocytes causes malignant transformation that leads to the development of HCC[5,6]. Tissue environment also plays a critical role in tumor formation[7]. Interaction of different cell types in the tumor stroma with components of the extracellular matrix (ECM), either directly or indirectly result in the acquisition of an abnormal phenotype that causes this transformation. Tumor stroma consists of fibroblasts [also referred to as “cancer-associated fibroblasts (CAFs)”], macrophages (liver resident KCs and other tumor-infiltrating cells), leukocytes, HSCs, endothelial cells, pericytes, neutrophils, and dendritic cells (DCs)[8]. Each of these cells produces growth factors, cytokines, chemokines, free radicals, and other tumorigenic substrates that contribute to tumor initiation and progression[9].
In HCC, CAFs are involved in tumor initiation and progression. They produce epidermal growth factor (EGF), hepatocyte growth factor, fibroblast growth factor, interleukin 6 (IL-6), chemokine (C-X-C motif) ligand 12 (CXCL12), and matrix metalloproteases (MMP-3 and MMP-9)[10]. They also produce IL-8, cyclooxygenase 2, and secreted protein acidic rich in cysteine to recruit and stimulate macrophage production, which can further increase the activation of CAFs through the secretion of tumor necrosis factor-α (TNF-α) and platelet-derived growth factor (PDGF)[11,12]. Tumor-associated macrophages (TAMs) are “polarized” into M2 mononuclear phagocyte-like cells by various cytokines [IL-4, IL-10, and transforming growth factor β (TGF-β)] present in the tumor microenvironment[13]. These M2-like TAMs, in turn, express cytokines (IL-10 and TGF-β), chemokines (CCL17, CCL22 and CCL24), vascular endothelial growth factor (VEGF), and EGF to recruit regulatory T cells (Tregs) and to promote angiogenesis[14,15]. KCs are able to impair cluster of differentiation 8+ (CD8+) cytotoxic T lymphocyte (CTL)-mediated immune responses through programmed death ligand 1[16]. Moreover, when stimulated with pro-inflammatory cytokines (IL-1β, TNF-α and PDGF), KCs and HSCs produce osteopontin that plays a pivotal role in various cell signaling pathways, which promote inflammation, tumor progression and metastasis[17,18]. DCs process antigens and present them to infiltrating CTLs by expressing them on their cell surface. These antigen presenting cells (APCs) possess high endocytic activity, and therefore, are critical for the induction of immune surveillance in tumors, and for immune evasion[19]. Such tumor-antigen specific CD8+ T cell responses were recently shown to suppress the recurrence of HCC[20].
In response to liver injury, HSCs transdifferentiate into myofibroblast-like cells and produce cytokines, chemokines, growth factors, and ECM[17]. This phenotypic transformation of HSCs is a key event in the development of hepatic fibrosis[21]. Hepatitis B virus (HBV)-encoded X protein, hepatitis C virus (HCV)-encoded non-structural proteins, MMP-9, PDGF, TGF-β, Janus kinase (JNK), insulin-like growth factor binding protein 5, and cathepsins (B and D) are potent inducers of HSC activation and proliferation that enhance liver fibrosis and carcinogenesis[17]. Endothelial cells express a variety of angiogenic receptors including VEGFR, EGFR, EGF homology domains-2 (Tie-2), PDGFR, and C-X-C chemokine receptors. Tumor-associated endothelial cells express high levels of TGF-β in HCC, which act as a chemoattractant for cluster of differentiation 105 (CD105, also known as endoglin), a type I cellular glycoprotein that is a port of the TGF-β receptor complex, to promote tumor angiogenesis[22]. It has been shown that CD105+ endothelial cells express increased angiogenesis activity with greater resistance to chemo-therapeutic agents and inhibitors of angiogenesis[23].
Infiltration of T cells into the tumor microenvironment is an important regulator of cancer progression. In HCC tissues, CD4+/CD25+ Tregs impair proliferation and activation of CTLs, degranulation, and production of granzymes (A and B), and perforin[24]. In a recent study, the infiltration of these Tregs into the tumors of HCC patients was found to correlate with an increase in tumor size[25]. Other studies have shown that low CD8+ T cell counts and high Treg numbers correlate with poor prognosis in HCC patients, especially after resection[26-28]. More recently, a 14-immune gene signature that drives the infiltration of lymphocytes into tumor has been identified[29]. This signature, which includes pro-inflammatory cytokines [TNF-α and interferon (IFN)-γ] and chemokines (CXCL10, CCL5 and CCL2), is a good predictor of patient survival at early tumor stages. In addition, dysfunctional regulation of immune response by excessive neutrophil activity was also reported as a poor prognostic indicator after resection of HCC[30].
To successfully detect and eliminate invading pathogens by discriminating self from non-self, the mammalian immune system has developed mechanisms that can be divided into two distinct components: the innate immunity and the adaptive immunity. In most multicellular organisms, the highly conserved innate immune system provides the first line of defense to limit infection by detecting pathogens using germline-encoded proteins[31]. The adaptive immunity, present only in vertebrates, detects non-self through the recognition of peptide antigens by receptors expressed on the surface of B and T cells[32]. The adaptive responses are much more diverse than the innate responses as each B and T lymphocyte clone expresses a distinct antigen receptor that arose by somatic gene rearrangement through a process of evolution[33]. Most often innate immune responses emanate from the host cell surface receptor with the recognition of conserved structural motifs termed pathogen-associated molecular patterns (PAMPs) on the surface of microorganisms. Toll-like receptors (TLRs), Nucleotide-binding and oligomerization domain-like receptors (NLRs) and RIG-I-like receptors (RLRs) are the key receptors that recognize a variety of PAMPs[34]. While TLRs can recognize bacteria, viruses, fungi and protozoa, NLRs and RLRs detect bacteria and viruses, respectively. All these pattern recognition receptors (PRRs) generate innate immune responses, either by acting alone or in combination with other receptors. The focus of the review is on the role of TLRs as most of the current knowledge on the role of innate immunity in liver diseases has been obtained from studies on the TLRs.
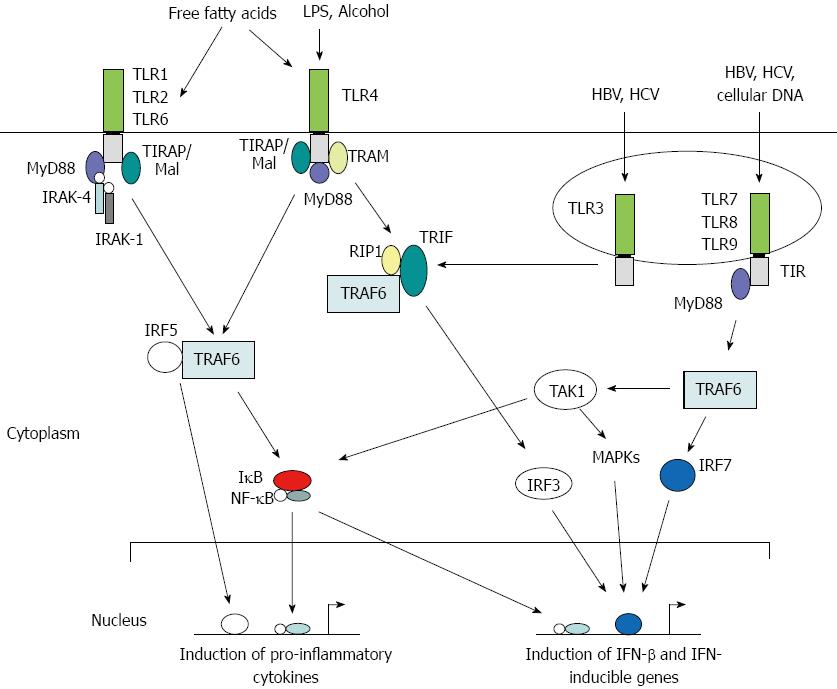
Toll was first identified as a gene important in the establishment of dorsal-ventral orientation during embryonic development in the fruit fly Drosophila melanogaster[35]. Later, it was found that the Toll protein plays a critical role in the fly’s immunity to fungal infections[35,36]. The first mammalian homolog of Toll, Toll-like receptor 4 (TLR4), was identified as a PRR required for adaptive immunity[37]. Subsequently, TLR4 and other TLRs were shown to play critical roles in generating innate immune responses against microbial pathogens in mammalian systems. To date, 11 human TLRs and 13 mouse TLRs have been identified, in addition to a number of TLRs in other vertebrates, that recognize a variety of PAMPs and trigger both innate and adaptive immune responses[32,38]. TLRs are membrane-bound proteins that contain varying numbers of extracellular leucine-rich repeats and a Toll-IL receptor (TIR) domain in the cytoplasmic region that are highly conserved (Figure 1). They recognize ligands through LRRs and transmit signals through their TIR domain via protein-protein interactions with cellular adaptor proteins triggering a cascade of signaling events such as phosphorylation of interleukin-1 receptor-associated kinase 1 (IRAK-1) and activation of Nuclear factor kappa B (NF-κB) or interferon regulatory factor 3 (IRF3), resulting in the production of immune mediators and IFN-inducible genes[39]. Thus, a direct or indirect association of a ligand with its cognate TLR serves as a signal to trigger an innate immune response. Each step along the TLR signaling pathways is tightly regulated by a complex mix of phosphorylation and targeted degradation, and sequestering of various signaling molecules is dependent upon the nature of the invading pathogen[40].
In general, vertebrate TLRs were classified into six distinct families based upon amino acid sequence homologies of LRRs[41]. Most mammalian cells express low levels of TLRs constitutively in a cell-type specific manner, and interestingly, they can be present in both membrane-bound and soluble forms. For example, the rainbow trout TLR5 is expressed constitutively as a membrane protein but upon induction with the bacterial flagellin, a soluble TLR5 is rapidly induced[42]. Normally, TLRs function as homodimers. However, some TLRs form heterodimers with other TLRs to recognize PAMPs. For instance, TLR2 associates either with TLR1 or TLR6 as a heterodimer to recognize triacylated lipoproteins and diacylated lipoproteins, respectively[32,38,40]. In addition, cellular membrane protein CD14 enhances the ligand recognition ability of TLR2[43].
In the healthy liver, TLR expression is detectable only at very low levels[44]. Eight TLRs are expressed in the mammalian liver with varying levels of expression on hepatocytes, KCs, HSCs and LSECs[45]. These TLRs not only recognize microbial PAMPs but also the damage-associated molecular patterns (DAMPs) of dying host cells[46]. Even though hepatocytes express all TLRs, they are capable of responding to TLR2 and TLR4 ligands only, and these responses are very weak in vivo[47]. Under inflammatory conditions, however, hepatocyte response to TLR2 ligands was significantly enhanced but the response to TLR4 ligands was still not detectable in these cells[48]. LSECs express mRNAs of TLRs 1-9, and respond to various TLR ligands by expressing TNF-α, IL-6, and IFN-β[49,50]. KCs express all TLRs and respond to a variety of ligands by producing TNF-α and IL-6[51,52]. When stimulated with ligands for TLRs 1, 2, 4 and 6, they produce IFN-γ and promote the proliferation of T cells[49]. In response to TLR3 and TLR4 ligands, they produce IFN-β, and for ligands against TLR1 and TLR8, they display a high level of major histocompatibility II expression. HSCs express low levels of TLR4 and TLR9, but activation of TLR4 has been shown to induce the expression of TLR2 as well[49]. In human HSCs, TLR4 activation results in the production of CCL2, CCL3 and CCL4[53], and their expression of TGF-β was implicated in the promotion of hepatic fibrosis[54]. Activation of TLR9 by DAMPs induces the differentiation of HSCs and increases the production of collagen[55].
Among the non-parenchymal cells, hepatic DCs can be classified into different subsets as plasmacytoid DCs, myeloid DCs, lymphoid DCs, natural killer DCs, and a mixture of lymphoid and myeloid DCs[56]. Differential expression of TLRs in these hepatic DCs varies according to the subset type and the species type. For example, in humans, pDCs express TLRs 1, 3 and 7 only, whereas other DC subsets express all TLRs except TLR9[57]. On the other hand, murine pDCs express TLRs 2, 4, 7 and 9 but not TLR3[58].
Hepatic lymphocyte population, which accounts for about 25% of non-parenchymal cells consists of B cells, NK cells, NKT cells, αβT cells and γδT cells[59]. In general, T cells are activated indirectly by TLRs through APCs[60], and NK cells that express TLRs 1 to 9, but not TLR5. They respond to various TLR ligands by producing IL-12[61]. Interestingly, expression of TLRs in B cells has no effect on antibody production, and on B cell memory responses[62]. However, TLR3 and TLR9 activation in CD4+ T cells enhances their proliferation[63], and TLR2 serves as a co-stimulatory receptor for antigen-specific T cell development and participates in T cell memory[64].
Significant amount of evidence in recent years has demonstrated the involvement of TLRs in the pathogenesis of various liver diseases. Most of this evidence comes from the overexpression of TLRs, activation of TLRs causing enhanced disease in animal models, single nucleotide polymorphisms (SNPs) in TLR-coding genes and their adapters linked to disease susceptibility, and TLR knockout mice being protected from disease[40]. Three most common risk factors for the development of HCC for which TLR involvement has demonstrated to play a critical role in the disease pathogenesis are discussed in this section.
Chronic hepatitis virus infection that affects almost half a billion people worldwide is a major risk factor for HCC. The infectivity of a given virus type varies according to the geographical location[2]. Co-infection with one or more viruses may also occur contributing to a higher risk of HCC, albeit it is rare[35]. HCV is the most common blood-borne infection in the United States, with nearly 20% of chronically infected individuals developing cirrhosis and HCC[65]. It is generally acknowledged that the humoral antibody response contributes to the clearance of circulating HBV particles and the prevention of viral spread within the host while the cellular immune response eliminates infected cells[66]. The T cell response to the HBV is vigorous, polyclonal and multispecific in acutely infected patients who successfully clear the virus, and relatively weak and narrowly focused in chronically infected patients, suggesting that clearance of HBV is T cell dependent[66]. Persistent HBV infection is characterized by chronic liver cell injury, regeneration, inflammation, widespread DNA damage and insertional deregulation of cellular growth control genes, which, collectively, lead to cirrhosis of the liver and HCC[66]. Other factors that could contribute to viral persistence are immunological tolerance, mutational epitope inactivation, T-cell receptor antagonism, incomplete down-regulation of viral replication, and infection of immunologically privileged tissues[66]. However, these pathways become apparent only in the setting of an ineffective immune response, which therefore, is the fundamental underlying cause[66]. In infected cells, the HBV capsid induces cytokine production via TLR2 activation. It was hypothesized that TLR2 activation is involved in viral clearance based on the observation that the administration of adefovir and entricitabine in HBV patients resulted in the up-regulation of TLR2 and reduction in the viral load[46]. In HepG2 cells, HBV triggers the production of cholesterol-metabolism genes via the TLR2 pathway[67], and inflammatory stress exacerbated hepatic cholesterol accumulation these cells and in mice by disrupting the PPAR-LXR-CYP7A1/ABCA1-mediated bile acid synthesis and cholesterol efflux[68]. Interestingly in HBV transgenic mice, activation of TLRs 3-5, 7, and 9, but not TLR2, inhibited HBV replication via IFN-α/β induction[69].
When immune responses were compared in macrophages of patients who spontaneously cleared HCV with those who were chronically infected, it was found that the TLR3 expression was significantly up-regulated in the former group[65]. Individuals who cleared the virus had an elevated expression of IFN-β and higher rate of STAT-1 phosphorylation. A significant association of intronic TLR3 SNP (rs13126816) in the clearance of HCV and the expression of TLR3 was found in this study, suggesting that an elevated innate immune response enhances HCV clearance and may offer a potential therapeutic option to increase viral clearance[65]. TLR2, in combination with TLR1 and TLR8, recognizes core and nonstructural 3 proteins of HCV in immune cells such as macrophages and monocytes, and triggers the production of pro-inflammatory immune mediators TNF-α, IL-6, IL-8, IL-10 and IL-1β[70]. On the other hand, HCV NS5 protein is recognized by TLR4 in both hepatocytes and B cells[71]. Prolonged activation of KCs in the HCV-infected liver by HCV-encoded proteins causes persistent inflammation resulting in severe liver damage and cancer[72]. It was reported recently that the expression of TLR2 and TLR4 was highly elevated in peripheral blood monocytes of HCV patients, and that the number of Tregs was significantly higher in these chronically infected individuals[73]. Similar correlation of TLR2 and TLR4 expression, and Treg numbers was also reported in HBV patients[74]. In a separate study, a strong correlation was also observed between TLR2/4 expression and TNF-α production causing hepatic inflammation in HCV patients[75]. Chronic infection with HBV and/or HCV, and imbalanced immunity contributes to severe inflammation, while TLR activation during this process might be a critical factor of this infection-induced prolonged inflammation. Activation of innate immune responses by viral proteins, and the interaction of HCV with cellular proteins to evade host’s immune responses as well as the role of SNPs in TLRs, their adaptors and cytokine genes in altering these immune responses have been extensively reviewed in the literature[76-78].
Excessive alcohol consumption that causes alcoholic liver disease (ALD) is a major risk factor for HCC worldwide[79]. ALD is an umbrella term used to describe a broad spectrum of liver abnormalities caused by alcohol that include simple steatosis, alcoholic hepatitis, fibrosis and cirrhosis, which can progress to HCC. The pathogenesis of ALD is characterized by processes such as ethanol metabolism-associated oxidative damage, glutathione depletion, abnormal methionine metabolism, ethanol-mediated induction of leakage of gut endotoxins, and inflammation[79]. Liver inflammation is known to occur with exposure to a variety of agents, including metabolites of alcohol[80]. If hepatic metabolism is impaired to any degree and fails to convert drugs and chemicals to non-reactive or non-immunogenic substances, the metabolic intermediates formed in hepatic tissues may cause liver damage[81]. In such cases, KCs and other cell types release cytokines and chemokines that result in the inflammation of the liver. This reaction coupled with deregulated hepatocyte proliferation can contribute to the pathogenesis of HCC[81].
Simple steatosis is a benign condition that progresses to alcoholic steatohepatitis (ASH) in about 10%-20% of cases and is associated with inflammation and liver injury caused by the innate immune responses[82]. Both MyD88-dependent and MyD88-independent pathways are activated in ASH, and studies with animal models have demonstrated the up-regulation of inflammatory cytokines in the serum, and activation of IFN and IFN-responsive genes in ASH[83,84]. Blocking of IL-1 receptor, which acts through MyD88, in advanced ASH was shown to confer protective effect in mice suggesting that IL-1 inhibitors may be used in the treatment of ALD[85].
Excessive alcohol consumption increases gut permeability and the translocation of bacteria-derived lipopolysaccharide (LPS, also known as endotoxin) from the gut to the liver[86]. In KCs, LPS interacts with TLR4 causing oxidative stress, and the production of pro-inflammatory cytokines and reactive oxygen species (ROS) that induce hepatocellular damage[83,87]. However, this effect was not abrogated in MyD88-deficient mice suggesting that MyD88-independent pathways are involved in NF-κB activation by alcohol[83]. Even though IRF-3 activation was not affected by chronic alcohol treatment, IRF-7 and IRF-3-inducible genes expression was significantly induced in KCs of alcohol-fed wild type mice[83]. The activation of TLR4 has also been demonstrated to occur during the alcohol and HCV synergism[88]. Furthermore, alcohol activates complements C3 and C5, following the production of TNF-α, and induces hepatocyte injury[89,90]. In addition to Gram-negative bacteria that produce LPS, gut is home to a large number of other microorganisms belonging to eukaryotes, prokaryotes, archaea, and viruses[91]. It is therefore likely that Gram-positive bacteria which produce lipoproteins and activate TLR2 signaling, and viruses that activate TLRs 3, 7, 8 and 9 may also leak from the gut to the liver. However, little is known about the activation of these TLRs during alcoholic liver injury.
NAFLD is the most common chronic liver disease that affects both adults and children worldwide[92]. Like ALD, NAFLD includes a broad spectrum of liver abnormalities ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), which can progress to cirrhosis and HCC. Of these, NASH is characterized by hepatocyte injury, inflammation, and fibrosis[92-94]. Most of these disease conditions are diagnosed at a late stage, and some patients also present for the first time with cirrhosis[92]. The development of cirrhosis is significantly higher in individuals with NASH when compared to patients with simple steatosis[92-94]. Consequently, liver-related mortality is also significantly higher in NASH patients.
NASH shares immunological characteristics with ASH such as the activation of innate immune responses, crosstalk between steatosis and inflammation, activation of TLR4 by LPS and fatty acids, complement activation, production of proinflammatory immune mediators, and alteration in NK and NKT cell number and activity[95,96]. In addition, activation of KCs in response to gut microbiota, activation of TLR signaling via both MyD88-dependent and -independent pathways, up-regulation of type I IFN and IFN-responsive genes occur in ASH and NASH[97-100]. However, recent studies have shown that NASH differs from ASH in certain aspects: insulin resistance, crosstalk between adipose tissue and the liver, dependence on MyD88 signaling, differential effects in mice due to MyD88 and IL-1 deficiency, and activation of inflammasome[96].
Progression of NASH is associated with the recruitment of T cells and T1 response leading to inflammation[101], and hepatic expression levels of inflammatory mediators are modified in morbidly obese patients even without pathohistological manifestations[102]. Accumulating evidence strongly suggests that inflammation causes the progression of NAFLD to NASH, and that innate immunity is involved in the inflammatory response. While TLR2 and TLR9 pathways are critical for the development of NAFLD[103], deletion of either TLR4 or its co-receptor MD-2 dampened (but not abolished) necroinflammatory activity of steatosis and fibrosis in a mouse model of NASH[104]. Furthermore, in NASH, gut microorganisms enter the liver because of the leakage in the intestinal mucosal barrier resulting in inflammation, due to the activation of TLR signaling by intestinal bacteria and their products such as LPS[45,93]. It is apparent from these reports that the activation of TLR signaling is an important factor in causing inflammation in NASH, and could be a crucial factor in the progression of NASH to cirrhosis. It was reported that the activation of TLR4 signaling and up-regulation of CD14 resulted in higher responsiveness to LPS and saturated fatty acids in KCs, and resulted in hepatic inflammation and liver complications in NASH patients[98,105].
Chronic liver damage caused by excessive inflammation due the exposure to various risk factors often results in the development of fibrosis-associated HCC[106]. Since the stimulation of TLR signaling pathways result in the production of proinflammatory immune mediators, it is likely that TLRs are involved in development and progression of hepatocarcinogenesis as well. The activation of TLR signaling in liver diseases leads to the activation of NF-κB and JNK pathways that are critical mediators of tumor-associated cytokine production. NF-κB activation induces the proliferation of tumor cells through the production of TNF-α[107]. Tumorigenic effect of JNK in HCC is mediated through the regulation of molecules involved in cell proliferation such as MMPs and cyclins[108]. Studies in animal models of HCC have shown that ROS-mediated JNK activation is critical for tumor development[109], and that elevated ROS levels induce hepatocyte cell death[110]. Moreover, hepatocyte-specific knockdown of the NF-κB inhibitor IκB kinase subunit NF-κB essential modulator causes spontaneous development of HCC in mice[111]. However, interestingly, hepatocyte-specific TGF-β-activated protein kinase 1-deficient mice had spontaneous hepatocyte cell death, compensatory proliferation, inflammatory cell infiltration, and fibrosis in the liver[102,112]. Collectively, these results demonstrate the involvement of NF-κB-mediated downstream molecules in cellular homeostasis and cancer development in the liver.
Dysregulation of TLR expression may shift the balance between the production of pro- and anti-inflammatory cytokines, and will have a profound effect on the risk of infection, chronic inflammation and cancer. Recently, Nishalke et al[113] showed that the frequency of TLR2 -196 to -714del allele was significantly higher in HCV-associated HCC patients than in HCV-infected individuals demonstrating that this deletion plays a role in HCC development. In addition, a number of SNPs have also been identified in every TLR gene. Some of these have been shown to enhance the susceptibility of various cancers in humans[114]. However, only a few reports have been published on the role of TLR SNPs in the pathogenesis of HCC. In one such study, Zhang et al[115], conducted a genome-wide genotyping of 440794 SNPs in chronic HBV carriers: 355 with HCC and 360 without HCC. They found that one intronic SNP rs17401966 present in the KIF1B gene on chromosome 1p36.22 was highly associated with HBV-associated HCC[115]. In a recent study, Junjie et al[116] investigated the association between SNPs of TLR2 and TLR9 genes, and the susceptibility of HCC in a cohort of 211 patients that included 172 HBV carriers. They found that two TLR2 SNPs rs3804099 C/T and rs3804100 C/T present in the same exon of TLR2 were associated with HCC, whereas TLR9 SNPs have no role in tumor development[116]. Additional studies are needed to fully understand the involvement of various TLR SNPs in hepatocarcinogenesis.
Overwhelming evidence on the involvement of TLR signaling pathways in many human diseases has led to the efforts to develop TLRs as vaccine adjuvants[117,118] and as therapeutic targets[119-121]. As discussed earlier, chronic inflammation, which plays a critical role in the progression of liver diseases and in the development of human cancers, is mediated by TLR activation. Hence, modulation of TLR pathways using various drugs, antibodies, microRNAs (miRNAs), and small molecules that function as TLR agonists and antagonists to reduce liver inflammation and to prevent the progression of liver diseases towards cancer is a promising strategy to combat HCC.
Initial efforts on the modulation TLR signaling with small TLR mimetics that act as agonists have produced mixed results. Using the TLR2 agonist Pam2Cys as a stimulant in vitro, it was shown that hepatoma cell lines HuH7 and HepG2 respond to HBV infection by producing IL-8 and by inhibiting viral replication[122]. However, in in vivo studies with HBV transgenic mice, it was found that ligands specific for TLRs 3, 4, 5, 7 and 9, but not TLR2, inhibited HBV replication in the liver[69]. On the other hand, intravenous administration of the TLR7 agonist isatoribine significantly reduced viral RNA in HCV patients independent of viral genotype[123]. The decrease in viral load in this study coincided with markers indicating a heightened antiviral immune state. In similar experiments, Weeratna et al[118] and Ma et al[124] tested TLR7 agonist R-848 and CpG oligodeoxynucleotides containing CpG motifs (CpG ODN) that act as the TLR9 agonists as adjuvants for HBV vaccination in mice using the HBV surface antigen (HBsAg) as a model antigen. This study was based on the notion that the immunostimulatory sequences (ISS) containing CpG motifs would elicit strong Th1 immune responses[125]. In their studies, both these groups found that the TLR agonists trigger immune responses that are beneficial to the host[118,124]. Along similar lines, Dynavax Corporation has developed a commercial vaccine (Heplisav) against HBV by combining a synthetic ISS sequence called ISS-1018 that acts as TLR9 agonist with the recombinant HBsAg. This vaccine induced seroprotective responses when administered in HBV patients[126]. Another CpG ODN containing TLR9, CPG7909, was also successfully tested as an adjuvant to HBV vaccine and is currently undergoing a phase 2 clinical trial[125,127].
In contrast to these TLR agonists, only a few studies have reported successful inhibition of TLR signaling using antagonists. For example, lipid A mimetics that bind directly to TLR4-MD2 were shown to inhibit LPS-mediated activation of TLR4 signaling both in vitro and in vivo[128]. TAK-242, another mimetic that targets the intracellular domain of TLR4, inhibited TLR4-mediated production of nitric oxide and TNF-α in murine and human cells by targeting TLR4-CD4 chimeric receptors[129]. Interestingly, however, TAK-242 had no effect on NF-κB activation mediated by MyD88, TIRAP, TRIF or TRAM. Recently, Cowden et al[130] tested two inhibitors of histamine H4 receptor that interacts with TLR4 and found that they reduced TNF-α production and LPS-induced inflammation in mouse livers.
In addition to the strategy of inhibiting TLRs, efforts are also being made to selectively inhibit their adaptors and downstream signaling molecules[131]. In such studies, Compound 4a inhibited the interaction of MyD88 protein with the TIR domain of TLRs and type I IL-1 receptor[132], whereas MyD88 inhibitors ST2825 and RO0884 blocked the recruitment of IRAK-1 and IRAK-4 in human cells[133,134]. Additionally, ST2825 also suppressed B cell proliferation and differentiation[133]. However, the efficacy of these molecules in reducing inflammation in the liver is yet to be determined.
MiRNAs are a class of small, noncoding RNAs that act as key regulators of many cellular processes. During the past decade, it has been well established that the aberrant expression of a large number of miRNAs correlate with disease severity and poor prognosis of HCC[135-144]. The expression of these “miRNA signatures” are unique for a given disease stage, and were useful in identifying patients with HCC who are likely to develop metastases and recurrence. In recent years, efforts to identify miRNAs involved in the regulation of innate immune genes has resulted in the identification of a few promising miRNA candidates that can be exploited for therapeutic purposes. For example, overexpression of miR-155 which is directly involved in the regulation of more than 30 innate immune genes[145] results in the significant reduction of MyD88 expression and IL-8 production induced by Helicobacter pylori[146]. Interestingly, in bone marrow-derived macrophages and RAW264.7 cells, IL-10 inhibits miR-155 expression after LPS stimulation and dampens inflammatory immune responses in a STAT-3 dependent manner[147].
When THP-1 monocytes were treated with ligands for TLRs 2, 4 and 5, production of TNF-α correlated inversely with the up-regulation of miR-146a[148], and when the tragets of miR-146a IRAK-1 or TRAF6 were knocked down, inflammatory responses to TLR2, TLR4 and TLR5 ligands were significantly reduced in these cells suggesting a role for miR-146a in LPS-induced cross-tolerance. Similarly, miR-132 and miR-212 were shown to be involved in TLR2-mediated cross-tolerance through IRAK4 modulation[149]. In addition, miR-146a disrupts the translation of TLR4-induced TNF-α production in these cells[150]. miR-146b exerts this effect in monocytes by negatively regulating TLR4 via a IL-10-mediated STAT3 dependent loop[151]. Another study on the expression profiling of endotoxin responsive genes in human monocytes has revealed that miR-146 regulates TLR and cytokine signaling in a NF-κB-dependent manner through a negative feedback regulation loop involving the down-regulation of IRAK-1 and TRAF6 protein levels[152]. In THP-1 cells, miR-146a also regulates TLR2 signaling by reducing IRAK-1 and phosphorylated IκBα expression[153].
In another study, miR-181a expression was found to be significantly elevated in mice stimulated with LPS and streptozotocin, which correlated strongly with the expression of inflammatory factors TNF-α, IL-6, IL-1β in macrophages suggesting that the inhibition of miR-181a could reduce TLR4-induced inflammation[154]. After stimulation with LPS, miR-92a significantly inhibited the activation of JNK/c-Jun pathway and the production of inflammatory cytokines in macrophages by directly targeting mitogen-activated protein kinase kinase 4[155], demonstrating that miR-92a is a negative regulator of TLR-triggered immune responses.
In other studies, Benakanakere et al[156] demonstrated that miR-105 binds directly to TLR2 and regulates its expression. When keratinocytes were challenged by a TLR2 agonist or when miR-105 was overexpressed, a strong inverse correlation between miR-105 expression and TLR2 protein levels was observed. Similar to miR-105, let-7i was recently shown to bind directly to TLR4 in cholangiocytes and to control its expression by translational repression[157]. In this study, let-7i mimics inhibited Cryptospiridium parvum induced TLR4 production. In dendritic cells of mice with colitis, the expression of miR-10a was found to be negatively regulated by the intestinal microbiota, which correlated inversely with the production of IL-12/IL-23p40[158]. This finding suggests that miR-10a targets IL-12/IL-23p40 to maintain immune homeostasis.
When stimulated with ligands for TLRs 2-4 miR-147 is induced in murine macrophages[159]. Its expression was found to be greater after the activation of TLR4 than that of TLR2 or TLR4, and was dependent on both MyD88 and TRIF. In vivo, TLR stimulation induced the expression of miR-147 as a negative-feedback loop mechanism to prevent excessive inflammatory responses[159]. Likewise, miR-145 targets the adaptor protein MAL, which facilitates the interaction between TLR and TLR4 with MyD88, and inhibits its expression[152]. Recently, Wendlandt et al[160] demonstrated that miR-200b and miR-200c reduced the expression of MyD88 but had no effect TLR4, IRAK-1 and TRAF6 in HEK293 cells. When miR-200b and miR-200c mimics were overexpressed, LPS-induced expression of IL-6, TNF-α, and CXCL9 was diminished, suggesting that these miRNAs regulate TLR4 signaling via MyD88-dependent manner[160]. Up-regulated miR-19a and miR-19b were able to inhibit the expression of suppressor of cytokine signaling 1, a gene important in the negative regulation of TLR signaling[161].
Programmed cell death 4 (PDCD4) is a pro-inflammatory protein that suppresses IL-10 production and activates NF-κB. Sheedy et al[162] found that PDCD4-deficient mice are protected from LPS-induced death and demonstrated that miR-21 targets PDCD4 after LPS stimulation to negatively regulate TLR4 pathway. Recently, Fabbri et al[163] reported that miR-21 and miR-29b secreted by lung cancer cells in exosomes function as TLR ligands. These miRNAs bind to and activate TLR7 and TLR8 signaling pathways in peritoneal macrophages cells, causing NF-κB activation and secretion of pro-metastatic inflammatory cytokines[163]. Secreted miR-21 was also found in the serum of HCC patients and in individuals with chronic hepatitis indicating that miR-21 is a key factor in hepatocarcinogenesis[164]. Several other circulating miRNAs have also been identified in the serum of patients with HCC[165-167]. Further studies are needed to determine whether any of these miRNAs can be used to modulate the TLR signaling pathways, and to alleviate inflammation in liver tissues.
Hepatocarcinogenesis is a multifactorial disease in which the expression of a large number of genes, proteins, and other molecules from diverse cellular processes is altered[2]. Growing evidence suggests that inflammation plays a critical role in the progression of liver diseases to HCC. Therefore, the use of combination therapy that targets multiple different steps and pathways, rather than a single test or a set of tests, to reduce inflammation is an appropriate therapeutic strategy to combat the development of HCC. Positive therapeutic outcomes achieved with sorafenib that can inhibit receptor tyrosine kinases of multiple signaling cascades[168], as well as evidence that a single molecule miR-26a can significantly reduce HCC without any toxicity[169] demonstrate the success of this multi-pronged approach.
Despite recent advances in the use of miRNAs either as antagomirs or in replacement therapy, the field of miRNA therapeutics for HCC is still in its infancy. Only a handful of successful outcomes have been reported so far[135]. One such success story was that of MiraVasen, which inhibited the activity of miR-122 and suppressed HCV viraemia with no evidence for viral resistance or side effects in chimpanzees[170]. This was the first miRNA-based therapeutic drug developed to treat a liver disease, and is currently being tested by Santaris Pharma (Hørsholm, Denmark) in phase 2 clinical trials[171]. The first successful demonstration of miRNA replacement to restore the expression levels of a down-regulated miRNA by delivery to the tumor was reported using a miR-26a-encoding AAV vector in a mouse model of HCC[169]. More recently, another miRNA down-regulated in HCC, miR-34a, has been successfully tested using this approach an orthotopic model of HCC[172]. In latter two cases, significant tumor reduction, dramatic protection from disease progression without toxicity, and prolonged survival of animals has been reported. While these reports were aimed to restore a loss of function, similar success has not yet been achieved for HCC treatment using the approaches to inhibit miRNA expression levels. In future investigations, it is important to exercise caution while designing clinical trials with miRNA mimics and TLR inhibitors for targeting multiple genes relevant to the liver diseases because of the concerns about potential toxicity in normal tissues, and as they can cause severe detrimental effects due to imbalances in TLR expression, and cellular functions that could cause immune paralysis leading to development of HCC.
| 1. | Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S206-S214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 405] [Article Influence: 25.3] [Reference Citation Analysis (2)] |
| 2. | Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 513] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 3. | Lang K, Danchenko N, Gondek K, Shah S, Thompson D. The burden of illness associated with hepatocellular carcinoma in the United States. J Hepatol. 2009;50:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Severi T, van Malenstein H, Verslype C, van Pelt JF. Tumor initiation and progression in hepatocellular carcinoma: risk factors, classification, and therapeutic targets. Acta Pharmacol Sin. 2010;31:1409-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4301] [Article Influence: 226.4] [Reference Citation Analysis (2)] |
| 6. | Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1576] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 7. | Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 2011;21:35-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 320] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 8. | Schrader J, Iredale JP. The inflammatory microenvironment of HCC - the plot becomes complex. J Hepatol. 2011;54:853-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Wu SD, Ma YS, Fang Y, Liu LL, Fu D, Shen XZ. Role of the microenvironment in hepatocellular carcinoma development and progression. Cancer Treat Rev. 2012;38:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 869] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 11. | Mueller L, Goumas FA, Affeldt M, Sandtner S, Gehling UM, Brilloff S, Walter J, Karnatz N, Lamszus K, Rogiers X. Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am J Pathol. 2007;171:1608-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Yang B, Du Z, Bai T, Gao YT, Wang YJ, Lou C, Wang FM, Bai Y. Aberrant methylation of SPARC in human hepatocellular carcinoma and its clinical implication. World J Gastroenterol. 2012;18:2043-2052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 14. | Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41:2522-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728-5739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 970] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 16. | Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067-8075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 17. | Leonardi GC, Candido S, Cervello M, Nicolosi D, Raiti F, Travali S, Spandidos DA, Libra M. The tumor microenvironment in hepatocellular carcinoma (review). Int J Oncol. 2012;40:1733-1747. [PubMed] |
| 18. | Ramaiah SK, Rittling S. Pathophysiological role of osteopontin in hepatic inflammation, toxicity, and cancer. Toxicol Sci. 2008;103:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Lin A, Schildknecht A, Nguyen LT, Ohashi PS. Dendritic cells integrate signals from the tumor microenvironment to modulate immunity and tumor growth. Immunol Lett. 2010;127:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, Sakaki M, Doi H, Uozumi S, Omori R. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2191] [Article Influence: 121.7] [Reference Citation Analysis (1)] |
| 22. | Benetti A, Berenzi A, Gambarotti M, Garrafa E, Gelati M, Dessy E, Portolani N, Piardi T, Giulini SM, Caruso A. Transforming growth factor-beta1 and CD105 promote the migration of hepatocellular carcinoma-derived endothelium. Cancer Res. 2008;68:8626-8634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Xiong YQ, Sun HC, Zhang W, Zhu XD, Zhuang PY, Zhang JB, Wang L, Wu WZ, Qin LX, Tang ZY. Human hepatocellular carcinoma tumor-derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clin Cancer Res. 2009;15:4838-4846. [PubMed] |
| 24. | Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 715] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 25. | Lee WC, Wu TJ, Chou HS, Yu MC, Hsu PY, Hsu HY, Wang CC. The impact of CD4+ CD25+ T cells in the tumor microenvironment of hepatocellular carcinoma. Surgery. 2012;151:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Wang F, Jing X, Li G, Wang T, Yang B, Zhu Z, Gao Y, Zhang Q, Yang Y, Wang Y. Foxp3+ regulatory T cells are associated with the natural history of chronic hepatitis B and poor prognosis of hepatocellular carcinoma. Liver Int. 2012;32:644-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, Zheng SS. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol. 2012;29:1817-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Mathai AM, Kapadia MJ, Alexander J, Kernochan LE, Swanson PE, Yeh MM. Role of Foxp3-positive tumor-infiltrating lymphocytes in the histologic features and clinical outcomes of hepatocellular carcinoma. Am J Surg Pathol. 2012;36:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, Weber A, Slankamenac K, Poon RT, Yang H. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012;61:427-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 30. | Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS, Xu YF. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 31. | Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 1776] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 32. | Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6034] [Cited by in RCA: 6395] [Article Influence: 290.7] [Reference Citation Analysis (0)] |
| 33. | Fearon DT. Seeking wisdom in innate immunity. Nature. 1997;388:323-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 164] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Creagh EM, O’Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 561] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 35. | Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 697] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 36. | Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 2640] [Article Influence: 88.0] [Reference Citation Analysis (4)] |
| 37. | Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3900] [Cited by in RCA: 3835] [Article Influence: 132.2] [Reference Citation Analysis (11)] |
| 38. | Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1590] [Article Influence: 79.5] [Reference Citation Analysis (14)] |
| 39. | Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8061] [Cited by in RCA: 8989] [Article Influence: 449.5] [Reference Citation Analysis (1)] |
| 40. | Coll RC, O’Neill LA. New insights into the regulation of signalling by toll-like receptors and nod-like receptors. J Innate Immun. 2010;2:406-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 41. | Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci USA. 2005;102:9577-9582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 857] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 42. | Tsujita T, Tsukada H, Nakao M, Oshiumi H, Matsumoto M, Seya T. Sensing bacterial flagellin by membrane and soluble orthologs of Toll-like receptor 5 in rainbow trout (Onchorhynchus mikiss). J Biol Chem. 2004;279:48588-48597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Henneke P, Takeuchi O, van Strijp JA, Guttormsen HK, Smith JA, Schromm AB, Espevik TA, Akira S, Nizet V, Kasper DL. Novel engagement of CD14 and multiple toll-like receptors by group B streptococci. J Immunol. 2001;167:7069-7076. [PubMed] |
| 44. | Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554-561. [PubMed] |
| 45. | Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 345] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 46. | Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: a contemporary view on liver diseases. Hepatology. 2006;44:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 47. | Liu S, Gallo DJ, Green AM, Williams DL, Gong X, Shapiro RA, Gambotto AA, Humphris EL, Vodovotz Y, Billiar TR. Role of toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immun. 2002;70:3433-3442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 48. | Matsumura T, Ito A, Takii T, Hayashi H, Onozaki K. Endotoxin and cytokine regulation of toll-like receptor (TLR) 2 and TLR4 gene expression in murine liver and hepatocytes. J Interferon Cytokine Res. 2000;20:915-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 144] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Wu J, Meng Z, Jiang M, Zhang E, Trippler M, Broering R, Bucchi A, Krux F, Dittmer U, Yang D. Toll-like receptor-induced innate immune responses in non-parenchymal liver cells are cell type-specific. Immunology. 2010;129:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 50. | Martin-Armas M, Simon-Santamaria J, Pettersen I, Moens U, Smedsrød B, Sveinbjørnsson B. Toll-like receptor 9 (TLR9) is present in murine liver sinusoidal endothelial cells (LSECs) and mediates the effect of CpG-oligonucleotides. J Hepatol. 2006;44:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Su GL, Klein RD, Aminlari A, Zhang HY, Steinstraesser L, Alarcon WH, Remick DG, Wang SC. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology. 2000;31:932-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 216] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 52. | Thobe BM, Frink M, Hildebrand F, Schwacha MG, Hubbard WJ, Choudhry MA, Chaudry IH. The role of MAPK in Kupffer cell toll-like receptor (TLR) 2-, TLR4-, and TLR9-mediated signaling following trauma-hemorrhage. J Cell Physiol. 2007;210:667-675. [PubMed] |
| 53. | Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 513] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 54. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1603] [Article Influence: 84.4] [Reference Citation Analysis (1)] |
| 55. | Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, Mehal WZ. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 56. | Hsu W, Shu SA, Gershwin E, Lian ZX. The current immune function of hepatic dendritic cells. Cell Mol Immunol. 2007;4:321-328. [PubMed] |
| 57. | Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 443] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 58. | Ma F, Zhang J, Zhang J, Zhang C. The TLR7 agonists imiquimod and gardiquimod improve DC-based immunotherapy for melanoma in mice. Cell Mol Immunol. 2010;7:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Chen Y, Sun R. Toll-like receptors in acute liver injury and regeneration. Int Immunopharmacol. 2011;11:1433-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Xu D, Liu H, Komai-Koma M. Direct and indirect role of Toll-like receptors in T cell mediated immunity. Cell Mol Immunol. 2004;1:239-246. [PubMed] |
| 61. | Sawaki J, Tsutsui H, Hayashi N, Yasuda K, Akira S, Tanizawa T, Nakanishi K. Type 1 cytokine/chemokine production by mouse NK cells following activation of their TLR/MyD88-mediated pathways. Int Immunol. 2007;19:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Meyer-Bahlburg A, Khim S, Rawlings DJ. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J Exp Med. 2007;204:3095-3101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 63. | Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065-6073. [PubMed] |
| 64. | Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA. 2004;101:3029-3034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 400] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 65. | Qian F, Bolen CR, Jing C, Wang X, Zheng W, Zhao H, Fikrig E, Bruce RD, Kleinstein SH, Montgomery RR. Impaired toll-like receptor 3-mediated immune responses from macrophages of patients chronically infected with hepatitis C virus. Clin Vaccine Immunol. 2013;20:146-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris). 2010;58:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 304] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 67. | Li YJ, Zhu P, Liang Y, Yin WG, Xiao JH. Hepatitis B virus induces expression of cholesterol metabolism-related genes via TLR2 in HepG2 cells. World J Gastroenterol. 2013;19:2262-2269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Chen Y, Chen Y, Zhao L, Chen Y, Mei M, Li Q, Huang A, Varghese Z, Moorhead JF, Ruan XZ. Inflammatory stress exacerbates hepatic cholesterol accumulation via disrupting cellular cholesterol export. J Gastroenterol Hepatol. 2012;27:974-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 69. | Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269-7272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 360] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 70. | Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, Szabo G. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 237] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 71. | Machida K, Cheng KT, Sung VM, Levine AM, Foung S, Lai MM. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol. 2006;80:866-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 72. | Hosomura N, Kono H, Tsuchiya M, Ishii K, Ogiku M, Matsuda M, Fujii H. HCV-related proteins activate Kupffer cells isolated from human liver tissues. Dig Dis Sci. 2011;56:1057-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Wang JP, Zhang Y, Wei X, Li J, Nan XP, Yu HT, Li Y, Wang PZ, Bai XF. Circulating Toll-like receptor (TLR) 2, TLR4, and regulatory T cells in patients with chronic hepatitis C. APMIS. 2010;118:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Zhang Y, Lian JQ, Huang CX, Wang JP, Wei X, Nan XP, Yu HT, Jiang LL, Wang XQ, Zhuang Y. Overexpression of Toll-like receptor 2/4 on monocytes modulates the activities of CD4(+)CD25(+) regulatory T cells in chronic hepatitis B virus infection. Virology. 2010;397:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Berzsenyi MD, Roberts SK, Preiss S, Woollard DJ, Beard MR, Skinner NA, Bowden DS, Visvanathan K. Hepatic TLR2 & TLR4 expression correlates with hepatic inflammation and TNF-α in HCV & HCV/HIV infection. J Viral Hepat. 2011;18:852-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Heim MH. Innate immunity and HCV. J Hepatol. 2013;58:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 77. | Imran M, Waheed Y, Manzoor S, Bilal M, Ashraf W, Ali M, Ashraf M. Interaction of Hepatitis C virus proteins with pattern recognition receptors. Virol J. 2012;9:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 78. | Sawhney R, Visvanathan K. Polymorphisms of toll-like receptors and their pathways in viral hepatitis. Antivir Ther. 2011;16:443-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1543] [Article Influence: 102.9] [Reference Citation Analysis (3)] |
| 80. | Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33:191-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 248] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 81. | Ajakaiye M, Jacob A, Wu R, Nicastro JM, Coppa GF, Wang P. Alcohol and hepatocyte-Kupffer cell interaction (review). Mol Med Rep. 2011;4:597-602. [PubMed] |
| 82. | Li Z, Diehl AM. Innate immunity in the liver. Curr Opin Gastroenterol. 2003;19:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 83. | Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 84. | Petrasek J, Dolganiuc A, Csak T, Nath B, Hritz I, Kodys K, Catalano D, Kurt-Jones E, Mandrekar P, Szabo G. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology. 2011;53:649-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 85. | Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323-34.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 627] [Article Influence: 39.2] [Reference Citation Analysis (1)] |
| 86. | Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 387] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 87. | Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 542] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 88. | Machida K, Tsukamoto H, Mkrtchyan H, Duan L, Dynnyk A, Liu HM, Asahina K, Govindarajan S, Ray R, Ou JH. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci USA. 2009;106:1548-1553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 89. | Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, Medof ME, Nagy LE. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology. 2007;132:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 90. | Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB, Nagy LE. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology. 2010;139:664-74, 674.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 91. | Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2236] [Cited by in RCA: 2652] [Article Influence: 189.4] [Reference Citation Analysis (0)] |
| 92. | Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1720] [Cited by in RCA: 1684] [Article Influence: 112.3] [Reference Citation Analysis (4)] |
| 93. | Farrell GC, van Rooyen D, Gan L, Chitturi S. NASH is an Inflammatory Disorder: Pathogenic, Prognostic and Therapeutic Implications. Gut Liver. 2012;6:149-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 94. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [PubMed] |
| 95. | Valenti L, Fracanzani AL, Fargion S. The immunopathogenesis of alcoholic and nonalcoholic steatohepatitis: two triggers for one disease? Semin Immunopathol. 2009;31:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Petrasek J, Csak T, Ganz M, Szabo G. Differences in innate immune signaling between alcoholic and non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2013;28 Suppl 1:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Csak T, Dolganiuc A, Kodys K, Nath B, Petrasek J, Bala S, Lippai D, Szabo G. Mitochondrial antiviral signaling protein defect links impaired antiviral response and liver injury in steatohepatitis in mice. Hepatology. 2011;53:1917-1931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 566] [Article Influence: 29.8] [Reference Citation Analysis (2)] |
| 99. | Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310-G1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 100. | Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 322] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 101. | Bertola A, Bonnafous S, Anty R, Patouraux S, Saint-Paul MC, Iannelli A, Gugenheim J, Barr J, Mato JM, Le Marchand-Brustel Y. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS One. 2010;5:e13577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 102. | Broering R, Lu M, Schlaak JF. Role of Toll-like receptors in liver health and disease. Clin Sci (Lond). 2011;121:415-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 103. | Wagnerberger S, Spruss A, Kanuri G, Volynets V, Stahl C, Bischoff SC, Bergheim I. Toll-like receptors 1-9 are elevated in livers with fructose-induced hepatic steatosis. Br J Nutr. 2012;107:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 104. | Csak T, Velayudham A, Hritz I, Petrasek J, Levin I, Lippai D, Catalano D, Mandrekar P, Dolganiuc A, Kurt-Jones E. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G433-G441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 105. | Szabo G, Velayudham A, Romics L, Mandrekar P. Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol Clin Exp Res. 2005;29:140S-145S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 106. | Maeda S. NF-κB, JNK, and TLR Signaling Pathways in Hepatocarcinogenesis. Gastroenterol Res Pract. 2010;2010:367694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 107. | Szlosarek PW, Balkwill FR. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol. 2003;4:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 277] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 108. | Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA. 2006;103:10544-10551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 354] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 109. | Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1403] [Cited by in RCA: 1480] [Article Influence: 70.5] [Reference Citation Analysis (4)] |
| 110. | Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 967] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 111. | Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 496] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 112. | Inokuchi S, Aoyama T, Miura K, Osterreicher CH, Kodama Y, Miyai K, Akira S, Brenner DA, Seki E. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci USA. 2010;107:844-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 113. | Nischalke HD, Coenen M, Berger C, Aldenhoff K, Müller T, Berg T, Krämer B, Körner C, Odenthal M, Schulze F. The toll-like receptor 2 (TLR2) -196 to -174 del/ins polymorphism affects viral loads and susceptibility to hepatocellular carcinoma in chronic hepatitis C. Int J Cancer. 2012;130:1470-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 114. | Kutikhin AG. Association of polymorphisms in TLR genes and in genes of the Toll-like receptor signaling pathway with cancer risk. Hum Immunol. 2011;72:1095-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 115. | Zhang H, Zhai Y, Hu Z, Wu C, Qian J, Jia W, Ma F, Huang W, Yu L, Yue W. Genome-wide association study identifies 1p36.22 as a new susceptibility locus for hepatocellular carcinoma in chronic hepatitis B virus carriers. Nat Genet. 2010;42:755-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 116. | Junjie X, Songyao J, Minmin S, Yanyan S, Baiyong S, Xiaxing D, Jiabin J, Xi Z, Hao C. The association between Toll-like receptor 2 single-nucleotide polymorphisms and hepatocellular carcinoma susceptibility. BMC Cancer. 2012;12:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 117. | Fujita Y, Taguchi H. Overview and outlook of Toll-like receptor ligand-antigen conjugate vaccines. Ther Deliv. 2012;3:749-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 118. | Weeratna RD, Makinen SR, McCluskie MJ, Davis HL. TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848). Vaccine. 2005;23:5263-5270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 119. | Hoebe K, Jiang Z, Georgel P, Tabeta K, Janssen E, Du X, Beutler B. TLR signaling pathways: opportunities for activation and blockade in pursuit of therapy. Curr Pharm Des. 2006;12:4123-4134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 120. | Vasileiou I, Kostopanagiotou G, Katsargyris A, Klonaris C, Perrea D, Theocharis S. Toll-like receptors: a novel target for therapeutic intervention in intestinal and hepatic ischemia-reperfusion injury? Expert Opin Ther Targets. 2010;14:839-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 121. | Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 689] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 122. | Thompson AJ, Colledge D, Rodgers S, Wilson R, Revill P, Desmond P, Mansell A, Visvanathan K, Locarnini S. Stimulation of the interleukin-1 receptor and Toll-like receptor 2 inhibits hepatitis B virus replication in hepatoma cell lines in vitro. Antivir Ther. 2009;14:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 123. | Horsmans Y, Berg T, Desager JP, Mueller T, Schott E, Fletcher SP, Steffy KR, Bauman LA, Kerr BM, Averett DR. Isatoribine, an agonist of TLR7, reduces plasma virus concentration in chronic hepatitis C infection. Hepatology. 2005;42:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 124. | Ma R, Du JL, Huang J, Wu CY. Additive effects of CpG ODN and R-848 as adjuvants on augmenting immune responses to HBsAg vaccination. Biochem Biophys Res Commun. 2007;361:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 125. | Gupta K, Cooper C. A review of the role of CpG oligodeoxynucleotides as toll-like receptor 9 agonists in prophylactic and therapeutic vaccine development in infectious diseases. Drugs R D. 2008;9:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 126. | Sung JJ, Lik-Yuen H. HBV-ISS (Dynavax). Curr Opin Mol Ther. 2006;8:150-155. [PubMed] |
| 127. | Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, Cameron DW, Heathcote J. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 267] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 128. | Rossignol DP, Lynn M. Antagonism of in vivo and ex vivo response to endotoxin by E5564, a synthetic lipid A analogue. J Endotoxin Res. 2002;8:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 129. | Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 130. | Cowden JM, Yu F, Challapalli M, Huang JF, Kim S, Fung-Leung WP, Ma JY, Riley JP, Zhang M, Dunford PJ. Antagonism of the histamine H4 receptor reduces LPS-induced TNF production in vivo. Inflamm Res. 2013;62:599-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 131. | Loiarro M, Ruggiero V, Sette C. Targeting the Toll-like receptor/interleukin 1 receptor pathway in human diseases: rational design of MyD88 inhibitors. Clin Lymphoma Myeloma Leuk. 2013;13:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 132. | Bartfai T, Behrens MM, Gaidarova S, Pemberton J, Shivanyuk A, Rebek J Jr. A low molecular weight mimic of the Toll/IL-1 receptor/resistance domain inhibits IL-1 receptor-mediated responses. Proc Natl Acad Sci USA. 2003;100:7971-7976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 133. | Loiarro M, Capolunghi F, Fantò N, Gallo G, Campo S, Arseni B, Carsetti R, Carminati P, De Santis R, Ruggiero V. Pivotal Advance: Inhibition of MyD88 dimerization and recruitment of IRAK1 and IRAK4 by a novel peptidomimetic compound. J Leukoc Biol. 2007;82:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 134. | Song KW, Talamas FX, Suttmann RT, Olson PS, Barnett JW, Lee SW, Thompson KD, Jin S, Hekmat-Nejad M, Cai TZ. The kinase activities of interleukin-1 receptor associated kinase (IRAK)-1 and 4 are redundant in the control of inflammatory cytokine expression in human cells. Mol Immunol. 2009;46:1458-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 135. | Aravalli RN. Development of microRNA therapeutics for hepatocellular carcinoma. Diagnostics. 2013;3:170-191. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 136. | Gramantieri L, Fornari F, Callegari E, Sabbioni S, Lanza G, Croce CM, Bolondi L, Negrini M. MicroRNA involvement in hepatocellular carcinoma. J Cell Mol Med. 2008;12:2189-2204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 137. | Ura S, Honda M, Yamashita T, Ueda T, Takatori H, Nishino R, Sunakozaka H, Sakai Y, Horimoto K, Kaneko S. Differential microRNA expression between hepatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology. 2009;49:1098-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 307] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 138. | Murakami Y, Yasuda T, Saigo K, Urashima T, Toyoda H, Okanoue T, Shimotohno K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25:2537-2545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 898] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 139. | Huang S, He X. The role of microRNAs in liver cancer progression. Br J Cancer. 2011;104:235-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 140. | Budhu A, Jia HL, Forgues M, Liu CG, Goldstein D, Lam A, Zanetti KA, Ye QH, Qin LX, Croce CM. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47:897-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 569] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 141. | Huang YS, Dai Y, Yu XF, Bao SY, Yin YB, Tang M, Hu CX. Microarray analysis of microRNA expression in hepatocellular carcinoma and non-tumorous tissues without viral hepatitis. J Gastroenterol Hepatol. 2008;23:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 142. | Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, Roberts LR, Schmittgen TD. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 425] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 143. | Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 543] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 144. | Li W, Xie L, He X, Li J, Tu K, Wei L, Wu J, Guo Y, Ma X, Zhang P. Diagnostic and prognostic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123:1616-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 231] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 145. | Gantier MP. New perspectives in MicroRNA regulation of innate immunity. J Interferon Cytokine Res. 2010;30:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 146. | Tang B, Xiao B, Liu Z, Li N, Zhu ED, Li BS, Xie QH, Zhuang Y, Zou QM, Mao XH. Identification of MyD88 as a novel target of miR-155, involved in negative regulation of Helicobacter pylori-induced inflammation. FEBS Lett. 2010;584:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 147. | McCoy CE, Sheedy FJ, Qualls JE, Doyle SL, Quinn SR, Murray PJ, O’Neill LA. IL-10 inhibits miR-155 induction by toll-like receptors. J Biol Chem. 2010;285:20492-20498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 148. | Nahid MA, Satoh M, Chan EK. Mechanistic role of microRNA-146a in endotoxin-induced differential cross-regulation of TLR signaling. J Immunol. 2011;186:1723-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 149. | Nahid MA, Yao B, Dominguez-Gutierrez PR, Kesavalu L, Satoh M, Chan EK. Regulation of TLR2-mediated tolerance and cross-tolerance through IRAK4 modulation by miR-132 and miR-212. J Immunol. 2013;190:1250-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 150. | El Gazzar M, Church A, Liu T, McCall CE. MicroRNA-146a regulates both transcription silencing and translation disruption of TNF-α during TLR4-induced gene reprogramming. J Leukoc Biol. 2011;90:509-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 151. | Curtale G, Mirolo M, Renzi TA, Rossato M, Bazzoni F, Locati M. Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci USA. 2013;110:11499-11504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 152. | Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481-12486. [PubMed] |
| 153. | Quinn EM, Wang JH, O’Callaghan G, Redmond HP. MicroRNA-146a is upregulated by and negatively regulates TLR2 signaling. PLoS One. 2013;8:e62232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 154. | Xie W, Li Z, Li M, Xu N, Zhang Y. miR-181a and inflammation: miRNA homeostasis response to inflammatory stimuli in vivo. Biochem Biophys Res Commun. 2013;430:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 155. | Lai L, Song Y, Liu Y, Chen Q, Han Q, Chen W, Pan T, Zhang Y, Cao X, Wang Q. MicroRNA-92a negatively regulates Toll-like receptor (TLR)-triggered inflammatory response in macrophages by targeting MKK4 kinase. J Biol Chem. 2013;288:7956-7967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 156. | Benakanakere MR, Li Q, Eskan MA, Singh AV, Zhao J, Galicia JC, Stathopoulou P, Knudsen TB, Kinane DF. Modulation of TLR2 protein expression by miR-105 in human oral keratinocytes. J Biol Chem. 2009;284:23107-23115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 157. | Chen XM, Splinter PL, O’Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J Biol Chem. 2007;282:28929-28938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 373] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 158. | Xue X, Feng T, Yao S, Wolf KJ, Liu CG, Liu X, Elson CO, Cong Y. Microbiota downregulates dendritic cell expression of miR-10a, which targets IL-12/IL-23p40. J Immunol. 2011;187:5879-5886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 159. | Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci USA. 2009;106:15819-15824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 375] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 160. | Wendlandt EB, Graff JW, Gioannini TL, McCaffrey AP, Wilson ME. The role of microRNAs miR-200b and miR-200c in TLR4 signaling and NF-κB activation. Innate Immun. 2012;18:846-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 161. | Pichiorri F, Suh SS, Ladetto M, Kuehl M, Palumbo T, Drandi D, Taccioli C, Zanesi N, Alder H, Hagan JP. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci USA. 2008;105:12885-12890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 458] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 162. | Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, Johnson DS, Chen Y, O’Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 808] [Article Influence: 47.5] [Reference Citation Analysis (14)] |
| 163. | Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109:E2110-E2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1301] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 164. | Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, Huang L, Li H, Tan W, Wang C. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 454] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 165. | Gailhouste L, Ochiya T. Cancer-related microRNAs and their role as tumor suppressors and oncogenes in hepatocellular carcinoma. Histol Histopathol. 2013;28:437-451. [PubMed] |
| 166. | Qi P, Cheng SQ, Wang H, Li N, Chen YF, Gao CF. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS One. 2011;6:e28486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 167. | Wong KF, Xu Z, Chen J, Lee NP, Luk JM. Circulating markers for prognosis of hepatocellular carcinoma. Expert Opin Med Diagn. 2013;7:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 168. | Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1394] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 169. | Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1388] [Cited by in RCA: 1372] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 170. | Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1294] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 171. | Lindow M, Kauppinen S. Discovering the first microRNA-targeted drug. J Cell Biol. 2012;199:407-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 172. | Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 541] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
P-Reviewer: Cao GW, Gangl A, Morise Z, Wang CC S-Editor: Ma YJ L- Editor: A E-Editor: Wang CHs