Published online Oct 28, 2013. doi: 10.3748/wjg.v19.i40.6825
Revised: July 27, 2013
Accepted: August 17, 2013
Published online: October 28, 2013
Processing time: 163 Days and 6.9 Hours
AIM: To explore the physiopathology and magnetic resonance imaging (MRI) findings in an animal model of acute arterial mesenteric ischemia (AAMI) with and without reperfusion.
METHODS: In this study, 8 adult Sprague-Dawley rats underwent superior mesenteric artery (SMA) ligation and were then randomly divided in two groups of 4. In group I, the ischemia was maintained for 8 h. In group II, 1-h after SMA occlusion, the ligation was removed by cutting the thread fixed on the back of the animal, and reperfusion was monitored for 8 h. MRI was performed using a 7-T system.
RESULTS: We found that, in the case of AAMI without reperfusion, spastic reflex ileus, hypotonic reflex ileus, free abdominal fluid and bowel wall thinning are present from the second hour, and bowel wall hyperintensity in T2-W sequences are present from the fourth hour. The reperfusion model shows the presence of early bowel wall hyperintensity in T2-W sequences after 1 h and bowel wall thickening from the second hour.
CONCLUSION: Our study has shown that MRI can assess pathological changes that occur in the small bowel and distinguish between the presence and absence of reperfusion after induced acute arterial ischemia.
Core tip: Diagnosis of acute arterial mesenteric ischemia depends on early detection and findings with regarding the presence or absence of reperfusion events. Distinguishing between these different conditions is crucial to improving outcome for the patient and represents a challenge for radiologists. The results of this preliminary study in an animal model provide for a time-based definition of the radiological findings in ischemia and reperfusion, showing that magnetic resonance imaging can adequately assess the different pathological changes that occur in acute arterial mesenteric ischemia with or without reperfusion.
- Citation: Saba L, Berritto D, Iacobellis F, Scaglione M, Castaldo S, Cozzolino S, Mazzei MA, Mizio VD, Grassi R. Acute arterial mesenteric ischemia and reperfusion: Macroscopic and MRI findings, preliminary report. World J Gastroenterol 2013; 19(40): 6825-6833
- URL: https://www.wjgnet.com/1007-9327/full/v19/i40/6825.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i40.6825
Mesenteric ischemia can have various causes[1]. One of the most threatening conditions is acute arterial mesenteric ischemia (AAMI), which is characterized by the acute occlusion of the arteries feeding the small bowel[2]. AAMI is considered a vascular emergency with a mortality rate of up to 60%-80%[3].
Mortality of patients with AAMI most likely remains high because ischemic changes of the abdominal organs are most frequently detected and diagnosed at a late stage when therapy is ineffective[4,5]. Multi-detector computed tomography angiography is currently considered the gold-standard for imaging mesenteric and colonic ischemia[6,7]. However, recent papers have shown that magnetic resonance imaging (MRI) has significant potential for detecting subtle changes that may affect the intestinal wall during the ischemic process[8].
Recently, some researchers[9] assessed the evolution of ischemic lesions using 7 Tesla-magnetic resonance imaging (7T-MRI) in an animal model of acute colonic ischemia. This work demonstrated that MRI can be used as a reliable diagnostic and grading technique in acute ischemic colitis, which would allow for the early identification of pathology.
The purpose of this study was to explore physiopathology and MRI findings of mesenteric ischemia in an animal model, with and without reperfusion, to define semiological changes of mesenteric ischemia from both a macroscopic and MRI point-of-view.
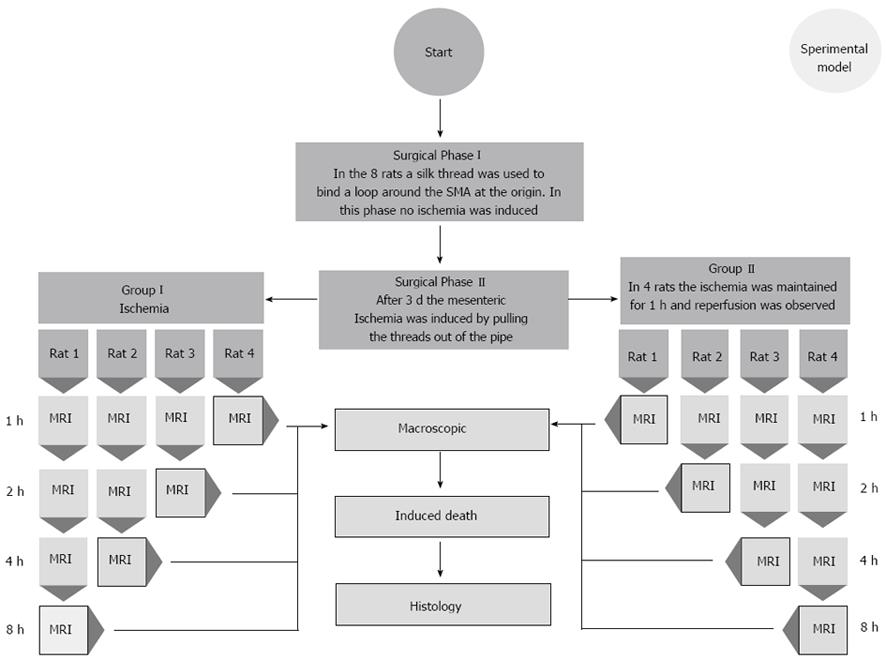
All procedures performed on animals were approved by the Animal Care and Use Committee of the Biotechnology Center of Cardarelli Hospital. Eight adult male Sprague-Dawley rats (250-340 g; Harlan, United States) were randomly divided in two groups of 4 rats each (group I and group II). The rats were maintained on a 12/12 h light/dark cycle and allowed free access to food and water. They were anesthetized with ketamine hydrochloride 100 mg/kg IM (CU ChemieUetikon GmbH, Lahr, Germany) and Domitor 0.25 mg/kg IM (Medethomidine hydrochloride, Pfizer, NY, United States) injections. Dolorex 0.1 mg/kg sc (Butarphanol, Intervet, Boxmeer, The Netherlands) was used immediately before the procedure to ensure intra-operative analgesia. Further injections of these drugs were provided throughout the operation to maintain a sufficient state of anesthesia. Each rat was allowed to breathe spontaneously. Body temperature was monitored with a rectal probe and maintained at 37.0 ± 0.5 °C with a heating blanket regulated by a homeothermic blanket control unit (Harvard Apparatus Limited, Edenbridge Kent, United Kingdom). After drug injection, rats were prepared for surgery via abdominal depilation. The area was then washed with povidone iodine and alcohol.
Surgery and drug administration were performed by a board-certified veterinarian with 5 years of experience in microsurgical and vascular techniques (Scaglione M). In all rats, acute SMA occlusion was induced using a two-step surgical procedure (Figure 1). After midline laparotomy, the bowel was exposed in the abdominal cavity and displaced to the left, allowing identification of the SMA. The bowel and the mesentery were drawn out of the abdominal cavity and a snapshot of exposed organs was taken (Nikon Coolpix S210, 8.0 Megapixels resolution, ISO 2000, Japan) as a basal image.
A 3/0 silk thread was used to wind a loop around the SMA at the origin, not yet tightening the loop. The tips of the thread were brought into a silicon pipe and, through it, carried out to the back of the animal, between its shoulders. The thread tips were attached to the pipe using medical plaster. Muscles and skin were closed in two layers using a 2/0 Vicryl thread. 10 mg/kg of Baytril 10% (Enrofloxacin 2.5%, Bayer AG, Leverkusen, Germany) was topically applied to the wounds to prevent infections. The animals were returned to their cages after awakening, and water and food were provided ad libitum. Three days after surgery, the rats underwent a second anesthetic treatment according to the same drug protocol, and acute mesenteric ischemia was induced by pulling the threads out of the pipe. In this way, the loop around the SMA was squeezed, and the arterial inflow through this artery was stopped. In group I rats, the ischemia was maintained for 8 h and during this time all animals were monitored at predetermined time points. In group II rats, the ligation was removed 1 h after SMA occlusion by cutting the thread fixed on the back of the animal; the following reperfusion was monitored for 8 h.
All animals underwent MRI of the abdomen using a 7-T micro-MRI scanner (BioSpec 70/16US, Bruker Medical Systems, Ettlingen, Germany). Two abdominal radiologists (Saba L and Grassi R), with 10 and 15 years of experience, respectively, assessed all 7T MR images by consensus. In all the rats, just before pulling the threads and occluding the vessel, a localizer scan along the three orthogonal planes [repetition time (TR) = 6.0 ms, echo time (TE) = 100.0 ms, field of view (FOV) = 8, Averages = 1, Flip Angle 30 deg, Matrix = 128, Slice thickness = 2.00 mm, Scan time = 12 s 800 ms] and a T2-weighted (T2-W) TurboRare sequence (TR = 4428.7 ms, TE = 36 ms; 180° flip angle; 38 slices; slice thickness 1 mm, interslice distance 1 mm, field of view, 6 cm; acquisition matrix, 256 × 256, scan time = 7 min 5 s 156 ms) were performed to serve as baseline images. Before and after the SMA occlusion, to insure the complete lack of flow in the vessels, a FLASH TOF 2D sequence was performed (TR = 12 ms, TE = 3.5 ms; 90° flip angle; 180 slices; slice thickness 0.4 mm, interslice distance 0.25 mm, field of view, 6 cm; acquisition matrix, 256 × 256, averages = 7, acquisition time 48 min 23 s 40 ms). Maximum intensity projection (MIP) images were reconstructed for each series of images to evaluate SMA occlusion.
For those rats in which lack of flow in the SMA after occlusion was confirmed, additional T2-W TurboRare sequences were performed at predetermined time-points to identify and characterize signs of bowel necrosis. For group II rats, a second FLASH TOF 2D sequence (TR = 12 ms, TE = 3.5 ms; 90° flip angle; 180 slices; slice thickness 0.4 mm, interslice distance 0.25 mm, field of view, 6 cm; acquisition matrix, 256 × 256, averages = 7, acquisition time 48 min 23 s 40 ms) after reperfusion was performed to verify the bloodflow through the SMA.
At each time-point in each rat, after opening the pre-existing midline laparotomy, the bowel and the mesentery were drawn out of the abdominal cavity, and a picture of the exposed organs was taken (Nikon Coolpix S210, 8.0 Megapixels, ISO 2000, Japan). The rat was then euthanized by an intrapulmonary injection of Tanax 0.5 mL (Enbutramide + Mebenzonium, Iodide + Tetracaine, Intervet/Shering-Plough Animal Healt, Boxmeer; The Netherlands).
MR images were assessed for the following parameters: (1) presence of free fluid in the abdomen; (2) gas filled dilated loops, known as hypotonic reflex ileus (HRI); (3) gas-fluid mixed stasis dilated loops (paralytic ileus, PI); (4) bowel wall thinning or thickening; (5) wall signal intensity on T2-W sequences; (6) mesenteric vessels; (7) wall pneumatosis; and (8) presence of free gas in the abdomen.
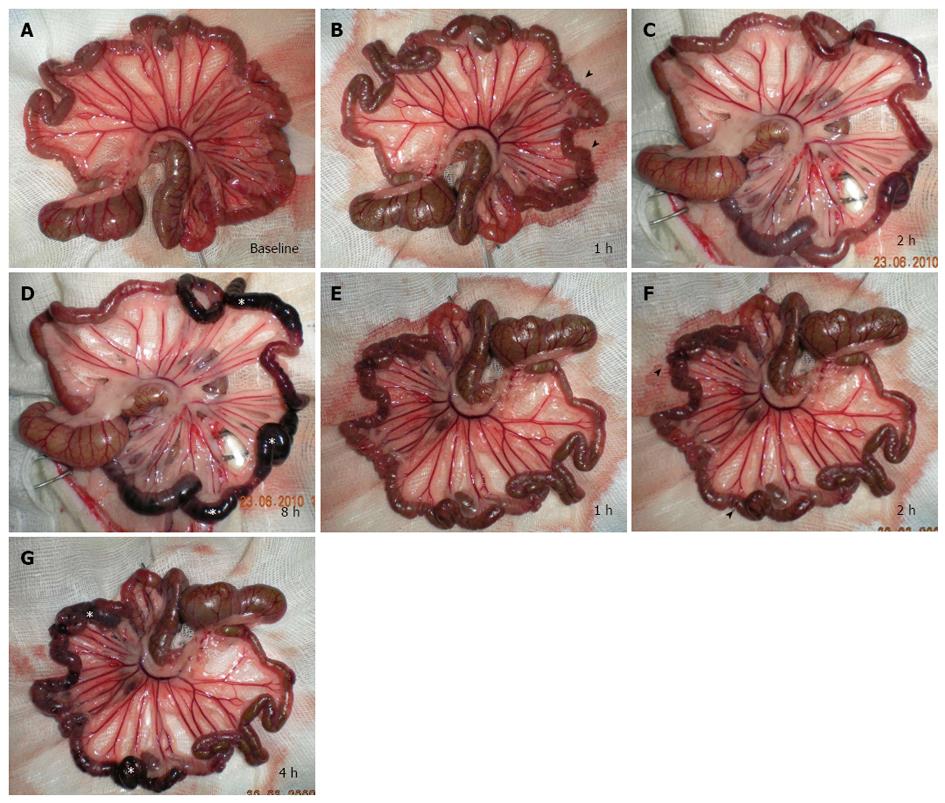
Immediately before the SMA ligation the bowel loops presented an average diameter of 1.5 mm (measured with a gauge), uniform serosa and rose-colored mesentery in all segments (Figure 2A). In all rats, 1 h after ischemia (Figure 2B), pale mesentery with thinning of mesenteric vessels and a spasm of the jejunal loops were evident, compared with the baseline assessment.
In group I rats, 2 h after acute SMA occlusion the spasm was replaced by definite HRI (Figure 2C). The ileal loops underwent the same pathological evolution, but the spasm was observed approximately 2 h after acute SMA occlusion, with transition to definite HRI after approximately 4 h. At this stage, a chromatic change from pink to dark red was observed in the loops of the middle portion of the ileum, then became charcoal black in the rat observed for 8 h, spreading to a cover larger ileal segment - both proximal and distal (Figure 2D). In group II rats, vascular congestion of mesenteric vessels was evident 1 hour after reperfusion (Figure 2E). After 2 h of reperfusion, damaged areas underwent chromatic change from pink to dark red, then became charcoal black at 4 h and growing more apparent in subsequent hours (Figure 2F and G).
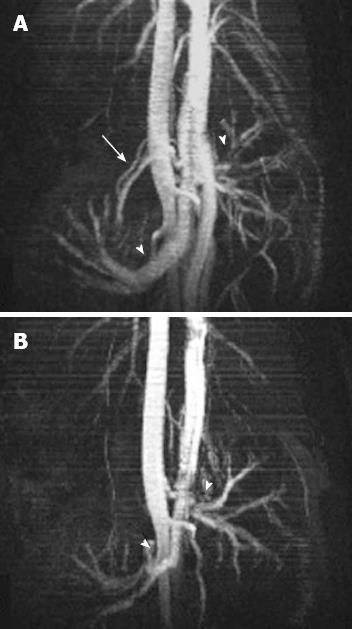
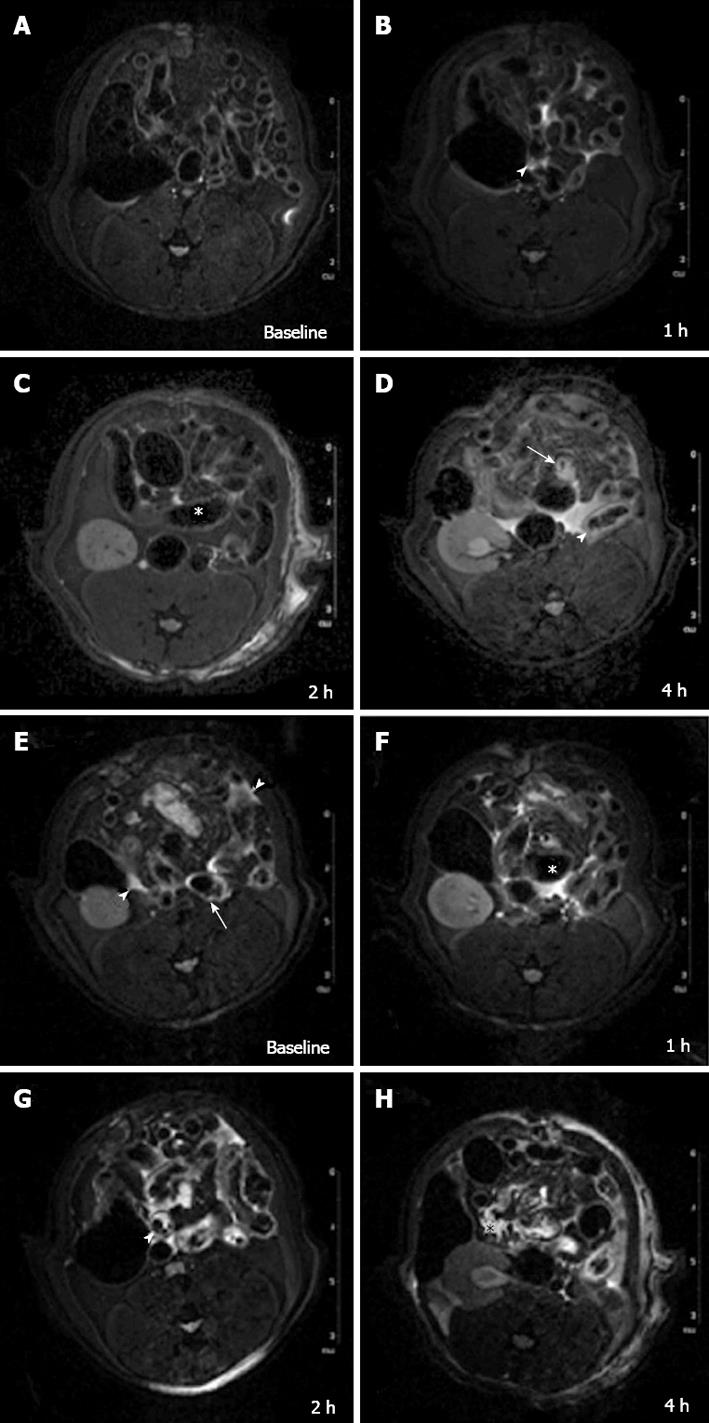
In all cases 7-T micro-MRI identified findings of intestinal ischemia. Immediately before inducing acute SMA obstruction, 3 d after laparotomy, no free gas within the abdominal cavity or signs of visceral or mesentery irritation were found. Compared with the baseline acquisition, the MR angiography sequence at 5 min showed that flow in the SMA had stopped (Figure 3). In group I (Table 1), 1 h after SMA ligation, T2-W sequences showed minimal change - a thin collection of fluid between the loops – compared with control scans (Figure 4A and B).
| 1-h | 2-h | 4-h | 8-h | ||
| Group I: Magnetic resonance imaging findings | |||||
| Rat 1 | Free fluid | 1 | 1 | 1 | 1 |
| Free gas | 0 | 0 | 0 | 0 | |
| HRI | 0 | 1 | 1 | 1 | |
| PI | 0 | 0 | 1 | 1 | |
| Bowel wall thinning | 0 | 1 | 1 | 1 | |
| Bowel wall thickening | 0 | 0 | 0 | 0 | |
| Wall signal (T2-W sequences) | 0 | 0 | 1 | 1 | |
| Wall pneumatosis | 0 | 0 | 0 | 0 | |
| Rat 2 | Free fluid | 1 | 1 | 1 | Rat dead |
| Free gas | 0 | 0 | 0 | ||
| HRI | 0 | 1 | 1 | ||
| PI | 0 | 0 | 1 | ||
| Bowel wall thinning | 0 | 1 | 1 | ||
| Bowel wall thickening | 0 | 0 | 0 | ||
| Wall signal (T2-W sequences) | 0 | 0 | 1 | ||
| Wall pneumatosis | 0 | 0 | 0 | ||
| Rat 3 | Free fluid | 1 | 1 | Rat dead | |
| Free gas | 0 | 0 | |||
| HRI | 0 | 1 | |||
| PI | 0 | 0 | |||
| Bowel wall thinning | 0 | 1 | |||
| Bowel wall thickening | 0 | 0 | |||
| Wall signal (T2-W sequences) | 0 | 0 | |||
| Wall pneumatosis | 0 | 0 | |||
| Rat 4 | Free fluid | 1 | Rat dead | ||
| Free gas | 0 | ||||
| HRI | 0 | ||||
| PI | 0 | ||||
| Bowel wall thinning | 0 | ||||
| Bowel wall thickening | 0 | ||||
| Wall signal (T2-W sequences) | 0 | ||||
| Wall pneumatosis | 0 | ||||
| Group II: Magnetic resonance imaging findings | |||||
| Rat 5 | Free fluid | 1 | 1 | 1 | 1 |
| Free gas | 0 | 0 | 0 | 0 | |
| HRI | 0 | 1 | 1 | 1 | |
| PI | 0 | 0 | 1 | 1 | |
| Bowel wall thinning | 0 | 0 | 0 | 0 | |
| Bowel wall thickening | 0 | 1 | 1 | 1 | |
| Wall signal (T2-W sequences) | 1 | 1 | 1 | 1 | |
| Wall pneumatosis | 0 | 0 | 0 | 0 | |
| Rat 6 | Free fluid | 1 | 1 | 1 | Rat dead |
| Free gas | 0 | 0 | 0 | ||
| HRI | 0 | 1 | 1 | ||
| PI | 0 | 0 | 1 | ||
| Bowel wall thinning | 0 | 0 | 0 | ||
| Bowel wall thickening | 0 | 1 | 1 | ||
| Wall signal (T2-W sequences) | 1 | 1 | 1 | ||
| Wall pneumatosis | 0 | 0 | 0 | ||
| Rat 7 | Free fluid | 1 | 1 | Rat dead | |
| Free gas | 0 | 0 | |||
| HRI | 0 | 1 | |||
| PI | 0 | 0 | |||
| Bowel wall thinning | 0 | 0 | |||
| Bowel wall thickening | 0 | 1 | |||
| Wall signal (T2-W sequences) | 1 | 1 | |||
| Wall pneumatosis | 0 | 0 | |||
| Rat 8 | Free fluid | 1 | Rat dead | ||
| Free gas | 0 | ||||
| HRI | 0 | ||||
| PI | 0 | ||||
| Bowel wall thinning | 0 | ||||
| Bowel wall thickening | 0 | ||||
| Wall signal (T2-W sequences) | 1 | ||||
| Wall pneumatosis | 0 | ||||
Dilation of numerous bowel loops (HRI) (mean diameter 4 mm, compared with 2.5 mm in baseline acquisition) and reduced wall thickness (average 0.5 mm compared with 1 mm in baseline acquisition) were already apparent at approximately 2 h of ischemia (Figure 4C). HRI was followed by PI (gas-liquid stasis) that was clearly evident 4 h after SMA ligation. A hyper-intense signal in the bowel wall was evident after 4 h in some loops (Figure 4D).
In group II (Table 1), an early hyper-intense signal of the bowel wall in some loops and a thin layer of peritoneal fluid were detected 1 h after reperfusion (Figure 4E). After 2 h, a small amount of peritoneal free fluid, bowel wall thickening (average thickness of 1.5 mm) and hyperintensity of the intestinal wall (Figure 4F) were observed. HRI in some loops and additional free fluid were related to previous findings. At 4 h, a PI (average diameter of intestinal lumen of 4.5 mm) with a larger amount of peritoneal fluid, a mild mesenteric engorgement and a hyper-intense signal of the intestinal wall became evident in many loops (Figure 4G and H). By comparing the different MRI signal characteristics of the rats that underwent the reperfusion versus those that did not undergo the reperfusion (Table 2), we found differences between the appearances of bowel wall thickening and wall signal (in T2-W).
| Time | 1-h | 2-h | 4-h | 8-h |
| Group I percentages | ||||
| Free fluid | 100% | 100% | 100% | 100% |
| Free gas | 0% | 0% | 0% | 0% |
| HRI | 0% | 100% | 100% | 100% |
| PI | 0% | 0% | 100% | 100% |
| Bowel wall thinning | 0% | 100% | 100% | 100% |
| Bowel wall thickening | 0% | 0% | 0% | 0% |
| Wall signal (T2-W sequences) | 0% | 0% | 100% | 100% |
| Wall pneumatosis | 0% | 0% | 0% | 0% |
| Group II percentages | ||||
| Free fluid | 100% | 100% | 100% | 100% |
| Free gas | 0% | 0% | 0% | 0% |
| HRI | 0% | 100% | 100% | 100% |
| PI | 0% | 0% | 100% | 100% |
| Bowel wall thinning | 0% | 0% | 0% | 0% |
| Bowel wall thickening | 0% | 100% | 100% | 100% |
| Wall signal (T2-W sequences) | 100% | 100% | 100% | 100% |
| Wall pneumatosis | 0% | 0% | 0% | 0% |
| Differences between the two groups | ||||
| Free fluid | No | No | No | No |
| FRee gas | No | No | No | No |
| HRI | No | No | No | No |
| PI | No | No | No | No |
| Bowel wall thinning | No | Yes | Yes | Yes |
| Bowel wall thickening | No | Yes | Yes | Yes |
| Wall signal (T2-W sequences) | Yes | Yes | No | No |
| Wall pneumatosis | No | No | No | No |
The morbidity and mortality of mesenteric ischemia are quite high[10]; some authors assert that this high mortality is associated with the low sensitivity of diagnostic tools, which may hinder a correct diagnosis in the early phase of ischemia[11,12]. Early identification of mesenteric ischemia is critical to plan an appropriate therapeutic approach. Moreover, different characteristics of the bowel corresponding to different pathogenesis are not clearly defined in imaging studies, adding further difficulties to obtaining an early and precise diagnosis[13].
We defined the progress of 2 different mechanisms of mesenteric ischemia - the acute occlusion of SMA and the acute occlusion of SMA followed by reperfusion - and investigated MRI findings in a time course. Most studies about ischemia/reperfusion reported in literature[14] analyze histological or biochemical alteration. In contrast, we focused our research on the development of radiological evidence for intestinal damage because diagnosis by imaging is crucial for the assessment of patients with acute mesenteric ischemia. Thus far, the chronological sequence of early changes that follow mesenteric ischemia and reperfusion has not been described, and the role of MRI diagnosis is still widely debated.
Intestinal ischemia occurs when there is an interruption of the blood supply to the bowel, or when there is inadequate organ perfusion due to conditions of shock or hypovolemia. The initial damage caused by ischemia could be worsened by oxidative damage during reperfusion. This clinical entity is known as ischemia/reperfusion (I/R) injury[15]. Previous studies on mesenteric I/R in felines reported that tissue lesions produced during reperfusion were greater than those produced during ischemia[16]. Despite decades of research in this area, I/R injury remains a clinically challenging problem[17-22]. Patients are particularly susceptible to the damaging effects of increased neutrophil activation following intestinal I/R[23]. Consequently, many cases of intestinal I/R develop into shock, multiple organ failure, and death[24,25].
The absence of reperfusion after acute SMA occlusion is characterized by clear macroscopic differences compared to AAMI with reperfusion. In the first case, there is a pale mesentery with thinning of mesenteric vessels and spasm of the jejunal loops due to the lack of blood flow and the contraction of the semilunar folds. With reperfusion, there is vascular congestion of the mesentery and bowel wall related to hypoxic damage of endothelial cells and subsequent blood extravasation following restoration of blood flow. These findings are in agreement with results obtained by previous authors[24-26].
Analyzing MRI signal characteristics, we found that the presence of free fluid in the abdomen (from the first hour) and HRI (from the second hour) are common and early findings in AAMI. It has been reported that a small amount of fluid is already visible 30 min after the ischemic event[24]; however, another study, in a porcine model, reported that free fluid is a late finding (detectable after 12 h)[25]. The presence of ascites is reported in up to 80% of cases[26]. HRI is a condition that occurs before PI, and it is considered an early reaction to the ischemic insult.
From the fourth hour, MRI also identified the presence of dilated loops with gas-fluid stasis (PI), reported in the literature in up to 65% of cases[25,27-29], and a hyper-intense signal from the bowel wall in some loops in T2-W sequences. These four findings (free fluid, HRI, dilated loops with gas fluids and bowel wall hyper-intensity) were present in all rats with mesenteric ischemia, with or without reperfusion.
We found important differences between the 2 mechanisms (mesenteric ischemia with or without reperfusion). First of all, the signal intensity of the wall in T2-W sequences: in those rats without reperfusion the signal intensity is normal at the first and second hour and becomes hyper-intense at the fourth and eighth. On the contrary, when we cause a reperfusion the hyper-intense signal is clearly visible form the first hour due to interstitial edema. Another MRI finding is the presence of bowel wall thickening due to blood extravasation and edema, which is visible from the second hour in rats with reperfusion but never found in rats without reperfusion. Bowel wall thickening was previously found in an animal model of mesenteric venous ischemia[30], suggesting that reperfusion produces hemorrhagic damage.
The early bowel wall thickening and hyper-intensity of the wall in T2-W sequence images may be considered signs of acute mesenteric ischemia with reperfusion. The presence of hyper-intense signal in T2-W images may be attributed to the edema of the submucosa caused by the release of vasoconstrictor amines that attract fluid from the bowel lumen[24,31]. The absence of mucosal thickening in arterial occlusion without reperfusion is interesting: indeed, the presence of wall thickening has been considered the most important feature of acute mesenteric ischemia[32] (with a prevalence of up to 96%). Nonetheless, we found that the wall thickening was only visible when the occlusion was followed by reperfusion. Further, our results showed that bowel wall thinning, detectable from the second hour after the SMA occlusion, is an extremely specific sign of occlusion without reperfusion. This finding was never detected in the cases with reperfusion. In the literature, bowel wall thinning seems to be heavily underestimated, as it is described in only 5% of cases[33].
The treatment of AAMI without reperfusion is significantly different from treatment of AAMI with reperfusion[34]. Because mesenteric ischemia with and without reperfusion have different therapeutic approaches, the power to distinguish between these conditions may have a great clinical importance[35,36]. Additionally, the radiological findings of mesenteric ischemia have different courses depending on whether the ischemic event followed by reperfusion: bowel wall thickening is a specific sign of reperfusion damage; acute SMA occlusion without reperfusion is characterized by bowel wall thinning. Although computer tomography remains a valid diagnostic tool for the visualization of mesenteric ischemia and for the evaluation of its damage, our study results suggest that MRI, which does not require the use of a contrast medium or ionizing radiation, can play an important role in the early diagnosis of this condition.
Because our model has some limitations, further investigation is necessary to verify whether these results can be translated to human beings. The first limitation is that we used a complete acute occlusion of the SMA, but in human beings it is possible to find partial occlusions. Second, the vessel ligation was performed at the vessel origin; this represents a potential bias because distal occlusions can occur. Other limitations include the small number of animals included in this preliminary report and the lack of histological analysis.
Acute arterial mesenteric ischemia is a life threatening vascular condition caused by the lack of arterial flow to the bowel, which can be occlusive or non-occlusive in origin. The initial damage caused by ischemia could be further worsened by oxidative damage during reperfusion. This entity is known as ischemia/reperfusion (I/R) injury. An early diagnosis of this condition is crucial to ensure a good outcome for the patient.
Although computer tomography (CT) remains a valid diagnostic tool for the visualization of mesenteric ischemia and for the evaluation of its damage, magnetic resonance imaging (MRI), which does not require the use of a contrast medium or ionizing radiation, can play an important role in the early diagnosis of this condition.
Despite decades of research in this area, ischemia/reperfusion injury remains a clinically challenging problem. Patients are particularly susceptible to the damaging effects of increased neutrophil activation following intestinal ischemia/reperfusion. To ensure a good prognosis to these patients an early diagnosis is requested and the prerequisite to do it consists in the knowledge of the timing of the lesions. Important differences between the 2 mechanisms (mesenteric ischemia with or without reperfusion) were found at MRI, regarding the timing of the following findings: the signal intensity of the wall in T2-W sequences and the presence of bowel wall thickening due to blood extravasation and edema. Although CT remains the gold standard in the evaluation of acute mesenteric ischemia, our results suggest that MRI, which does not require the use of a contrast medium or ionizing radiation, could in future play an important role in the early diagnosis of this condition.
This study suggests the following points: (1) The reperfusion of the mesenteric region, after 1 h of induced acute arterial ischemia, is characterized by early bowel wall hyperintensity in T2-W sequences, from the first hour, and bowel wall thickening, from the second hour; (2) Bowel wall thinning is a specific sign of acute superior mesenteric artery occlusion without reperfusion, detectable from the second hour; and (3) MRI can assess the various pathological changes that occur in the small bowel after induced acute arterial mesenteric ischemia with and without reperfusion.
Acute arterial mesenteric ischemia/infarction: lack of blood flow to the small bowel caused by a pathologic constriction or obstruction of its blood vessels, or an absence of blood circulation that can lead to ischemic damage of the bowel (involving part of the wall) or infarction (full thickness necrosis). 7T microMRI: Non-invasive preclinical studies method of demonstrating internal anatomy based on the principle that atomic nuclei in a strong magnetic field (7 Tesla) absorb pulses of radiofrequency energy and emit them as radiowaves which can be reconstructed into computerized images.
The paper presents some new methodology concerning the detection of subtle changes that occurs during the acute arterial mesenteric ischemia and ischemia-reperfusion pathological process. The paper is well designed and the results are meaningful and conceivable.
| 1. | Furukawa A, Kanasaki S, Kono N, Wakamiya M, Tanaka T, Takahashi M, Murata K. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192:408-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Barmase M, Kang M, Wig J, Kochhar R, Gupta R, Khandelwal N. Role of multidetector CT angiography in the evaluation of suspected mesenteric ischemia. Eur J Radiol. 2011;80:e582-e587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Debus ES, Müller-Hülsbeck S, Kölbel T, Larena-Avellaneda A. Intestinal ischemia. Int J Colorectal Dis. 2011;26:1087-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Wyers MC. Acute mesenteric ischemia: diagnostic approach and surgical treatment. Semin Vasc Surg. 2010;23:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Herbert GS, Steele SR. Acute and chronic mesenteric ischemia. Surg Clin North Am. 2007;87:1115-1134, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Romano S, Romano L, Grassi R. Multidetector row computed tomography findings from ischemia to infarction of the large bowel. Eur J Radiol. 2007;61:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Saba L, Mallarini G. Computed tomographic imaging findings of bowel ischemia. J Comput Assist Tomogr. 2008;32:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Lauenstein TC, Ajaj W, Narin B, Göhde SC, Kröger K, Debatin JF, Rühm SG. MR imaging of apparent small-bowel perfusion for diagnosing mesenteric ischemia: feasibility study. Radiology. 2005;234:569-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Iacobellis F, Berritto D, Somma F, Cavaliere C, Corona M, Cozzolino S, Fulciniti F, Cappabianca S, Rotondo A, Grassi R. Magnetic resonance imaging: a new tool for diagnosis of acute ischemic colitis? World J Gastroenterol. 2012;18:1496-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Kaleya RN, Sammartano RJ, Boley SJ. Aggressive approach to acute mesenteric ischemia. Surg Clin North Am. 1992;72:157-182. [PubMed] |
| 11. | Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg. 1978;15:1-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 114] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Saba L, Mallarini G. Spiral computed tomography imaging of bowel ischemia: a literature review. Panminerva Med. 2007;49:35-41. [PubMed] |
| 13. | Paterno F, Longo WE. The etiology and pathogenesis of vascular disorders of the intestine. Radiol Clin North Am. 2008;46:877-885, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 14. | Boros M, Takaichi S, Hatanaka K. Ischemic time-dependent microvascular changes and reperfusion injury in the rat small intestine. J Surg Res. 1995;59:311-320. [PubMed] |
| 15. | Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359-1377. [PubMed] |
| 16. | Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250:G749-G753. [PubMed] |
| 17. | Köksoy C, Kuzu MA, Kuzu I, Ergün H, Gürhan I. Role of tumour necrosis factor in lung injury caused by intestinal ischaemia-reperfusion. Br J Surg. 2001;88:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Souza DG, Cara DC, Cassali GD, Coutinho SF, Silveira MR, Andrade SP, Poole SP, Teixeira MM. Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries following ischaemia of the superior mesenteric artery in the rat. Br J Pharmacol. 2000;131:1800-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 400] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 20. | Pierro A, Eaton S. Intestinal ischemia reperfusion injury and multisystem organ failure. Semin Pediatr Surg. 2004;13:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir Bras. 2005;20:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Kalia N, Brown NJ, Wood RF, Hopkinson K, Fairburn B, Pockley AG. Effects of intestinal ischemia-reperfusion injury on rat peripheral blood neutrophil activation. Dig Dis Sci. 2003;48:1677-1684. [PubMed] |
| 23. | Stallion A, Kou TD, Latifi SQ, Miller KA, Dahms BB, Dudgeon DL, Levine AD. Ischemia/reperfusion: a clinically relevant model of intestinal injury yielding systemic inflammation. J Pediatr Surg. 2005;40:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Berritto D, Somma F, Landi N, Cavaliere C, Corona M, Russo S, Fulciniti F, Cappabianca S, Rotondo A, Grassi R. Seven-Tesla micro-MRI in early detection of acute arterial ischaemia: evolution of findings in an in vivo rat model. Radiol Med. 2011;116:829-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Klein HM, Klosterhalfen B, Kinzel S, Jansen A, Seggewiss C, Weghaus P, Kamp M, Töns C, Günther RW. CT and MRI of experimentally induced mesenteric ischemia in a porcine model. J Comput Assist Tomogr. 1996;20:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Taourel PG, Deneuville M, Pradel JA, Régent D, Bruel JM. Acute mesenteric ischemia: diagnosis with contrast-enhanced CT. Radiology. 1996;199:632-636. [PubMed] |
| 27. | Mazzei MA, Mazzei FG, Marrelli D, Imbriaco G, Guerrini S, Vindigni C, Civitelli S, Roviello F, Grassi R, Volterrani L. Computed tomographic evaluation of mesentery: diagnostic value in acute mesenteric ischemia. J Comput Assist Tomogr. 2012;36:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Wiesner W, Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226:635-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 250] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 29. | Alpern MB, Glazer GM, Francis IR. Ischemic or infarcted bowel: CT findings. Radiology. 1988;166:149-152. [PubMed] |
| 30. | Somma F, Berritto D, Iacobellis F, Landi N, Cavaliere C, Corona M, Russo S, Di Mizio R, Rotondo A, Grassi R. 7T μMRI of mesenteric venous ischemia in a rat model: timing of the appearance of findings. Magn Reson Imaging. 2013;31:408-413. [PubMed] |
| 31. | Chou CK. CT manifestations of bowel ischemia. AJR Am J Roentgenol. 2002;178:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Bartnicke BJ, Balfe DM. CT appearance of intestinal ischemia and intramural hemorrhage. Radiol Clin North Am. 1994;32:845-860. [PubMed] |
| 33. | Grassi R, Pinto A, Romano L, Rossi G, de Ritis R, Laporta A, Rotondo A. Twenty-six consecutive patients with acute superior mesenteric infarction. Comparison of conventional radiology, ultrasonography, and computerized tomography. Radiol Med. 1997;93:699-703. [PubMed] |
| 34. | Newton WB, Sagransky MJ, Andrews JS, Hansen KJ, Corriere MA, Goodney PP, Edwards MS. Outcomes of revascularized acute mesenteric ischemia in the American College of Surgeons National Surgical Quality Improvement Program database. Am Surg. 2011;77:832-838. [PubMed] |
| 35. | Liu S, Sun Q, Tao H, Sun X. Oral administration of mannitol may be an effective treatment for ischemia-reperfusion injury. Med Hypotheses. 2010;75:620-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Takizawa Y, Kitazato T, Kishimoto H, Tomita M, Hayashi M. Effects of antioxidants on drug absorption in in vivo intestinal ischemia/reperfusion. Eur J Drug Metab Pharmacokinet. 2011;35:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
P- Reviewers Boros M, Camara CR, Han JY, Mallet R S- Editor Zhai HH L- Editor A E- Editor Wu HL