Published online Oct 14, 2013. doi: 10.3748/wjg.v19.i38.6383
Revised: July 24, 2013
Accepted: August 5, 2013
Published online: October 14, 2013
Processing time: 98 Days and 13.5 Hours
Globally, gastric cancer is the 4th most frequently diagnosed cancer and the 2nd leading cause of death from cancer, with an estimated 990000 new cases and 738000 deaths registered in 2008. In the advanced setting, standard chemotherapies protocols acquired an important role since last decades in prolong survival. Moreover, recent advances in molecular therapies provided a new interesting weapon to treat advanced gastric cancer through anti-human epidermal growth factor receptor 2 (HER2) therapies. Trastuzumab, an anti-HER2 monoclonal antibody, was the first target drug in the metastatic setting that showed benefit in overall survival when in association with platinum-5-fluorouracil based chemotherapy. Further, HER2 overexpression analysis acquired a main role in predict response for trastuzumab in this field. Thus, we conducted a review that will discuss the main points concerning trastuzumab and HER2 in gastric cancer, providing a comprehensive overview of molecular mechanisms and novel trials involved.
Core tip: Advanced gastric cancer is a very aggressive disease thought the standard chemotherapy protocols available. In 2010, Trastuzumab for Gastric Cancer trial showed that the combination of trastuzumab, could be considered a new standard option for patients with human epidermal growth factor receptor 2 (HER2) positive advanced gastric and gastro-esophageal junction cancer. Thus, a new era emerged for those patients due to the interesting possibility in prolong survival in a personalized setting (HER2 positive). Our manuscript will provide an overview of the molecular mechanisms involved and also promising targeted therapies in this field.
- Citation: Luis M, Tavares A, Carvalho LS, Lara-Santos L, Araújo A, Mello RA. Personalizing therapies for gastric cancer: Molecular mechanisms and novel targeted therapies. World J Gastroenterol 2013; 19(38): 6383-6397
- URL: https://www.wjgnet.com/1007-9327/full/v19/i38/6383.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i38.6383
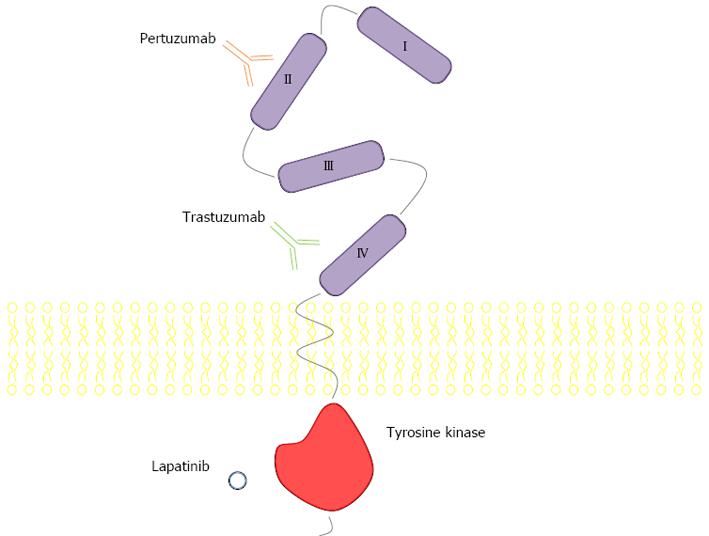
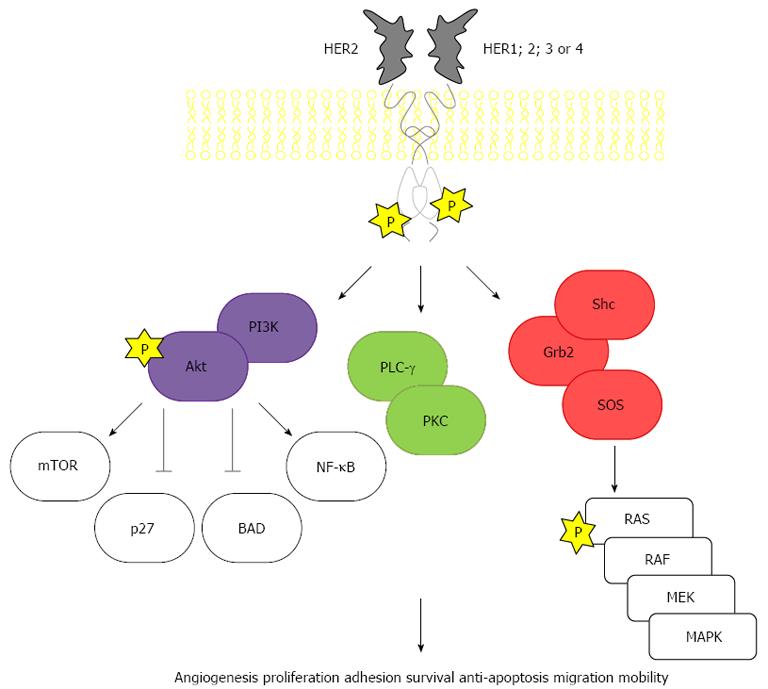
Gastric cancer has been described since 3000 BC in ancient Egypt. One of the first epidemiologic studies on cancer, with data spanning from 1760 to 1839, pointed gastric cancer as the most common and lethal. In modern times, it remains one of the most important forms of cancer, with different geographic, ethnic and socio-economic distributions. Incidence is particularly high in Japan, China, South Korea, Chile and Costa Rica. The large regional variations in incidence possibly reflect different prevalences of Helicobacter pylori infection, which is responsible for more than 60% of gastric cancer globally. Globally, gastric cancer is the 4th most frequently diagnosed cancer and the 2nd leading cause of death from cancer, with an estimated 990000 new cases and 738000 deaths registered in 2008[1]. The human epidermal growth factor receptor 2 (HER2) protein, a 185 kDa protein (p185) encoded by a gene located on chromosome 17q21 is a transmembrane tyrosine kinase receptor with an extracellular ligand-binding domain; a short transmembrane domain and an intracellular domain with kinase activity (Figure 1). It belongs to the epidermal growth factor receptor (EGFR) family of growth factors comprising four structurally related members, HER1 or ErbB1, also known as EGFR, HER2 or ErbB2, HER3 or ErbB3 and HER4 or ErbB4. Activation occurs through homo- or heterodimerization induced by ligands. HER2 is designated an orphan receptor which is believed to homodimerize independently of a ligand or to heterodimerize with another ligand-bound member of the EGFR family. Activation triggers a cascade of events that involves autophosphorilation and activation of the tyrosine kinase domain, Ras/Raf/mitogen-activated protein kinase pathway, phospholipase C-γ and phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) (Figure 2). HER2 receptors have also been found in nuclear localization, where they act as transcription factors for cycline D1 and p53[2,3]. Therefore, HER2 (also known as c-erbB-2/neu) acts as an oncogene involved in the regulation of cell proliferation, differentiation, motility and apoptosis[4-8]. Heterodimers of HER2 with other members of the HER family, particularly with HER3, are the most mitogenic dimers and HER2 increases the affinity of EGFR, HER3 and HER4 to their ligands[9-12].
Analysing the molecular structure of HER2 allows new insights into approaching it as a potential therapeutic target (Figures 1 and 3). The extracellular domain of the receptor is subdivided into four subdomains. Whereas subdomains II and IV are involved in the process of dimerization, subdomains I and III are the binding sites for pertuzumab and trastuzumab respectively, two of the most well studied HER2 inhibitors which will be discussed further on. The transmembrane domain of HER2 plays an important role in the process of dimerization and oncogenic mutations in this region were described. The intracellular domain contains the active enzyme site which activates different downstream pathways by phosphorylation[13-16].
The importance of addressing HER2 as a therapeutic target is underscored by a number of molecular and pathological findings. Amplified HER2 relates to processes of carcinogenesis and adverse pathologic features such as tumor size, invasion and metastastatic spread; the level of HER2 gene expression is much higher in cancer cells than that in non-malignant adult cells[17]. HER2 overexpression has been reported in breast, lung, salivary gland, ovary, colon, prostate and pancreatic cancers[18,19].
About 10%-34% of invasive breast cancers present HER2 overexpression. Trastuzumab has shown survival advantage in early and metastatic disease and is now a part of standard care. HER2 overexpression stands as a poor prognosis marker for chemo- and endocrine therapy but at the same time as a positive predictive marker for treatment with trastuzumab. Furthermore, trastuzumab proved to be effective as adjuvant treatment in breast cancer with HER2 overexpression, with different chemotherapy regimens[20-26]. In gastric cancer, the prognostic role of HER2 overexpression remains controversial. The most important prognostic factor for gastric cancer is the tumor node metastasis (TNM) stage[20,27]. Initial works addressing the prognostic significance of HER2 overexpression reported a negative effect on overall survival (OS)[28,29]. However, conflicting results regarding the prognostic value of HER2 have been published more recently. Some studies found a negative effect of HER2 on prognosis with reduction in OS[17,20,29-36], others found no relationship[37-40] and a trend towards improved survival was found in one cohort[41]. A comprehensive review by Jørgensen et al[42] found that the majority of publications that fulfilled the selection criteria for the analysis, associated HER2-positive status with poor survival and clinicopathological characteristics such as serosal invasion, lymph node metastases, disease stage or distant metastases. Chua et al[43] recently reviewed 49 studies with data regarding the relation of HER2 with clinicopathological variables and survival and concluded that HER2 overexpression is associated with poor survival; results pertaining other variables were not conclusive. HER2 overexpression has also been suggested as a molecular abnormality in the development of intestinal type gastric cancer and HER2 expression increases with disease progression, leading to the suggestion that the initial timing of this event probably occurs in early stages. Barros-Silva et al[20] found overexpression and amplification in both components of mixed tumors (intestinal and diffuse components) and HER2 amplification in early stages, supporting this idea of amplification in an early stage of carcinogenesis. Further support arises from the high levels of concordance between primary tumors and paired metastatic sites found by some authors, suggesting HER2 amplification as an early event and not acquired at a later moment by cells with metastatic potential[44]. Kataoka et al[45] found no HER2 positivity in the diffuse component of mixed type cases, but also found HER2 overexpression in early TNM T1a cases, pointing towards an early event[30,46]. Although these data tend to establish HER2 as a potential negative prognostic factor in gastric cancer, the relation seems not to be as consistent as in breast cancer[42]. In fact, more recent studies demonstrate no significant prognostic relationship. In a study involving 381 metastatic gastric cancer patients, Yanjigian et al[47] found that patients with HER2-positive gastric cancer had longer median OS compared with HER2-negative gastric cancer patients, but on multivariate analysis HER2 status was not an independent prognostic factor. Terashima et al[48] found no correlation with OS in 829 stage II/III resected gastric cancer patients. Hsu et al[49] investigated 1036 gastric cancer patients undergoing curative-intent resection. Although HER2 positivity emerged as a favourable prognostic factor for stage III-IV gastric cancer on univariate analysis, it did not on multivariate adjustment.
Despite these conflicting results, it seems likely that HER2 is not associated with an adverse prognosis in gastric cancer in an extent similar to breast cancer; nevertheless, inhibition of the HER2 pathway in patients with HER2 amplification demonstrated clinical benefits. In this review, we will address the main advances in the treatment of advanced gastric cancer, focusing on the novel biomarkers and target therapies concerning HER2 signalling pathways.
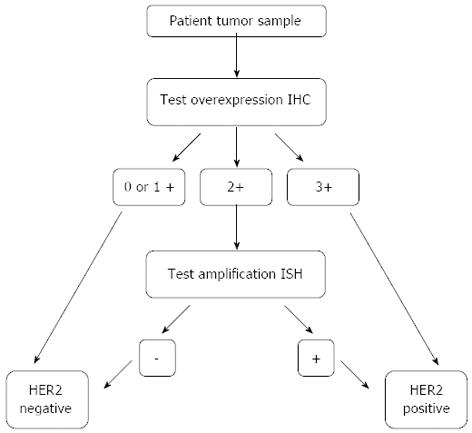
A precise analysis is necessary in order to address the status of HER2 expression in gastric cancer, which constitutes an essential step to select the patients feasible to treatment with HER2 target therapies. Techniques include primarily immunohistochemistry (ICH) and in situ hybridization (ISH), which constitute standard techniques also used in the current practice of HER2-status determination in breast cancer. Current evidence suggests the need to adopt the methods used in breast cancer in order to address HER2 expression in gastric cancer[50]. Considering the different biologic origin of the tissue, the high density of glandular structures needs to be understood in its context. In gastric tissue, ICH staining for HER2 occurs typically on the basolateral membrane and less so on the luminal aspect of the cell, conferring an U-shaped appearance to the staining whereas completeness of the membrane staining is the rule for higher scores in breast cancer. Another difference concerns the heterogeneity of immunostaining which is rare in breast, but frequent in gastric tumors. ICH should be used as primary test; cases with ICH score 3+ are candidates for HER2 directed therapy, 2+ scoring cases should be re-tested using ISH; in the case of ISH positivity patients are eligible for these therapeutic modality[51].
In January 2010, the European Medicines Agency granted approval to trastuzumab plus chemotherapy in the treatment of with IHC 3+ or 2+/metastatic adenocarcinoma of the stomach or gastro-esophageal junction (GEJ)[52]. The United States Food and Drug Administration approved trastuzumab for HER2 overexpressing patients, without further specification[53].
Trastuzumab is a fully humanized monoclonal antibody that binds to the extracellular domain of the receptor, acting by blockage of the HER2 receptor cleavage, inhibition of dimerization, as well as by the induction of antibody-dependent cellular cytotoxicity (ADCC), increasing endocytosis of the receptor and possibly through anti-angiogenic effects[54-56]. It was developed in the 1990s, after murine monoclonal antibodies directed to the extracellular domain of HER2 were produced and evaluated in cell lines and xenografts[57-59].
Overexpression of HER2 in gastric cancer cells was first reported in 1986 by Sakai et al[60] and Fukushige et al[61]. Preclinical models of gastric cancer were successful in demonstrating the inhibitory effect of trastuzumab on human gastric cancer cell lines in vitro and in mice xenografts in vivo, with additive and synergistic antineoplastic effects in combination with chemotherapy[59,62-65]. A study by Tanner et al[17] points out a gastric cancer cell line that was as sensitive to trastuzumab as a breast cancer cell line, both of them with amplified HER2, while Matsui et al[63] reported suppression of tumor growth in a xenograft model. Enhanced antineoplastic effects were observed with capecitabine, cisplatin, docetaxel, paclitaxel and irinotecan[62], and a further synergistic effect with cisplatin has been found by Kim et al[64].
Although information on the specific pathways involved is scarce, HER2 has been shown to be amplified in gastric cancer and HER2 is progressively regarded as an important biomarker and driver of cancerization in gastric cancer, with studies pointing out amplification or overexpression in 7%-34% of tumors, mainly in the intestinal type and in GEJ and proximal tumors[17,27,66].
Cortés-Funes et al[67] presented preliminary results of a phase II study involving 21 chemothreapy-naïve patients with HER2 overexpressing locally advanced or metastatic gastric cancer. Trastuzumab at a loading dose of 8 mg/kg and maintenance dose of 6 mg/kg and cisplatin 75 mg/m2 were administered every 21 d until progression, unacceptable toxicity or withdrawal of consent. Response rate was of 35%, with 17% of patients achieving stabilization. The tolerability profile was favourable; no grade 4 toxicity was observed and most the frequent grade 3 events were asthenia, nausea or vomiting, diarrhea, hiporexia and neutropenia. Data from another preliminary phase II study involving 16 gastric cancer patients were presented by Egamberdiev et al[68]. Trastuzumab 6 mg/kg was administered once in addition to cisplatin 100 mg/m2 during 3 d + fluorouracil (5-FU) 1000 mg/m2 3 d + leicovirin 100 mg/m2 3 d, every 3 wk. Authors reported an objective response rate of 54.5% in the combined therapy group vs 33.3% in the chemotherapy-only group and a median remission duration of 8.3 mo vs 5.2 mo. In a recent phase II study carried out by Grávalos et al[69], chemo-naïve patients with non-resectable advanced or metastatic gastric or GEJ adenocarcinoma overexpressing HER2 were treated with trastuzumab 8 mg/kg as loading dose and 6 mg/kg in subsequent cycles + cisplatin 75 mg/m2 every 3 wk. Twenty-two out of 228 patients (9.6%) enrolled had HER2 overexpression. An overall response rate of 32% was found, with disease control achieved in 64% of patients; median time to progression was 5.1 mo. No grade 4 toxicities occurred, whereas most frequent grade 3 adverse events were asthenia, neutropenia, anorexia, diarrhoea and abdominal pain. Interestingly, higher baseline HER2 extracellular domain levels associated with better response to therapy.
In more recent studies, HER2 overexpression was found to be lower than previously reported, especially in distant gastric cancers[70]. Resectable gastric cancer has reported HER2-positive ratios of 8.1% and 11.7%, suggesting that in resectable gastric cancer HER2 positive status might be less frequent than in advanced gastric cancer[71,72].
The phase III ToGA trial constitutes a milestone, establishing trastuzumab as the first biological therapy that demonstrated survival benefits in gastric cancer[62,63]. ToGA was a multicenter, international trial, undertaken in 24 countries[73]. It evaluated the combination of trastuzumab with standard chemotherapy (cisplatin + either capecitabine or 5-FU) in advanced (inoperable locally advanced, recurrent or metastatic) HER2-positive gastric cancer as a first-line therapy vs chemotherapy alone. Patients were treated with six cycles of chemotherapy in both treatment arms, with patients in the experimental arm continuing to be treated with trastuzumab until disease progression. Cisplatin 80 mg/m² was given on day 1 by intravenous infusion. Capecitabine 1000 mg/m² was given orally twice a day for 2 wk followed by a 1-wk rest or 5-FU 800 mg/m² per day was given by continuous infusion on days 1-5 of each cycle. Trastuzumab was given intravenously at a loading dose of 8 mg/kg on day 1 of the first cycle, followed by 6 mg/kg afterwards.
The primary objective of the study was to compare OS in both arms, and the secondary objectives were to compare progression-free survival (PFS), time to progression, overall response rate, disease control, duration of response and quality of life between the two treatment arms. Among 3665 tumor tissue specimens screened for HER2 positivity, 22% were HER2 positive (34% of the intestinal type vs 6% of diffuse and 20% of mixed types). Assessment was done with IHC and fluorescence ISH (FISH), according to Figure 3. The highest rate was observed in 34% of GEJ cancer and 20% of gastric cancer samples[74], which is in conformity with other studies where positivity rates for the GEJ are between 24%-35% and in gastric cancer samples comprise 9.5%-21.0%[17,27,75-77].
The combination of trastuzumab with chemotherapy in advanced HER2-positive cancer patients led to significantly better OS compared to the same chemo-therapeutic regimen alone (median OS in the combination therapy group was 13.8 mo against 11.1 mo in the chemotherapy-alone group). This effect was observed in patients with intestinal type gastric cancer but not in those with diffuse type gastric cancer[73,78]. Median PFS (6.7 mo vs 5.5 mo) and radiological response rate (47% vs 35%,) also improved with trastuzumab therapy. Exploring these data further, a sub-group analysis of the ToGA study which excluded patients with IHC 0-1+ FISH+ disease, found a main gain in median survival of 4.2 mo, comparable to the figures in breast cancer[28]. In fact, patients with strongest HER2 expression (IHC 3+ FISH+) gained the greatest benefit, with a median survival of 17.9 mo in patients treated with trastuzumab vs 12.3 mo with chemotherapy alone.
A summary of selected clinical trials of trastuzumab in gastro-esophageal cancer can be found in Table 1.
| Ref. | Phase | Treatment | n | OS (mo) | PFS (mo) | RR | CR | PR |
| Bang et al[73] | III | 5-FU + cisplatin or capecitabine + cisplatin | 290 | 11.1 | 5.5 | 34.50% | NA | NA |
| Trastuzumab + 5-FU + cisplatin or trastuzumab + capecitabine + cisplatin | 294 | 13.8 | 6.7 | 47.30% | NA | NA | ||
| Cortés-Funes et al[67] | II | Trastuzumab + cisplatin | 21 | NA | NA | 41.10% | 5.80% | 35.00% |
| Egamberdiev et al[68] | II | Trastuzumab + leucovirin + cisplatin + 5-FU | 16 | NA | 8.3 | 54.50% | NA | NA |
| Leucovirin + cisplatin + 5-FU | 18 | NA | 5.2 | 33.30% | NA | NA | ||
| Grávalos et al[69] | II | Trastuzumab + cisplatin | 22 | NA | 5.1 | 32.00% | NA | NA |
In this behalf, it is important to consider the possible benefits of trastuzumab in the adjuvant setting for earlier stages of the disease; however activity of targeted therapeutics in advanced disease should not automatically be extrapolated into the adjuvant setting, as results may be misleading[79]. Trials have been initiated which intend to investigate anti-HER2 therapeutics in this setting[80,81]. Early onset gastric cancer (presenting at or under the age of 45) seems to have lower HER2 overexpression than in late onset cases, with possible different molecular genetic pathways[82-84]. Ongoing clinical trials with trastuzumab can be found in Table 2.
| Setting, therapy line | ID | Phase | n | Treatment combined with trastuzumab | Primary EP | Status |
| Operable disease | NCT01196390 | III | 480 | Carboplatin, paclitaxel, radical radiotherapy | PFS | Recruiting |
| NCT01472029 | II | 53 | 5-FU, leucovorin, docetaxel, oxaliplatin | Rate of CR | Recruiting | |
| NCT01130337 | II | 45 | Oxaliplatin, capecitabine | PFS | Active, not recruiting | |
| Advanced first line | NCT01450696 | III | 400 | Cisplatin, capecitabine | OS | Recruiting |
| NCT01503983 | II | 51 | Oxaliplatin, capecitabine | OS | Recruiting | |
| NCT01461057 | II | 30 | Cisplatin, capecitabine, pertuzumab | Safety | Active, not recruiting | |
| NCT01396707 | II | 56 | Oxaliplatin, capecitabine | RR | Recruiting | |
| NCT01364493 | II | 51 | Oxaliplatin, capecitabine | RR | Recruiting | |
| NCT01359397 | II | 80 | Docetaxel, oxaliplatin, capecitabine, bevacizumab | PFS | Recruiting | |
| NCT01228045 | II | 30 | Cisplatin, S-1 | RR | Unknown | |
| NCT01191697 | II | 36 | Oxaliplatin, capecitabine, bevacizumab | RR | Recruiting | |
| Advanced second line | NCT01402401 | II | 48 | AUY922 | RR | Terminated |
From a clinical perspective, data known from breast cancer suggest that trastuzumab administration after disease progression might have benefits in OS[23,85]. In an observational of study of 623 patients, median time to progression was longer in patients who continued trastuzumab beyond progression than in those who stopped (10.2 mo vs 7.1 mo)[86]. Data from an interventional study involving 156 patients revealed OS rates of 20.4 mo vs 25.5 mo and response rates of 27.0% vs 48.1% in patients who stopped and continued trastuzumab beyond progression, respectively. Continuation of trastuzumab beyond progression was not associated with increased toxicity[87]. However, the issue is still a matter of debate, as increasing therapeutic options pose a challenge on the best possible sequencing and combinations of these interventions[88-90].
Perioperative chemotherapy regimens have shown promising results in gastric cancer. The MAGIC trial randomized over 500 patients to either surgery alone or perioperative chemotherapy consisting of epirubicin, cisplatin and fluorouracil (3 cycles before and 3 cycles after surgery). This triplet therapy demonstrated a decrease in tumor size and improved PFS and OS in comparison with surgery alone[91,92]. In addition, some data indicate that response to neoadjuvant treatment is a major predictive factor of survival after curative surgical resection[93].
Although there is no trial so far reporting results on the role of trastuzumab in the neoadjuvant setting, a number of case reports with trastuzumab-containing neoadjuvant chemotherapy regimens have been published, with interesting outcomes; complete pathological responses were attained in 2 cases and a partial response with tumor mass reduction allowing for an extensive surgery in another case[94-96].
Most data regarding the pharmacokinetic and pharmacodynamic profiles of trastuzumab stem from studies in breast cancer. A low systemic clearance (5.15 ± 2.45 mL/kg per day) and volume of distribution (44 mL/kg) have been described. Serum minimum concentrations of 10 μg/mL are needed to attain anti-proliferative effects and ADCC. With the usual loading dose of 4 mg/kg followed by 2 mg/kg per week, trastuzumab achieves and maintains serum minimum concentrations greater than 20 μg/mL. Recent results demonstrate that trastuzumab 6 mg/kg every 3 wk lead to the same plasma trough levels as trastuzumab 2 mg/kg weekly. Trastuzumab has been found not to exhibit dose-related nonlinear pharmacokinetics and the value of half-life of trastuzumab has an estimated value of 28.5 d[97,98]. No relevant drug interactions have been reported to date and elimination pathways remain largely unknown[99]. An extensive review about the pharmacodynamic and pharmacokinetic profiles, tolerability and dosage of trastuzumab in gastric cancer has been elaborated by Croxtall et al[100]. Targeted delivery systems involving anti-HER2 antibody mediated nano-scaled systems, drug conjugates, and fusion proteins are under active investigation[4,101,102].
The most commonly described adverse events with trastuzumab are infusion-related, described as fever, rigors, chills, nausea, dyspnea, and hypotension, and are present in about 40% of patients after the first administration and in 5% with subsequent treatment[103]. Trastuzumab has been extensively evaluated in breast cancer with a wide range of chemotherapeutic agents showing no significant overlapping toxicity, with one important exception, regarding an increased risk of cardiotoxicity. Trastuzumab-related cardiac dysfunction is largely reversible on withdrawal of the antibody. However, significant cardiopathy such as valvular heart disease, angina pectoris, previous transmiocardial infarction and heart failure with left ventricular ejection fraction (LVEF) < 50% are generally regarded as counter-indications for trastuzumab use[28]. With the chemotherapy doublet regimen evaluated in the ToGA trial, trastuzumab contributed with little added toxicity; no increase in chemotherapy related grade 3-4 toxicities (68% both arms) or cardiac events (6% both arms) were found. Nonetheless the number of patients with cardiac dysfunction (considered a ≥ 10% drop in LVEF to an absolute value < 50%) was low in both arms (5% trastuzumab + chemotherapy vs 1% chemotherapy alone). The European Society for Medical Oncology[104], issued a statement regarding the cardiac monitoring of patients receiving trastuzumab. Clinical evaluation and assessment of cardiovascular risk factors and comorbidities should be performed in every patient proposed for treatment with trastuzumab[105]. While screening algorithms for trastuzumab-induced cardiomyopathy provide guidance, patient-based strategies of surveillance remain important. Many clinical trials involving patients with metastatic breast cancer include a screening study to document the baseline LVEF, followed by serial monitoring at 8- to-16-wk intervals[106].
In the ToGA trial, serious adverse events were reported in 32% of patients treated with trastuzumab + chemotherapy and 28% in the chemotherapy group; with treatment-related mortality of 3% and 1% respectively. The adverse events were similar between both groups, with no difference in the overall rate of adverse events. Nausea, neutropenia, vomiting, and anorexia were the most frequently reported adverse events. Patients treated with trastuzumab + chemotherapy had slightly higher rates of diarrhoea, stomatitis, anemia, thrombocytopenia, fatigue, chills, weight loss, pyrexia, mucosal inflammation, and nasopharyngitis[73]. In a phase II study with trastuzumab and cisplatin as first-line therapy in GEJ and gastric cancer, trastuzumab showed a favourable toxicity profile[69].
Whilst data regarding mechanisms of resistance to trastuzumab in gastroesophageal cancer is scarce, important information can be retrieved from previous knowledge in the treatment of breast cancer. Primary resistance to single-agent trastuzumab in HER2-overexpressing metastatic breast carcinomas is described in 66%-88% of cases, with resistance eventually ensuing after a relatively short treatment period; in fact, the majority of patients who achieve an initial response to trastuzumab-based regimens develop resistance within 1 year (PFS between 6.7 and 7.4 mo)[85,107-109].
Proposed resistance mechanisms include aberrations in the PI3K/AKT/mTOR pathway with or without loss of the phosphatase and tensin homologue protein (PTEN) tumor suppressor gene, accumulation of truncated forms of the HER2 receptor that lack the extracellular trastuzumab-binding domain (collectively known as p95HER2), loss of phosphatase, activation of other tyrosine kinase receptors including the insulin-like growth factor receptor (IGF-1R), increased expression of membrane-associated glycoprotein (MUC1 and MUC4) and cyclin E overexpression[85,109-111].
PTEN inhibits PI3K, thereby inhibiting the PI3K/AKT/mTOR pathway. Loss of this tumor suppressor gene leads to at least partial resistance to trastuzumab. Indeed, both PIK3 mutations and PTEN loss were associated with inferior PFS and OS in a retrospective study of 256 HER2-positive metastatic breast cancer patients treated with trastuzumab[112]. A potential role for PI3K, AKT or mTOR inhibitors seems to exist, since these agents preclude the constitutive activation of this pathway, reversing PTEN loss-induced trastuzumab resistance[113-116].
Truncated forms of HER2 which arise through the proteolytic shedding of the extracellular domain of full-length HER2 or by alternative translation initiation from two methionine residues are the predominant HER2 forms in some tumors. The biological function of p95HER2 has not been fully characterized, though overexpression of p95HER2 has been shown to lead to growth of tumor xenografts in nude mice. The p95HER2 protein has kinase activity, and this activity is required for tumor growth; however, the mechanisms involved and its possible relationship with those used by full-length HER2 are still unknown. Importantly, since p95HER2 lacks the binding site for trastuzumab, it conveys resistance to this antibody. p95HER2 is expressed in approximately 30% of HER2-positive breast tumors and is correlated with poor disease-free survival and increased nodal metastasis when compared with patients that express full-length HER2[110,117]. p95HER2 can therefore be seen as a prognostic and predictive biomarker in breast cancer. In one study analysing 93 metastatic breast tumors, patients overexpressing p95HER2 were found to have a higher incidence of lung metastases and had significantly shorter PFS and OS with trastuzumab treatment in comparison with patients expressing only the full-length receptor[118]. Tumors that express p95HER2 may be resistant to trastuzumab but sensitive to the inhibitory effects of lapatinib, a low-molecular-weight dual tyrosine kinase inhibitor (TKI) of HER1/2 that has activity in patients with HER2-expressing tumors that are resistant to trastuzumab. Combination of trastuzumab with lapatinib has been evaluated in women with HER2-positive, trastuzumab-refractory metastatic breast cancer. Lapatinib with trastuzumab was superior to lapatinib alone in clinical benefit: complete response, partial response, and stable disease for ≥ 24 wk was observed in 24.7% of patients in the combination arm vs 12.4% in the monotherapy arm[119,120]. According to some authors this combination could provide a chemotherapy-free option after first line chemotherapy + trastuzumab[109].
Increased signalling through other receptor TKIs including EGFR, HER3, MET and IGF-1R has been found in cells resistant to HER2-targeting treatments[109]. PI3K/AKT/mTOR pathway activation through upregulation of HER3 signalling was demonstrated after exposure of breast cancer cells to HER TKIs[121]. On the other hand, pertuzumab, a HER2-HER3 dimerization inhibitor has demonstrated activity against trastuzumab resistant breast cancer cells[122]. Taking this findings into account, HER3 seems to play an important role in the mechanism of trastuzumab resistance.
In preclinical studies, co-expression of HER2 and IGF-1R in breast cancer cells resulted in loss of sensitivity to trastuzumab, conversely, inhibiting ligand-mediated activation of IGF-1R restored sensitivity to trastuzumab, therefore pointing towards a possible strategy to reduce or delay trastuzumab resistance[123,124].
Strategies to overcome trastuzumab resistance imply the important fact that many HER2-positive gastric tumors retain dependency on downstream signalling via the HER2 pathway. Therefore, besides other anti-HER2 agents (described in the following section), a focus on targeting these downstream signalling molecules has emerged[125,126]. Implied targets include mTOR inhibitors, HSP90 inhibitors and MET inhibitors; particularly interesting data exists concerning the possibility to combine some of these agents with anti-HER2 agents on which a patient has progressed, as the potential to reverse resistance to trastuzumab has been demonstrated[127-129].
Lapatinib is a dual TKI active on both EGFR and HER2, with known activity in trastuzumab resistant advanced breast cancer; data suggests that there is no cross-resistance with trastuzumab and lapatinib restored trastuzumab sensitivity in preclinical models[28,130,131]. Wainberg et al[132] evaluated the effect of lapatinib in HER2-amplified cell lines and xenograft models, concluding that lapatinib inhibits the growth of HER2-amplified cancer cell lines, induces cell cycle arrest and apoptosis and acts synergistically with trastuzumab.
It is approved as combination therapy with capecitabine for patients with HER2-overexpressing breast cancer with prior progression on trastuzumab, an anthracycline and a taxane[133]. In a phase II trial conducted by Galsky et al[134], patients with HER2 amplified gastro-esophageal, bladder, ovarian, or uterine tumors were enrolled into a double-blinded randomized discontinuation study of lapatinib 1500 mg per os a day. Of a total of 141 patients screened, 32 patients with HER2 amplified tumors were enrolled in the study. At 3 mo, 1 (3%) patient had a complete response (CR), 9 (28%) had stable disease, 20 (63%) had progressive disease, and 2 (6%) were unknown. Unfortunately, due to low response rate and slow enrolment, the study had to be closed early. Concerning gastro-esophageal cancer, a modest CR rate of 6.25% was reported. A phase II study with lapatinib as first-line therapy in 47 patients with advanced gastric cancer showed modest single-agent activity, with 12% response rate, 20% disease stabilization, 7% of patients experiencing partial response and a median OS of 5 mo, less than that seen with conventional cytotoxic chemotherapy[135]. Another phase II study of lapatinib monotherapy in patients with HER2-overexpressing GEJ or esophageal cancer reported limited single-agent activity, with no objective responses and stable disease in 8% of pacients[136]. Lapatinib in conjunction with capecitabine in the first line treatment of HER2 positive metastatic gastric cancer setting was addressed in a multicenter phase II trial, reporting a response rate of 22% and stable disease rate of 45%[137]. In another phase II trial, partial response of 24% and stable disease in 34% of patients was reported with lapatinib + capecitabine. Most frequent grade 3 and 4 side effects were anorexia, hand-foot syndrome, anemia and nausea; no significant cardiotoxicity was reported[138]. Two phase III studies evaluating the role of lapatinib in combination with chemotherapy in advanced esophagogastric cancer are currently being conducted, the LOGIC trial[139,140] (combination of lapatinib with oxaliplatin and capecitabine as first-line treatment) and the TYTAN trial[141,142] (lapatinib in combination with weekly paclitaxel in second-line setting). OS results from the LOGIC trial are expected in 2014. Data from the TYTAN trial were presented at ASCO GI 2013. 430 patients were randomized, with an OS of 11 mo for the experimental arm vs 8.9 mo for the paclitaxel-alone arm; the subgroup of patients with HER2 3+ expression score attained an OS of 14 mo.
As previously stated, dual blockade with lapatinib and trastuzumab in metastatic breast cancer patients that progressed on trastuzumab-containing regimens improved PFS and clinical response rate[120]; a clinical case reported durable stable disease in a patient treated with this strategy despite progression during prior chemotherapy with trastuzumab[143].
Pertuzumab is a monoclonal antibody targeting HER2 in domain II (Figure 1), preventing formation of the highly mitogenic HER2/HER3 dimer. Available data stem mostly from breast cancer. As with trastuzumab, the antibody is not effective in patients without amplification of HER2[144]. In the phase III CLEOPATRA study, 808 patients with HER2-positive metastatic breast cancer received placebo + trastuzumab + docetaxel (control group) or pertuzumab + trastuzumab + docetaxel (pertuzumab group). Median PFS was 12.4 mo in the control group vs 18.5 mo in the pertuzumab group. The hazard ratio for the addition of pertuzumab to docetaxel + trastuzumab for PFS was 0.62, with moderate toxicity added by the second antibody[145]. Pre-clinical results show potentiation of trastuzumab antitumour activity when combined with pertuzumab[146]. Pertuzumab is currently under investigation in a phase II study, in the first line gastric setting in combination with trastuzumab and platinum-fluoropyrimidine based chemotherapy[147].
Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate, which combines trastuzumab with the targeted delivery of the cytotoxic agent DM1, a derivative of maytansine, and a potent antimicrotubule agent. As systemic therapy, gastrointestinal toxicity limits the therapeutic usefulness of the agent[109]. In xenograft models, T-DM1 was found more effective than trastuzumab alone, with positive results independent of the tumor burden at therapy initiation, or preceding treatment with trastuzumab[101]. In a phase II study by Burris et al[148], T-DM1 had robust single-agent activity in patients with heavily pre-treated, HER2-positive metastatic breast cancer, with a favourable toxicity profile. In breast cancer, the EMILIA trial assigned patients with HER2-positive advanced breast cancer, previously treated with trastuzumab and a taxane, to T-DM1 or lapatinib + capecitabine. Median PFS was 9.6 mo with T-DM1 vs 6.4 mo with lapatinib plus capecitabine; with an objective response rate of 43.6% for T-DM1[149]. Taken together, results from preclinical studies and breast cancer clinical trials point out T-DM1 as a promising agent to be evaluated in gastro-esophageal cancer. Currently, a phase II-III study is ongoing to evaluate T-DM1 vs taxane in patients with previously treated locally advanced or metastatic HER2+ gastric and GEJ cancer.
Irreversible small molecule pan-HER TKIs causes tumor regression in HER2-overexpressing human gastric cancer xenograft models. They act by inhibition of HER family receptor phosphorylation and blocking of hetero-dimerization among them. Pre-clinical data reveal a synergistic effect with other molecular targeted agents (including trastuzumab) and chemotherapeutic agents. Currently investigated pan-HER TKIs include dacomitinib and afatinib[150,151].
Selected ongoing clinical trials exploring other anti-HER2 agents can be found in Table 3.
| Setting, therapy line | ID | Phase | n | Treatment | Primary EP | Status |
| Operable disease | NCT00450203 | III | 370 | Lapatinib, epirubicin, cisplatin, capecitabine | OS | Recruiting |
| Advanced first line | NCT00680901 | III | 535 | Lapatinib, oxaliplatin, capecitabine | OS | Active, not recruiting |
| LOGiC | ||||||
| NCT01395537 | II | 43 | Lapatinib, carboplatin, paclitaxel | Safety, RR | Active, not recruiting | |
| NCT01123473 | II | 192 | Lapatinib, epirubicin, cisplatin, capecitabine, 5-FU | PFS | Unknown | |
| NCT00526669 | II | 67 | Lapatinib, capecitabine | RR | Active, not recruiting | |
| Advanced second line | NCT00486954 | III | 273 | Lapatinib, paclitaxel | OS | Completed |
| TYTAN | ||||||
| NCT01522768 | II | 27 | Afatinib | RR | Recruiting | |
| NCT01152853 | II | 28 | Dacomitinib | PFS | Unknown | |
| NCT01145404 | II | 76 | Lapatinib, capecitabine | RR | Active, not recruiting |
HER2 vaccines, both DNA and peptide-based, are actively researched in the field of breast cancer and results indicate a possible future role for this modality in combination with other HER2 targeted therapies. A phase I study carried out by Hamilton et al[152] combined HER2 immunization with lapatinib found this combination to be safe and immunogenic, however, the anticancer activity of immunization-induced antibodies is still not well characterized. Successful repression of the HER2 gene by the means of adenovirus constructs rises expectations for possible applications in cancer treatment[153]. Radioimmunotherapy is another possible application of HER2 directed homing, with authors currently evaluating 212Pb immunoconjugates with trastuzumab in intraperitoneal application after preclinical studies showed interesting results[154,155].
Now, times are changing. New strategies had been developed and implemented for advanced gastric cancer treatment. HER2 acquired a main role in gastric cancer management and current is also mandatory in order to predict trastuzumab response in association with standard platinum-based chemotherapy. Furthermore, others drugs are in developing to overcome resistance to trastuzumab, serious treatment-related toxicities and also help oncologists to improve treatments approaches. In future, genomic profiles will probably be part of clinical routines for personalizing therapies and improve outcomes of those patients.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25606] [Article Influence: 1707.1] [Reference Citation Analysis (11)] |
| 2. | Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 824] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 3. | Williams CC, Allison JG, Vidal GA, Burow ME, Beckman BS, Marrero L, Jones FE. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol. 2004;167:469-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Release. 2010;146:264-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 419] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 5. | Rüschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G, Chenard MP, Penault-Llorca F, Nagelmeier I, Schlake W. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457:299-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 372] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 6. | Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, Seeburg PH, Libermann TA, Schlessinger J, Francke U. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132-1139. [PubMed] |
| 7. | Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644-1646. [PubMed] |
| 8. | Popescu NC, King CR, Kraus MH. Localization of the human erbB-2 gene on normal and rearranged chromosomes 17 to bands q12-21.32. Genomics. 1989;4:362-366. [PubMed] |
| 9. | Pinkas-Kramarski R, Shelly M, Glathe S, Ratzkin BJ, Yarden Y. Neu differentiation factor/neuregulin isoforms activate distinct receptor combinations. J Biol Chem. 1996;271:19029-19032. [PubMed] |
| 10. | Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem. 1999;274:8865-8874. [PubMed] |
| 11. | Hendriks BS, Orr G, Wells A, Wiley HS, Lauffenburger DA. Parsing ERK activation reveals quantitatively equivalent contributions from epidermal growth factor receptor and HER2 in human mammary epithelial cells. J Biol Chem. 2005;280:6157-6169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Park JW, Neve RM, Szollosi J, Benz CC. Unraveling the biologic and clinical complexities of HER2. Clin Breast Cancer. 2008;8:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Kofler M, Jorissen RN, Nice EC, Burgess AW. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11:495-505. [PubMed] |
| 15. | Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65:1566-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 548] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 16. | Telesco SE, Radhakrishnan R. Atomistic insights into regulatory mechanisms of the HER2 tyrosine kinase domain: a molecular dynamics study. Biophys J. 2009;96:2321-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 532] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 18. | Niehans GA, Singleton TP, Dykoski D, Kiang DT. Stability of HER-2/neu expression over time and at multiple metastatic sites. J Natl Cancer Inst. 1993;85:1230-1235. [PubMed] |
| 19. | Yan SY, Hu Y, Fan JG, Tao GQ, Lu YM, Cai X, Yu BH, Du YQ. Clinicopathologic significance of HER-2/neu protein expression and gene amplification in gastric carcinoma. World J Gastroenterol. 2011;17:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Barros-Silva JD, Leitão D, Afonso L, Vieira J, Dinis-Ribeiro M, Fragoso M, Bento MJ, Santos L, Ferreira P, Rêgo S. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer. 2009;100:487-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Dowsett M, Bartlett J, Ellis IO, Salter J, Hills M, Mallon E, Watters AD, Cooke T, Paish C, Wencyk PM. Correlation between immunohistochemistry (HercepTest) and fluorescence in situ hybridization (FISH) for HER-2 in 426 breast carcinomas from 37 centres. J Pathol. 2003;199:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 178] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8204] [Cited by in RCA: 8267] [Article Influence: 330.7] [Reference Citation Analysis (11)] |
| 23. | Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3742] [Cited by in RCA: 3728] [Article Influence: 177.5] [Reference Citation Analysis (0)] |
| 24. | Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4010] [Cited by in RCA: 3963] [Article Influence: 188.7] [Reference Citation Analysis (14)] |
| 25. | Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, Utriainen T, Turpeenniemi-Hujanen T, Jyrkkiö S, Möykkynen K. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27:5685-5692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 342] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 26. | Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1061] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 27. | Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 904] [Article Influence: 50.2] [Reference Citation Analysis (2)] |
| 28. | Okines AF, Cunningham D. Trastuzumab in gastric cancer. Eur J Cancer. 2010;46:1949-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Yonemura Y, Ninomiya I, Yamaguchi A, Fushida S, Kimura H, Ohoyama S, Miyazaki I, Endou Y, Tanaka M, Sasaki T. Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res. 1991;51:1034-1038. [PubMed] |
| 30. | Mizutani T, Onda M, Tokunaga A, Yamanaka N, Sugisaki Y. Relationship of C-erbB-2 protein expression and gene amplification to invasion and metastasis in human gastric cancer. Cancer. 1993;72:2083-2088. [PubMed] |
| 31. | Motojima K, Furui J, Kohara N, Izawa K, Kanematsu T, Shiku H. erbB-2 expression in well-differentiated adenocarcinoma of the stomach predicts shorter survival after curative resection. Surgery. 1994;115:349-354. [PubMed] |
| 32. | Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirao T, Nakano H. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894-1902. [PubMed] |
| 33. | Allgayer H, Babic R, Gruetzner KU, Tarabichi A, Schildberg FW, Heiss MM. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol. 2000;18:2201-2209. [PubMed] |
| 34. | Pinto-de-Sousa J, David L, Almeida R, Leitão D, Preto JR, Seixas M, Pimenta A. c-erb B-2 expression is associated with tumor location and venous invasion and influences survival of patients with gastric carcinoma. Int J Surg Pathol. 2002;10:247-256. [PubMed] |
| 35. | Uchino S, Tsuda H, Maruyama K, Kinoshita T, Sasako M, Saito T, Kobayashi M, Hirohashi S. Overexpression of c-erbB-2 protein in gastric cancer. Its correlation with long-term survival of patients. Cancer. 1993;72:3179-3184. [PubMed] |
| 36. | Ananiev J, Gulubova M, Manolova I, Tchernev G. Prognostic significance of HER2/neu expression in gastric cancer. Wien Klin Wochenschr. 2011;123:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Tateishi M, Toda T, Minamisono Y, Nagasaki S. Clinicopathological significance of c-erbB-2 protein expression in human gastric carcinoma. J Surg Oncol. 1992;49:209-212. [PubMed] |
| 38. | Ohguri T, Sato Y, Koizumi W, Saigenji K, Kameya T. An immunohistochemical study of c-erbB-2 protein in gastric carcinomas and lymph-node metastases: is the c-erbB-2 protein really a prognostic indicator? Int J Cancer. 1993;53:75-79. [PubMed] |
| 39. | Lee HR, Kim JH, Uhm HD, Ahn JB, Rha SY, Cho JY, Lee JI, Lee KH, Chung HC, Roh JK. Overexpression of c-ErbB-2 protein in gastric cancer by immunohistochemical stain. Oncology. 1996;53:192-197. [PubMed] |
| 40. | Sasano H, Date F, Imatani A, Asaki S, Nagura H. Double immunostaining for c-erbB-2 and p53 in human stomach cancer cells. Hum Pathol. 1993;24:584-589. [PubMed] |
| 41. | Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 131] [Reference Citation Analysis (0)] |
| 42. | Jørgensen JT, Hersom M. HER2 as a Prognostic Marker in Gastric Cancer - A Systematic Analysis of Data from the Literature. J Cancer. 2012;3:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 43. | Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. Int J Cancer. 2012;130:2845-2856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 44. | Bozzetti C, Negri FV, Lagrasta CA, Crafa P, Bassano C, Tamagnini I, Gardini G, Nizzoli R, Leonardi F, Gasparro D. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer. 2011;104:1372-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 45. | Kataoka Y, Okabe H, Yoshizawa A, Minamiguchi S, Yoshimura K, Haga H, Sakai Y. HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer. 2013;16:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Fornaro L, Lucchesi M, Caparello C, Vasile E, Caponi S, Ginocchi L, Masi G, Falcone A. Anti-HER agents in gastric cancer: from bench to bedside. Nat Rev Gastroenterol Hepatol. 2011;8:369-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jäger E, Altmannsberger HM, Robinson E, Tafe LJ, Tang LH. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol. 2012;23:2656-2662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 274] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 48. | Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, Katai H, Sano T, Imamura H, Sasako M. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992-6000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 49. | Hsu JT, Chen TC, Tseng JH, Chiu CT, Liu KH, Yeh CN, Hwang TL, Jan YY, Yeh TS. Impact of HER-2 overexpression/amplification on the prognosis of gastric cancer patients undergoing resection: a single-center study of 1,036 patients. Oncologist. 2011;16:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 450] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 51. | Albarello L, Pecciarini L, Doglioni C. HER2 testing in gastric cancer. Adv Anat Pathol. 2011;18:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 52. | European Medicines Agency. Assessment Report for Herceptin 2010. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000278/WC500074921.pdf. |
| 53. | Food and Drug Administration. U.S. BL 103792 Supplement: Trastuzumab Genentech, Inc 2010; Available from: www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf. |
| 54. | Stoss O, Nagelmeier I, Zielinski D, Rüschoff J. The ToGA (Trastuzumab for GAstric Cancer) Trial: Importance from a Biomarker Perspective. Available from: http://www.dako.com/28830_connection_15_the_toga_trastuzumab_for_gastric_cancer_trial_importance_from_a_biomarker_perspective_1.pdf. |
| 55. | Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523-1530. [PubMed] |
| 56. | Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 915] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 57. | Fendly BM, Winget M, Hudziak RM, Lipari MT, Napier MA, Ullrich A. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res. 1990;50:1550-1558. [PubMed] |
| 58. | Hancock MC, Langton BC, Chan T, Toy P, Monahan JJ, Mischak RP, Shawver LK. A monoclonal antibody against the c-erbB-2 protein enhances the cytotoxicity of cis-diamminedichloroplatinum against human breast and ovarian tumor cell lines. Cancer Res. 1991;51:4575-4580. [PubMed] |
| 59. | Kasprzyk PG, Song SU, Di Fiore PP, King CR. Therapy of an animal model of human gastric cancer using a combination of anti-erbB-2 monoclonal antibodies. Cancer Res. 1992;52:2771-2776. [PubMed] |
| 60. | Sakai K, Mori S, Kawamoto T, Taniguchi S, Kobori O, Morioka Y, Kuroki T, Kano K. Expression of epidermal growth factor receptors on normal human gastric epithelia and gastric carcinomas. J Natl Cancer Inst. 1986;77:1047-1052. [PubMed] |
| 61. | Fukushige S, Matsubara K, Yoshida M, Sasaki M, Suzuki T, Semba K, Toyoshima K, Yamamoto T. Localization of a novel v-erbB-related gene, c-erbB-2, on human chromosome 17 and its amplification in a gastric cancer cell line. Mol Cell Biol. 1986;6:955-958. [PubMed] |
| 62. | Fujimoto-Ouchi K, Sekiguchi F, Yasuno H, Moriya Y, Mori K, Tanaka Y. Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother Pharmacol. 2007;59:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 63. | Matsui Y, Inomata M, Tojigamori M, Sonoda K, Shiraishi N, Kitano S. Suppression of tumor growth in human gastric cancer with HER2 overexpression by an anti-HER2 antibody in a murine model. Int J Oncol. 2005;27:681-685. [PubMed] |
| 64. | Kim SY, Kim HP, Kim YJ, Oh do Y, Im SA, Lee D, Jong HS, Kim TY, Bang YJ. Trastuzumab inhibits the growth of human gastric cancer cell lines with HER2 amplification synergistically with cisplatin. Int J Oncol. 2008;32:89-95. [PubMed] |
| 65. | Gong SJ, Jin CJ, Rha SY, Chung HC. Growth inhibitory effects of trastuzumab and chemotherapeutic drugs in gastric cancer cell lines. Cancer Lett. 2004;214:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Bang Y, Chung H, Xu J, Lordick F, Sawaki A, Lipatov O, Al-Sakaff N, See CG, Rueschoff J, Van Cutsem E. Pathological features of advanced gastric cancer (GC): Relationship to human epidermal growth factor receptor 2 (HER2) positivity in the global screening programme of the ToGA trial. J Clin Oncol. 2009;27 Suppl 15:Abstr4556. |
| 67. | Cortés-Funes H, Rivera F, Alés I, Márquez A, Velasco A, Colomer R, García-Carbonero R, Sastre J, Guerra J, Grávalos C. Phase II of trastuzumab and cisplatin in patients with advanced gastric cancer with HER2/neu overexpression/amplification. J Clin Oncol. 2007;25:1-2. |
| 68. | Egamberdiev D, Djuraev M, Tuydjanova K, Nematov O. Our experience in the use of trastuzumab in patients with advanced stomach cancer. Ann Oncol. 2010;21 Suppl 8:S839. |
| 69. | Grávalos C, Gómez-Martín C, Rivera F, Alés I, Queralt B, Márquez A, Jiménez U, Alonso V, García-Carbonero R, Sastre J. Phase II study of trastuzumab and cisplatin as first-line therapy in patients with HER2-positive advanced gastric or gastroesophageal junction cancer. Clin Transl Oncol. 2011;13:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Boers JE, Meeuwissen H, Methorst N. HER2 status in gastro-oesophageal adenocarcinomas assessed by two rabbit monoclonal antibodies (SP3 and 4B5) and two in situ hybridization methods (FISH and SISH). Histopathology. 2011;58:383-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 71. | He C, Bian XY, Ni XZ, Shen DP, Shen YY, Liu H, Shen ZY, Liu Q. Correlation of human epidermal growth factor receptor 2 expression with clinicopathological characteristics and prognosis in gastric cancer. World J Gastroenterol. 2013;19:2171-2178. [PubMed] |
| 72. | Kunz PL, Mojtahed A, Fisher GA, Ford JM, Chang DT, Balise RR, Bangs CD, Cherry AM, Pai RK. HER2 expression in gastric and gastroesophageal junction adenocarcinoma in a US population: clinicopathologic analysis with proposed approach to HER2 assessment. Appl Immunohistochem Mol Morphol. 2012;20:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 73. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5532] [Article Influence: 345.8] [Reference Citation Analysis (3)] |
| 74. | De Vita F, Giuliani F, Silvestris N, Catalano G, Ciardiello F, Orditura M. Human epidermal growth factor receptor 2 (HER2) in gastric cancer: a new therapeutic target. Cancer Treat Rev. 2010;36 Suppl 3:S11-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Moelans CB, van Diest PJ, Milne AN, Offerhaus GJ. Her-2/neu testing and therapy in gastroesophageal adenocarcinoma. Patholog Res Int. 2011;2011:674182. [PubMed] |
| 76. | Hede K. Gastric cancer: trastuzumab trial results spur search for other targets. J Natl Cancer Inst. 2009;101:1306-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Polkowski W, van Sandick JW, Offerhaus GJ, ten Kate FJ, Mulder J, Obertop H, van Lanschot JJ. Prognostic value of Laurén classification and c-erbB-2 oncogene overexpression in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol. 1999;6:290-297. [PubMed] |
| 78. | Yamashita K, Sakuramoto S, Watanabe M. Genomic and epigenetic profiles of gastric cancer: potential diagnostic and therapeutic applications. Surg Today. 2011;41:24-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Bystricky B, Okines A, Cunningham D. Targeting Her-2 in gastric cancer - incorporation of trastuzumab into the treatment of operable disease. Gastrointestinal Cancer: Targets and Therapy. 2011;1:41-52. |
| 80. | Radiation Therapy Oncology Group. Radiation Therapy, Paclitaxel, and Carboplatin With or Without Trastuzumab in Treating Patients With Esophageal Cancer. 2010. Available from: http://clinicaltrials.gov/ct2/show/NCT0119639. |
| 81. | Hoffmann-La Roche. A Study of Capecitabine (Xeloda) in Combination With Trastuzumab (Herceptin) and Oxaliplatine in Patients With Resectable Gastric Cancer. 2010. Available from: http://clinicaltrials.gov/ct2/show/NCT01130337. |
| 82. | Carvalho R, Milne AN, van Rees BP, Caspers E, Cirnes L, Figueiredo C, Offerhaus GJ, Weterman MA. Early-onset gastric carcinomas display molecular characteristics distinct from gastric carcinomas occurring at a later age. J Pathol. 2004;204:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Milne AN, Carvalho R, Morsink FM, Musler AR, de Leng WW, Ristimäki A, Offerhaus GJ. Early-onset gastric cancers have a different molecular expression profile than conventional gastric cancers. Mod Pathol. 2006;19:564-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 84. | Milne AN, Sitarz R, Carvalho R, Carneiro F, Offerhaus GJ. Early onset gastric cancer: on the road to unraveling gastric carcinogenesis. Curr Mol Med. 2007;7:15-28. [PubMed] |
| 85. | Rose JS, Bekaii-Saab TS. New developments in the treatment of metastatic gastric cancer: focus on trastuzumab. Onco Targets Ther. 2011;4:21-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 86. | Extra JM, Antoine EC, Vincent-Salomon A, Delozier T, Kerbrat P, Bethune-Volters A, Guastalla JP, Spielmann M, Mauriac L, Misset JL. Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study. Oncologist. 2010;15:799-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 87. | von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann M, Bauer W. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J Clin Oncol. 2009;27:1999-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 516] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 88. | Jahanzeb M. Continuing trastuzumab beyond progression. J Clin Oncol. 2009;27:1935-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Valabrega G, Aglietta M, Montemurro F. Trastuzumab beyond disease progression: case closed? J Clin Oncol. 2009;27:e121-e12; author reply e121-e12;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 90. | Boku N. HER2-positive gastric cancer. Gastric Cancer. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 264] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 91. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4739] [Article Influence: 237.0] [Reference Citation Analysis (7)] |
| 92. | Okines AF, Thompson LC, Cunningham D, Wotherspoon A, Reis-Filho JS, Langley RE, Waddell TS, Noor D, Eltahir Z, Wong R. Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann Oncol. 2013;24:1253-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229:303-308. [PubMed] |
| 94. | Sbitti Y, Essaidi I, Debbagh A, Kadiri H, Oukabli M, Moussaid Y, Slimani K, Fetohi M, Elkaoui H, Albouzidi A. Is there any advantage to combined trastuzumab and chemotherapy in perioperative setting her 2neu positive localized gastric adenocarcinoma? World J Surg Oncol. 2011;9:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Wang J, Saukel GW, Garberoglio CA, Srikureja W, Hsueh CT. Pathological complete response after neoadjuvant chemotherapy with trastuzumab-containing regimen in gastric cancer: a case report. J Hematol Oncol. 2010;3:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Khaledy C, Ashouri S, Hiyama D, Sadeghi S. Trastuzumab based Neoadjuvant chemotherapy for Locally Advanced HER2 Over Expressing Gastric Adenocarcinoma. Proceedings of UCLA Healthcare. 2013;17. |
| 97. | Harris KA, Washington CB, Lieberman G, Lu JF, Mass R, Bruno R. A population pharmacokinetic model for trastuzumab (Herceptin) and implications for clinical dosing. Proc Am Soc Clin Oncol. 2002;21:Abstr488. |
| 98. | Leyland-Jones B, Gelmon K, Ayoub JP, Arnold A, Verma S, Dias R, Ghahramani P. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol. 2003;21:3965-3971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 99. | Levêque D, Gigou L, Bergerat JP. Clinical pharmacology of trastuzumab. Curr Clin Pharmacol. 2008;3:51-55. [PubMed] |
| 100. | Croxtall JD, McKeage K. Trastuzumab: in HER2-positive metastatic gastric cancer. Drugs. 2010;70:2259-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Barok M, Tanner M, Köninki K, Isola J. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett. 2011;306:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 102. | Zhou XX, Ji F, Zhao JL, Cheng LF, Xu CF. Anti-cancer activity of anti-p185HER-2 ricin A chain immunotoxin on gastric cancer cells. J Gastroenterol Hepatol. 2010;25:1266-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 103. | Meza-Junco J, Au HJ, Sawyer MB. Critical appraisal of trastuzumab in treatment of advanced stomach cancer. Cancer Manag Res. 2011;3:57-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 104. | Bovelli D, Plataniotis G, Roila F. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21 Suppl 5:v277-v282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 288] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 105. | Fiuza M, Magalhães A. Trastuzumab and Cardiotoxicity. Cardiotoxicity of Oncologic Treatments: InTech. Rijeka: InTech Editor 2012; 122-152. |
| 106. | Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1954] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 107. | Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11:263-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 108. | Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 646] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 109. | Okines AF, Cunningham D. Trastuzumab: a novel standard option for patients with HER-2-positive advanced gastric or gastro-oesophageal junction cancer. Therap Adv Gastroenterol. 2012;5:301-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 110. | de Mello RA, de Vasconcelos A, Ribeiro RA, Pousa I, Afonso N, Pereira D, Rodrigues H. Insight into p95HER2 in breast cancer: molecular mechanisms and targeted therapies. Recent Pat DNA Gene Seq. 2012;6:56-63. [PubMed] |
| 111. | Fessler SP, Wotkowicz MT, Mahanta SK, Bamdad C. MUC1* is a determinant of trastuzumab (Herceptin) resistance in breast cancer cells. Breast Cancer Res Treat. 2009;118:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 112. | Razis E, Bobos M, Kotoula V, Eleftheraki AG, Kalofonos HP, Pavlakis K, Papakostas P, Aravantinos G, Rigakos G, Efstratiou I. Evaluation of the association of PIK3CA mutations and PTEN loss with efficacy of trastuzumab therapy in metastatic breast cancer. Breast Cancer Res Treat. 2011;128:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 113. | Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1374] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 114. | Lu CH, Wyszomierski SL, Tseng LM, Sun MH, Lan KH, Neal CL, Mills GB, Hortobagyi GN, Esteva FJ, Yu D. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883-5888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 115. | Andre F, Campone M, O’Regan R, Manlius C, Massacesi C, Sahmoud T, Mukhopadhyay P, Soria JC, Naughton M, Hurvitz SA. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010;28:5110-5115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 116. | Morrow PK, Wulf GM, Ensor J, Booser DJ, Moore JA, Flores PR, Xiong Y, Zhang S, Krop IE, Winer EP. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126-3132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 117. | Sáez R, Molina MA, Ramsey EE, Rojo F, Keenan EJ, Albanell J, Lluch A, García-Conde J, Baselga J, Clinton GM. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res. 2006;12:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 118. | Sperinde J, Jin X, Banerjee J, Penuel E, Saha A, Diedrich G, Huang W, Leitzel K, Weidler J, Ali SM. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin Cancer Res. 2010;16:4226-4235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 119. | Gajria D, Gonzalez J, Feigin K, Patil S, Chen C, Theodoulou M, Drullinsky P, D’Andrea G, Lake D, Norton L. Phase II trial of a novel capecitabine dosing schedule in combination with lapatinib for the treatment of patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2012;131:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 120. | Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 715] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 121. | Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 791] [Cited by in RCA: 775] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 122. | Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878-5887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 504] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 123. | Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst. 2001;93:1852-1857. [PubMed] |
| 124. | Browne BC, Crown J, Venkatesan N, Duffy MJ, Clynes M, Slamon D, O’Donovan N. Inhibition of IGF1R activity enhances response to trastuzumab in HER-2-positive breast cancer cells. Ann Oncol. 2011;22:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 125. | Pazo Cid RA, Antón A. Advanced HER2-positive gastric cancer: current and future targeted therapies. Crit Rev Oncol Hematol. 2013;85:350-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 126. | Smyth EC, Cunningham D. Targeted therapy for gastric cancer. Curr Treat Options Oncol. 2012;13:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 127. | Van Cutsem E, Yeh K, Bang Y. Phase III trial of everolimus (EVE) in previously treated patients with advanced gastric cancer (AGC): GRANITE-1. J Clin Oncol. 2012;30 Suppl 4:Abstr LBA3. |
| 128. | Lu C, Liu D, Jin J, Deokar H, Zhang Y, Buolamwini JK, Yu X, Yan C, Chen X. Inhibition of gastric tumor growth by a novel Hsp90 inhibitor. Biochem Pharmacol. 2013;85:1246-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 129. | Nahta R, O’Regan RM. Evolving strategies for overcoming resistance to HER2-directed therapy: targeting the PI3K/Akt/mTOR pathway. Clin Breast Cancer. 2010;10 Suppl 3:S72-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 130. | Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2515] [Cited by in RCA: 2489] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 131. | Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909-4919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 405] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 132. | Wainberg ZA, Anghel A, Desai AJ, Ayala R, Luo T, Safran B, Fejzo MS, Hecht JR, Slamon DJ, Finn RS. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res. 2010;16:1509-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 133. | Zagouri F, Papadimitriou CA, Dimopoulos MA, Pectasides D. Molecularly targeted therapies in unresectable-metastatic gastric cancer: a systematic review. Cancer Treat Rev. 2011;37:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 134. | Galsky MD, Von Hoff DD, Neubauer M, Anderson T, Fleming M, Nagarwala Y, Mahoney JM, Midwinter D, Vocila L, Zaks TZ. Target-specific, histology-independent, randomized discontinuation study of lapatinib in patients with HER2-amplified solid tumors. Invest New Drugs. 2012;30:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 135. | Iqbal S, Goldman B, Fenoglio-Preiser CM, Lenz HJ, Zhang W, Danenberg KD, Shibata SI, Blanke CD. Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol. 2011;22:2610-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (2)] |
| 136. | Hecht J, Urba S, Koehler M. Lapatinib monotherapy in recurrent upper gastrointestinal malignancy: phase II efficacy and biomarker analyses. ASCO Proceedings of the Gastrointestinal Cancers Symposium. 2008;43:Abstr48. |
| 137. | Pishvaian M, Sakaeva D, Hsieh R. A global, multi-center phase II trial of lapatinib plus capecitabine in gastric cancer. J Clin Oncol. 2011;29 Suppl 4:Abstr 88. |
| 138. | Lenz H, Zhang J, Kemner AM, Kaneko T, Yang D, Franklin N. Lapatinib capecitabine in advanced gastric cancer: An open-label phase II study of non ERBB2-targeted disease. Ann Oncol. 2010;21 Suppl 8:S817. |
| 139. | Lenz H; LOGiC. Lapatinib Optimization Study in ErbB2 (HER2) Positive Gastric Cancer: A Phase III Global, Blinded Study Designed to Evaluate Clinical Endpoints and Safety of Chemotherapy Plus Lapatinib 2008. Available from: http://clinicaltrials.gov/ct2/show/NCT00680901. |
| 140. | Hecht JR, Urba SG, Koehler M, Ellis C, Gagnon R, Kemner A. Lapatinib monotherapy in recurrent upper gastrointestinal malignancy: Phase II efficacy and biomarker analyses. Proceedings of the Gastrointestinal Cancers Symposium American Society of Clinical Oncology; 2008, Jan 25-27; Orlando Florida, USA. Alexandria: Proc ASCO GI 2008; 43. |
| 141. | Kline GS. Lapatinib in Combination With Weekly Paclitaxel in Patients With ErB2 Amplified Advanced Gastric Cancer 2007. Available from: http://clinicaltrials.gov/ct2/show/NCT00486954. |
| 142. | Satoh T, Bang Y, Wang J, Xu J, Chung HC, Yeh K, Chen J, Mukaiyama A, Yoshida P, Ohtsu A. Interim safety analysis from TYTAN: A phase III Asian study of lapatinib in combination with paclitaxel as second-line therapy in gastric cancer. J Clin Oncol. 2010;28:4057. |
| 143. | Shitara K, Mizota A, Yatabe Y, Kondo C, Nomura M, Yokota T, Takahari D, Ura T, Muro K. Lapatinib plus trastuzumab for a patient with heavily pre-treated gastric cancer that progressed after trastuzumab. Jpn J Clin Oncol. 2011;41:663-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 144. | Gianni L, Lladó A, Bianchi G, Cortes J, Kellokumpu-Lehtinen PL, Cameron DA, Miles D, Salvagni S, Wardley A, Goeminne JC. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 145. | Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1741] [Cited by in RCA: 1892] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 146. | Yamashita-Kashima Y, Iijima S, Yorozu K, Furugaki K, Kurasawa M, Ohta M, Fujimoto-Ouchi K. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res. 2011;17:5060-5070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 147. | Hoffmann-La Roche. A Study of Pertuzumab in Combination With Trastuzumab and Chemotherapy in Patients With HER2-Positive Advanced Gastric Cancer 2011. Available from: http://clinicaltrials.gov/ct2/show/NCT01461057. |
| 148. | Burris HA, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, Tan-Chiu E, Krop IE, Michaelson RA, Girish S. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 561] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 149. | Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 2870] [Article Influence: 205.0] [Reference Citation Analysis (0)] |
| 150. | Nam HJ, Ching KA, Kan J, Kim HP, Han SW, Im SA, Kim TY, Christensen JG, Oh DY, Bang YJ. Evaluation of the antitumor effects and mechanisms of PF00299804, a pan-HER inhibitor, alone or in combination with chemotherapy or targeted agents in gastric cancer. Mol Cancer Ther. 2012;11:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 151. | Lin NU, Winer EP, Wheatley D, Carey LA, Houston S, Mendelson D, Munster P, Frakes L, Kelly S, Garcia AA. A phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab. Breast Cancer Res Treat. 2012;133:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 152. | Hamilton E, Blackwell K, Hobeika AC, Clay TM, Broadwater G, Ren XR, Chen W, Castro H, Lehmann F, Spector N. Phase 1 clinical trial of HER2-specific immunotherapy with concomitant HER2 kinase inhibition [corrected]. J Transl Med. 2012;10:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 153. | Loewenstein PM, Green M. Expression of the Adenovirus Early Gene 1A Transcription-Repression Domain Alone Downregulates HER2 and Results in the Death of Human Breast Cancer Cells Upregulated for the HER2 Proto-Oncogene. Genes Cancer. 2011;2:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 154. | Yong KJ, Milenic DE, Baidoo KE, Brechbiel MW. (212)Pb-radioimmunotherapy induces G(2) cell-cycle arrest and delays DNA damage repair in tumor xenografts in a model for disseminated intraperitoneal disease. Mol Cancer Ther. 2012;11:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 155. | Milenic DE, Wong KJ, Baidoo KE, Nayak TK, Regino CA, Garmestani K, Brechbiel MW. Targeting HER2: a report on the in vitro and in vivo pre-clinical data supporting trastuzumab as a radioimmunoconjugate for clinical trials. MAbs. 2010;2:550-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
P- Reviewer Aoyagi K S- Editor Gou SX L- Editor A E- Editor Zhang DN