CONFOCAL LASER ENDOMICROSCOPY
Confocal laser endomicroscopy (CLE) is a newly introduced procedure which allows to capture the images of “virtual histology” of the gastrointestinal mucosa during endoscopy[1,2], so offering the opportunity to get the “real time” visualization of the pathology of the mucosal epithelium with its cellular and subcellular structures[3,4]. At present, CLE can be performed with 2 devices: one integrated into an endoscope (Pentax, Tokio, Japan, herein termed e-CLE) and one as a mini-probe through the scope (p-CLE; Cellvizio, Mauna Kea Technologies, Paris, France). Confocal microscopy consists of focusing a laser ray onto the mucosal surface and filtering the returned light by means of a small pinhole which rejects out-of-focus light. The illumination and detection systems are in the same focal plane and are termed “confocal”. After passing the pinhole, the fluorescent light is detected by a photo-detection, transforming the light signal into an electrical one that is recorded by a computer. All detected signals from the illuminated spot are captured and measured. As the laser scans over the plane of interest, a whole image is obtained pixel-by-pixel and line-by-line, whereas the brightness of a resulting image pixel corresponds to the relative intensity of detected fluorescent light. The gray-scale image created is an optical section representing one focal plane within the examined specimen. Real-time confocal laser scanning microscopy-sequences (duration 1 min are recorded and stored digitally for later evaluation. CLE evaluation and its relative high-quality images have shown high agreement with the real histology of the tissue, so opening a wide spectrum of potential applications, all focused on the possibility of reducing and/or targeting the biopsies during endoscopy[5,6]. The current potential indications for CLE imaging are broad and include almost all the cases in which endoscopic biopsy is needed.
At now, various studies have addressed the potential usefulness of CLE in diagnostic work-up of inflammatory bowel disease (IBD), with particular interest to ulcerative colitis (UC)[7-9]. In effect, all the studies concerning the application of CLE in the UC context have shown that this technique can have a potential role in assessing the extension and the activity of disease and in targeting biopsies, reducing the number of useless biopsies and improving the early detection of dysplasia[10-13]. In the field of UC, the most frequent alterations in crypt architecture are represented by dilation of crypt openings, more irregular arrangement of crypts, enlarged spaces between crypt, crypt destruction and/or crypt fusion, and crypt abscess with fluorescein leaks into the crypt lumen (therefore making the lumen brighter than the surrounding epithelium). Microvascular alterations are mainly represented by dilated, prominent branching vessels (Figures 1 and 2). The study by Watanabe et al[14], including 17 patients with active UC compared with 14 controls, showed that CLE images provided equivalent information to histopathology with respect to definition of the main histological outcomes (crypt architecture, capillaries and inflammatory cells). On these bases, a new classification of inflammatory activity in UC using CLE has been proposed[15], which comprises the assessment of crypt architecture, microvascular alterations and fluorescein leakage.
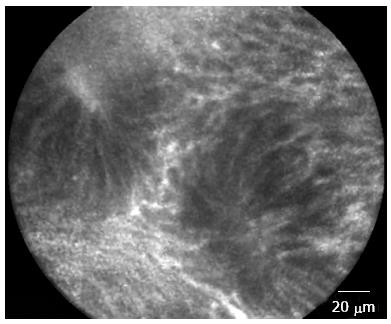
Figure 1 Rectal mucosa of patient in remission from ulcerative colitis.
Irregular alignment of crypt, crypt distortion and fusion with reduced amount of goblet cells.
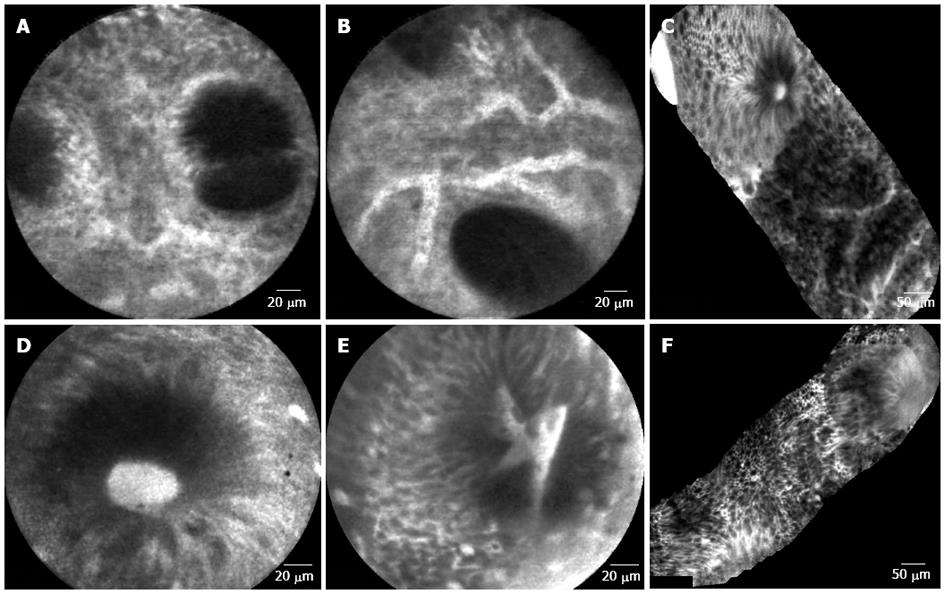
Figure 2 Colonic mucosa.
A: Patient in remission from ulcerative colitis. Crypt distortion and fusion and many capillaries visible in the lamina propria; B: Patient in remission from ulcerative colitis. Enlarged spaces between crypts and dilated prominent branching vessels; C: In patient with active ulcerative colitis (distal colitis). Image of colonic mucosa showing the switch from normal mucosa (top of the figure) to inflamed mucosa Inflamed mucosa showing irregular arrangement of crypts, crypt fusion and capillaries alterations; D: In patient with active ulcerative colitis. Dilated and bright crypt lumen (fluorescein leakage) with intact epithelium; E: In patient with active ulcerative colitis. Dilated, irregular and bright crypt lumen (fluorescein leakage) with partially intact epithelium; F: In patient with highly active ulcerative colitis (Mayo CU3). Crypts distortion and destruction, crypt abscess and crypts replacement by diffuse necrosis.
One of the most important diagnostic goals in the management of patients with UC, especially of those who present risk factors for cancer development, should be the “real-time” endoscopic identification and diagnosis of dysplasia/neoplasia, as this would reduce the number of unnecessary biopsies with their associated time and costs[16,17]. Starting from these assumptions, Kiesslich et al[18] have shown for the first time that the diagnosis of dysplasia/neoplasia in UC could be maximized by using both pan-chromoendoscopy (CE) and targeted CLE, with high values of diagnostic accuracy (sensitivity 94%, specificity 98%). This result has been recently confirmed, although with less remarkable diagnostic values, by van den Broek et al[19], who reported a diagnostic accuracy of 81% when comparing CLE with narrow-band imaging plus high-definition endoscopy (diagnostic accuracy 92%). In accordance with these reports, a recent paper produced by our group, exploring the efficacy of the combined application of CE and targeted p-CLE in diagnosing dysplasia in longstanding UC in the “real-life”, has underlined the high diagnostic accuracy of such a procedure compared to standard histology (sensitivity 100%, specificity 90%, positive predictive value 83% and negative predictive value 100%)[20] (Figure 3). Giving value to all the above-mentioned contributions, the combination of CE and CLE corresponds to a diagnostic gain of 3- to 5-fold for detecting dysplasia/neoplasia than conventional colonoscopy[21]. The diagnostic gain is mainly due to CE application which could dramatically decreased (about of 10 times) the number of biopsies when just circumscribed suspicious lesions on CE would have been targeted; if only CLE-suspected neoplastic lesions had undergone biopsy after CE, the mean number of biopsies would be further reduced.
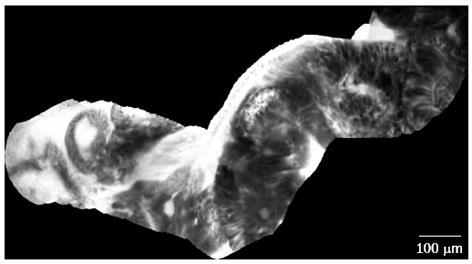
Figure 3 Dysplasia-associated lesional mass in long-standing ulcerative colitis.
Image of colonic mucosa evidencing the switch from the inflamed mucosa, to the neoplastic mucosa. Inflamed mucosa is characterized by crypts fusion and distortion, dilation of crypt openings, enlarged spaces between crypts, and microvascular alterations with fluorescein leaks into the crypt lumen. Dysplastic mucosa (right corner) is characterized by “dark” cells, irregular architectural patterns with villiform structures and a “dark” epithelial border.
More recently, Neumann et al[22] have explored the clinical utility of CLE also in 76 patients affected by Crohn’s disease (CD), particularly determining whether the disease activity can be graded by using CLE. In effect, a relevant percentage of patients with active CD presented an increased colonic crypt tortuosity, enlarged crypt lumen, microerosions, augmented vascularization and increased cellular infiltrates. Starting from these considerations, these authors proposed a CLE score for assessing CD activity in vitro, with such a score having of potential utility for predicting the course of CD and the response to medical therapy.
CLE application in IBD has been evaluated even under a prognostic view. A nice work by Kiesslich et al[23] have shown that “cell shedding” and “barrier loss” detected by CLE are able to predict relapse of IBD and have potential role as diagnostic tool for the management of the disease. In this paper, the sensitivity, specificity and accuracy for the “CLE grading system” to predict a flare were 62.5%, 91.2% and 79%, respectively. Interestingly, a recent paper by Turcotte et al[24] confirmed the high prognostic power of CLE in predicting the course for other relevant clinical end-points for patients affected by IBD. In particular, increased epithelial gaps in the small intestine as determined by CLE were a predictive factor for future hospitalization or surgery in IBD patients.
Going to another potential indication of this procedure in diagnostic work-up of IBD patients, CLE has been effectively used in diagnosing a biliary dysplasia/neoplasia in patients with primary sclerosing cholangitis (PSC), a pathological condition frequently associated with IBD, with a coexisting bile duct stricture[25]. In effect, Heif et al[25] showed a high diagnostic accuracy in detecting the presence of a bile duct neoplasia in 15 PSC patients with 21 dominant stenoses (sensitivity 100%; specificity 61%; positive predictive value 22%; negative predictive value 100%). This paper has opened the doors to a further potential application of CLE in IBD.
Unfortunately, some relevant limitations reduced the current application of CLE in general practice: the need for a learning curve, the cost of the equipment, the need for an extra-time (about 30 min) to enhanced colonoscopy and, not less important, a number of medical-legal issues. Furthermore, the promising results in the literature derived from a little number of trials and still from a few experienced centers and, as a consequence, these cannot be generalized easily.
In our mind, among these limitations, the need for an adequate learning curve represents the less relevant topic. In effect, as shown in previous papers, the operator’s endoscopic expertise and learning curve represent the crucial issues and main limitation for the routine application of this endoscopic technique. However, a recent report has highlighted that the ability to accurately interpret CLE images for predicting neoplastic lesions can be learned rapidly by a range of GI specialists[26]; similarly, the ability to acquire high-quality CLE images can also be learned quickly.
About medical-legal issues, mainly regarding the application of CLE for surveillance endoscopy in UC, the principal matter is represented by the fact that endoscopists would make a histological diagnosis without the confirmation by a pathologist and would decide during the endoscopy if performing or not multiple biopsies. At now, this kind of diagnostic approach is not reported by the current guidelines[27] and should be applied only within a controlled trial formally approved by an ethical committee.
Concluding, new multicenter studies are needed to assess the real cost-effectiveness of CLE technique for IBD. In our opinion, when balancing the interesting diagnostic advantage of CLE in clinical practice with its important realistic limitation, the wide application of this procedure in the current endoscopic practice still appears to be more a dream then a reality.