Published online Sep 7, 2013. doi: 10.3748/wjg.v19.i33.5493
Revised: May 24, 2013
Accepted: June 19, 2013
Published online: September 7, 2013
Processing time: 132 Days and 15.2 Hours
AIM: To compare the bowel cleansing efficacy, tolerability and acceptability of split 2-L polyethylene glycol (PEG)-citrate-simethicone (PEG-CS) plus bisacodyl (BIS) vs 4-L PEG for fecal occult blood test-positive screening colonoscopy.
METHODS: This was a randomised, observer-blind comparative study. Two hundred and sixty-four subjects underwent screening colonoscopy (mean age 62.5 ± 7.4 years, male 61.7%). The primary objective of the study was to compare the bowel cleansing efficacy of the two preparations. Interventions: BIS plus PEG-CS: 3 tablets of 5-mg BIS at 16:00, PEG-CS 1-L at 19:00 and 1-L at 7:00, 4-L PEG: 3-L at 17:00, and 1-L at 7:00. Colonoscopy was carried out after 11:00, at least 3 h after the completion of bowel preparation. Bowel cleansing was evaluated using the Harefield Cleansing Scale.
RESULTS: Bowel preparation was successful for 92.8% of subjects in the PEG-CS group and for 92.1% of subjects in the 4-L PEG (RR = 1.01; 95%CI: 0.94-1.08). BIS + PEG-CS was better tolerated than 4-L PEG. A greater rate of patients in the BIS + PEG-CS group had no difficulty and/or were willing to repeat the same preparation compared to split-dose 4-L PEG group. Subjects in the BIS + PEG-CS group rated the prep as good or satisfactory in 90.6% as compared to 77% in the 4-L PEG (P = 0.003). Subjects receiving BIS + PEG-CS stated they fully adhered to instructions drinking all the 2-L solution in 97.1% compared with 87.3% in the 4-L PEG (P = 0.003).
CONCLUSION: BIS plus split 2-L PEG-CS was as effective as but better tolerated and accepted than split 4-L PEG for screening colonoscopy. This new procedure may increase the positive attitude and participation to colorectal cancer screening colonoscopy.
Core tip: Colorectal cancer ranks as the most common newly-diagnosed cancer in Europe and the second most common cause of cancer death in Europe. A new colon cleansing procedure based on bisacodyl plus polyethylene glycol (PEG) with citrates and simethicone administered as split dose has the same efficacy but superior tolerability and acceptance to split conventional 4-L PEG. This new procedure may increase the positive attitude and participation to colorectal cancer screening colonoscopy.
- Citation: Valiante F, Bellumat A, De Bona M, De Boni M. Bisacodyl plus split 2-L polyethylene glycol-citrate-simethicone improves quality of bowel preparation before screening colonoscopy. World J Gastroenterol 2013; 19(33): 5493-5499
- URL: https://www.wjgnet.com/1007-9327/full/v19/i33/5493.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i33.5493
Colorectal cancer (CRC) ranks as the most common newly-diagnosed cancer in Europe and the second most common cause of cancer death in Europe[1]. Screening for early detection and removal of premalignant adenomas or localized cancer is crucial to reduce morbidity and mortality associated with CRC[2,3]. Colonoscopy is the current gold standard when non-invasive methods are positive (i.e., faecal occult blood test, FOBT) in colorectal cancer population screening programs (> 50 years in Italy) and is also recommended and used as a primary screening modality[4,5]. The success of colonoscopy is largely dependent on the level of bowel cleansing[6]. Adequate visualization of the colonic mucosa requires a clean colon with no solid or residual brown liquid that could mask a potential lesion. It has been demonstrated that inadequate bowel preparation is associated with lower adenoma detection rates, incomplete colonoscopy or more technically difficult procedure[7-10]. A major concern is that detection of lesions in the right colon can be missed due to inadequate bowel preparation[11]. The quality of bowel preparation depends on the compliance of the patient, the type of bowel preparation and the timing of ingestion[12].
Polyethylene glycol (PEG) solutions are widely used as they are safe and effective. However the large volume (4 L) to be taken may be a considerable burden for the patient. In clinical practice the reduced tolerability due to large volume and salty taste of PEG solutions may lead to low adherence to the instructions by patients, they drink less than the correct amount with the result of suboptimal efficacy[13].
Different low-volume formulations have been used such as sodium phosphate and magnesium citrates. They appear to be better tolerated but these solutions should be used with caution in frail patients or patients with renal failure as they can induce dehydration or electrolyte imbalance[11]. A more recent option is the addition of ascorbates to the PEG solution or the use of bisacodyl (BIS) for low-volume bowel preparation[14,15].
Also timing and dose administration improve the overall performance and acceptance of bowel preparation[16,17]. A split dose regimen, in which the first half dose is taken on the day before and the second half dose on the day of procedure have been shown to be more effective for colon cleansing than single full dose administration on the day before. The split dosing rule appears to be valid for any type of bowel preparation[18-21]. A new iso-osmotic sulphate-free PEG electrolyte preparation with citrate and simethicone (PEG-CS) is commercially available to be used with BIS tablets to achieve optimal colon cleansing with threefold mechanism of action[22,23]. BIS has a stimulant effect on the colonic motility, while PEG and citrates act as osmotic agents and simethicone favors the foam coalescence improving mucosal visibility[24-27]. We therefore compared the efficacy, tolerability and compliance of the new low volume procedure with BIS plus PEG-CS solution versus standard 4-L PEG in patients undergoing screening colonoscopy.
Eligible subjects were those referred to colonoscopy as a second level examination following positive FOBT or as a follow-up for adenomatous polyps in the CRC screening promoted by the Veneto region, from December 2009 to January 2011.
Subjects were excluded if they had a history of hypersensitivity to PEG or any other ingredient of products used in the study and other labeled contraindications of the commercially available products.
This study was conducted according to the principles of the Declaration of Helsinki after obtaining approval from the Institutional Review Board. Written informed consent was obtained from each subject.
All patients were instructed to follow a low-fiber diet for the three days preceding colonoscopy and to drink only clear fluids after starting the bowel preparation.
PEG-CS is an iso-osmotic low volume sulphate-free bowel preparation consisting of PEG 4000, citric acid, sodium citrate, sodium chloride, potassium chloride, simethicone and flavoring agents supplied in four 64.5 g sachets (Lovol-esse, Promefarm). The powder for oral solution must be dissolved in 500 mL of water. This product is combined with BIS 5-mg tablets (Lovoldyl, Promefarm) for full bowel preparation before colonoscopy. In this study, a split dose regimen was used: BIS 15 mg at 16:00 and PEG-CS 1 L at 19:00 the day before. On the day of colonoscopy, PEG-CS 1 L at 7:00 for colonoscopy after 11:00.
The reference preparation was standard PEG electrolyte solution (Isocolan, Bracco), given as split dosing, i.e., 3 L at 17:00 and 1 L at 7:00 for colonoscopy after 11:00 (Table 1).
| PEG-CS | 4-L PEG | |
| Active ingredients | Bisacodyl, PEG, citrates, simethicone | PEG, sodium sulphate |
| Product description | 4 sachets; each containing PEG 4000 60.7 g, sodium citrate 1.066 g, citric acid 1.25 g, simethicone 80 mg | 8 sachets; each containing PEG 4000 29.5 g and sodium sulphate 2.843 g |
| Total volume | 2-L | 4-L |
| Electrolytes | Sodium chloride, potassium chloride | Sodium bicarbonate, sodium chloride, potassium chloride |
| Osmolality (mOsmol/kg) | 293 | 288 |
| Mixed with | Water | Water |
| Diet prior to colonoscopy | Clear liquid after starting solution intake | Clear liquid after starting solution intake |
| Timing of intake | 1-L of solution at 19:00 the day prior to procedure 1-L of solution at 7:00 the day of the exam | 3-L of solution at 17:00 the day prior to procedure 1-L of solution at 7:00 the day of the exam |
| Additional agents | 15 mg bisacodyl (3 tablets) at 16.00 the day prior to procedure |
This was a randomised comparative investigator-blind study including consecutive outpatients undergoing screening or follow-up colonoscopy at the Department of Gastroenterology and Digestive Endoscopy at Santa Maria del Prato Hospital in Feltre (Belluno, Italy).
At the time of registration, subjects were randomly allocated in a 1:1 ratio to receive PEG-CS plus BIS or the standard PEG 4-L. Randomization was computer-generated; eligible patients were sequentially numbered and received the corresponding preparation by a nurse in order to ensure adequate concealment.
Study medications were supplied to subjects using the commercially available preparations. Study investigators were kept blinded to study medications and subjects were asked not to reveal the preparation used.
Subjects visited the departments on 2 occasions: enrolment (randomization) and on the day of colonoscopy.
Medical history including concomitant medications, physical examination and vital signs were taken at baseline and on the day of colonoscopy. Subjects received oral and written instructions on the use of the bowel preparation including dietary advice consisting of a 3-d low-fibre diet followed by clear liquids on the day before colonoscopy.
Safety evaluation was based on reporting of adverse events and adverse drug reactions using a standard questionnaire during the visit before colonoscopy.
On the day of colonoscopy the patients were asked to fill a further questionnaire to provide information about whether or not they experienced gastrointestinal (GI) symptoms such as nausea, bloating, and abdominal discomfort, the amount of solution actually taken, difficulty to complete the preparation (3-point scale), taste (3-point scale), willingness to repeat the same preparation in the future (yes or no).
Bowel preparation was evaluated using a 5-point bowel cleansing rating scale for each colonic segment (caecum/ascending colon, transverse, descending and sigmoid colon, rectum)[12]. The overall quality of cleansing was based on the assessment of the individual segments using the grade A = all segment clean (i.e., scores of 3 or 4 in all segments); B = brown liquid or removable semi-solid residue (i.e., score of 2) in 1 or more segments; C = semi-solid only partially removable in at least one segment (i.e., score of 1); D = presence of solid stool that can not be removed (i.e., score of 0). In case of D the exam has to be repeated.
Although we used a validated rating scale, the degree of cleansing remains a matter of personal judgment. In order to minimize this potential issue, four experienced endoscopists (> 5000 procedures in their career) participated in this study after training with the same rating scale by using a set of endophotographs of different segments with various degrees of cleansing.
In the primary analysis, successful bowel cleansing was considered as overall cleansing score equal to A or B.
The primary objective of the study was to compare the degree of cleansing of the bowel preparations. It was also assumed that the low volume prep might improve tolerability and acceptability.
The study was designed as a non-inferiority study and sample size was based on an expected rate of successful bowel cleansing of 80% for both groups. Based on practical considerations, the non-inferiority limit was specified as 15%. In order to reach a 80% statistical power with a significant level of 5%, and taking into account a drop-out rate of 10%, no less than 136 patients were needed in each arm.
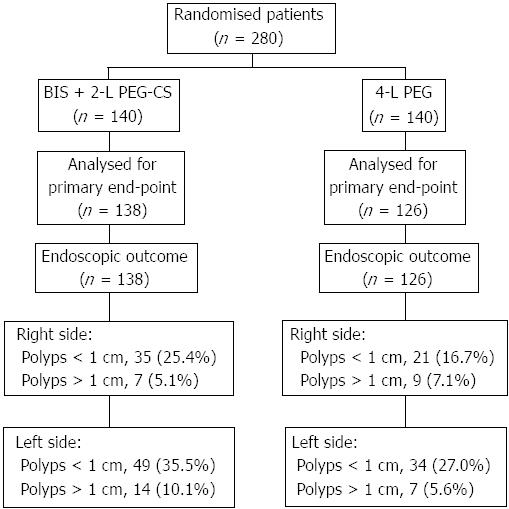
Overall, 280 patients were randomly allocated to receive BIS plus PEG-CS (n = 140) or standard 4-L PEG (n = 140). Colonoscopy data were not available for sixteen patients who did not show up and no information was available with regard to bowel preparation. Therefore 264 patients were included in the analysis of the primary outcome (138 in the PEG-CS and 126 in the 4-L PEG) (Figure 1).
Comparison of demographic characteristics at baseline show no significant differences between the two treatment groups [PEG-CS: male 59.4%; age 63.6 ± 7.1 years; body mass index (BMI) 27.3 kg/m2; 4-L PEG: male 64.3%; age 61.3 ± 7.7 years; BMI 27.7 ± 4.5 kg/m2)].
Bowel preparation was successful (grade A + B) for 92.8% of subjects in the PEG-CS group and for 92.1% of subjects in the 4-L PEG (RR = 1.01; 95%CI: 0.94-1.08) (Table 2). There was no statistical difference with regard to the primary outcome of the study also for the grade A alone between the two groups. Bowel cleansing scores according to colonic segment are shown in Table 3. The rates of excellent score were higher for 4-L PEG in the caecum/ascending colon (P < 0.02) and in the sigmoid colon (P < 0.02) compared with PEG-CS.
| Outcome | BIS + 2-L PEG-CS (n = 138) | 4-L PEG (n = 126) | P value |
| Bowel cleansing | |||
| A (all segments as excellent or good) | 107 (77.5) | 105 (83.3) | NS |
| B (at least one segment as fair) | 21 (15.2) | 11 (8.7) | |
| C (at least one segment as poor) | 7 (5.1) | 8 (6.3) | |
| D (exam not completed) | 3 (2.2) | 2 (1.6) | |
| Successful (A + B) | 128 (92.8) | 116 (92.1) | NS |
| Unsuccessful (C + D) | 10 (7.2) | 10 (7.9) | |
| Tolerability | |||
| Nausea | 27 (19.6) | 26 (20.6) | 0.575 |
| Bloating | 11 (8.0) | 33 (26.2) | < 0.001 |
| Abdominal discomfort | 13 (9.4) | 5 (4.0) | 0.079 |
| Overall (any of previous ones) | 45 (32.6) | 57 (45.2) | 0.035 |
| Acceptance | |||
| Good | 70 (50.7) | 30 (23.8) | < 0.001 |
| Satisfactory | 55 (39.9) | 67 (53.2) | |
| Not acceptable | 13 (9.4) | 22 (23.0) | |
| Compliance | |||
| 100% of solution drunk | 134 (97.1) | 110 (87.3) | 0.010 |
| 75% of solution drunk | 4 (2.9) | 15 (11.9) | |
| 50% or less of solution drunk | 0 (0.0) | 1 (0.8) |
| Caecum/ascending colon | Transverse colon | Descending colon | Sigmoid colon | Rectum | ||||||
| BIS + 2-L PEG-CS (n = 138) | 4-L PEG(n = 126) | BIS + 2-L PEG-CS (n = 138) | 4-L PEG(n = 126) | BIS + 2-L PEG-CS (n = 138) | 4-L PEG(n = 126) | BIS + 2-L PEG-CS (n = 138) | 4-L PEG(n = 126) | BIS + 2-L PEG-CS (n = 138) | 4-L PEG(n = 126) | |
| Score | ||||||||||
| Excellent | 37.00% | 52.40% | 52.20% | 57.90% | 51.40% | 59.50% | 45.70% | 60.30% | 40.60% | 51.60% |
| Good | 50.70% | 37.30% | 38.40% | 34.90% | 37.00% | 31.00% | 39.90% | 28.60% | 43.50% | 39.70% |
| Fair | 7.20% | 4.00% | 5.10% | 2.40% | 6.50% | 5.60% | 9.40% | 5.60% | 12.30% | 4.00% |
| Poor | 2.90% | 4.80% | 2.20% | 3.20% | 5.10% | 3.20% | 5.10% | 4.80% | 3.60% | 4.00% |
| Missing | 2.20% | 1.60% | 2.20% | 1.60% | 0.00% | 0.80% | 0.00% | 0.80% | 0.00% | 0.80% |
A numerically higher number of polyps was observed both in the right and left colon for PEG-CS than 4-L PEG though no statistical difference was found between groups. No adverse events were reported in the study.
The rate of patients with bloating or any GI volume-related symptoms was significantly lower following 2-L PEG-CS + BIS than 4-L PEG (Table 2).
A greater rate of patients in the PEG-CS + BIS had no difficulty and/or were willing to repeat the same preparation than split-dose 4-L PEG. Subjects in the PEG-CS group rated the prep as good or satisfactory in 90.6% as compared to 77% in the 4-L PEG, P < 0.01) (Table 2).
Subjects receiving PEG-CS stated they fully adhered to instructions drinking all the 2-L solution in 97.1% compared with 87.3% in the 4-L PEG (P < 0.01) (Table 2).
This study shows that a new isosmotic 2-L PEG formulation with citrate and simethicone plus BIS tablets was as effective as split 4-L PEG and electrolytes for bowel cleansing in subjects undergoing FOBT-positive screening colonoscopy. The low-volume formulation was associated with better tolerability, acceptability and compliance to instructions received for bowel preparation. Complete bowel preparation is an important component to ensure high quality in colonoscopy and minimize the risk of missing polyps and lesions[9,28]. Current PEG-based bowel preparations are safe but inadequate bowel preparation is common with about 25% of patients with inadequate colon cleansing and about 4%-5% of patients who have to repeat colonoscopy[7,8]. However the large volume of fluids, salty taste and difficulty to complete the preparation remain a deterrent for patients undergoing colonoscopy and to a greater extent for asymptomatic subjects invited to a screening CRC program.
Efforts have been made by the pharmaceutical industry to satisfy the need to reduce the burden and make PEG bowel preparation easier and more acceptable for patients without changing the level of efficacy and safety.
The new procedure based on BIS tablets and split PEG-CS appears to be a valuable option. In our study it provided a level of overall cleansing similar to split 4-L PEG. Previous studies have shown that BIS tablets plus 2-L PEG-CS given the day before is equally as effective and safe as 4-L PEG for bowel cleansing before colonoscopy[22,23]. There are important differences with earlier trials, regarding the PEG-formulation and the dose regimen.
First, the formulation of the low-volume PEG solution is different from the standard 4-L PEG. PEG-CS is sulphate-free, contains new active ingredients (citric acid, sodium citrate and simethicone) and an higher concentration of PEG per litre of reconstituted solution than the traditional PEG formulation. From our data, there is no clue to determine the relative contribution of BIS tablets or any other ingredient of the bowel preparation for bowel cleansing.
Second, this study compared the split dosing regimen for both the low-volume and reference bowel preparation. According to ACG guidelines, split dose bowel preparation enhances the quality of bowel preparation and therefore is now recommended for all patients undergoing screening or surveillance colonoscopy[2]. When a part of the bowel preparation is taken within 4-8 h of colonoscopy, there is a better cleansing of caecum and the ascending colon compared with traditional dosing schedule of the day before. With such regimen the long interval of > 12 h between bowel preparation and colonoscopy allows the flow of intestinal secretion across the ileo-caecum valve and yellow fluid cover mucosa of the right colon. For the full-dose, we have used an unequal split (PEG 3-L the day before and 1-L the day before the same day) which have been shown to be effective but more feasible as it allows to perform colonoscopy shortly in the morning[29].
In our study BIS tablets were taken in the afternoon before, 1 L of PEG-CS was taken in the evening and 1 L in the morning about 4 h before colonoscopy. The important finding of this study is that for the first time a 2-L PEG preparation administered as a split-dose was shown to be as globally effective as 4-L PEG. Although this finding needs to be confirmed in future trials, a better cleansing in the right colon may favor the detection of small or flat lesions which are more likely to remain undetected compared to other sites of the colon[9,10].
Any new low-volume bowel preparations should also be evaluated on the grounds of safety, tolerability, acceptance and compliance.
PEG-CS is an osmotically balanced PEG solution and therefore it is less likely to induce electrolyte imbalance as compared to bowel preparations based on sodium phosphate, magnesium phosphate or hyperosmotic PEG solutions. Based on vital signs, haemodynamic data and lack of extra-intestinal adverse events, no safety issue was identified in this study for both bowel preparations.
Similarly to standard 4-L PEG no issues of safety, in particular electrolyte imbalance and dehydration are associated with the new formulation.
The new formulation was significantly better tolerated and accepted by patients. A reduced rate of bloating and cumulative volume-related GI symptoms were observed in BIS plus PEG-CS than in the reference group. This is not surprising as a much lower amount of non-absorbable fluid must be taken for each session with PEG-CS (1-L only) than traditional PEG-formulation. The new formulation was also shown to be less difficult to complete, of pleasant taste and patients were more willing to repeat it for future examination. Although split-dosing itself may increase acceptance of bowel preparation, the features of this low-volume colon cleansing procedure improved acceptance, compliance and adhesion to bowel preparation instructions.
This low-volume bowel preparation may be a first option for patients who poorly tolerated and accepted large volume bowel preparations for colonoscopy. Better tolerability, acceptance and compliance to bowel preparation may increase the attitude and uptake to screening colonoscopy. This requires a high level of quality as the objective of the examination is to detect even small but potentially dangerous lesions which may progress to cancer.
This single-centre study was carried out in a homogenous group of patients undergoing screening colonoscopy. It should be noted that the high rate of adequate bowel cleansing in both groups is largely due to the fact the sample group was made of motivated subjects aged between 50 and 69 years who came for a second level examination after being found positive to occult blood. Our finding may have important implication for the general population or elderly people who may have lower levels of motivation to undergo colonoscopy also due to bowel preparation.
There are concerns about ischemic colitis related to BIS. To date, no reports of ischemic colitis were observed in the clinical trials reviewed and in the post-marketing pharmacovigilance according to the manufacturer. A causal relationship between use of BIS for colon cleansing and ischemic colitis remains to be established.
Over the last years the pharmaceutical industry has tried to offer bowel preparations which are better accepted without compromising efficacy and safety. Although further evidence is needed, it seems from our study that an important advance toward optimal and easy bowel preparation has been made.
In conclusion, this study evaluated a new low-volume bowel preparation for FOBT-positive screening colonoscopy. BIS plus PEG-CS was as effective but better tolerated and accepted than split 4-L PEG. Bowel preparation before colonoscopy has for a long time been an issue for our patients. Now progress has been made towards better tolerability, acceptance and compliance of bowel preparation. Reducing the burden for healthy subjects may improve their attitude and maximize the benefits of screening colonoscopy.
Promefarm provided supply of study medications.
Quality of bowel preparation is essential to identify lesions in the colon. The running time between the last dose of bowel preparation and the exam has been shown to play a key role toward the ideal bowel cleansing.
Split dose regimen with a fraction of bowel preparation taken the day of the exam may be an effective approach in clinical practice.
A new colon cleansing procedure based on bisacodyl (BIS) plus polyethylene glycol (PEG) with citrates and simethicone (PEG-CS) administered as split dose has the same efficacy but superior tolerability and acceptance to split conventional 4-L PEG.
The study results suggest that the split dose of the low volume PEG-CS after BIS increases the patient attitude and acceptance to colorectal cancer screening colonoscopy.
This is a well designed and written study that adds to the authors’ understanding of optimal bowel preparation regimens.
| 1. | Brenner H, Bouvier AM, Foschi R, Hackl M, Larsen IK, Lemmens V, Mangone L, Francisci S. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: the EUROCARE study. Int J Cancer. 2012;131:1649-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2382] [Article Influence: 170.1] [Reference Citation Analysis (2)] |
| 4. | Parente F, Marino B, Ardizzoia A, Ucci G, Ilardo A, Limonta F, Villani P, Moretti R, Zucchi A, Cremaschini M. Impact of a population-based colorectal cancer screening program on local health services demand in Italy: a 7-year survey in a northern province. Am J Gastroenterol. 2011;106:1986-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Zorzi M, Fedato C, Naldoni C, Sassatelli R, Sassoli De’ Bianchi P, Senore C, Visioli CB, Cogo C. Screening for colorectal cancer in Italy: 2007 survey. Epidemiol Prev. 2009;33:57-74. [PubMed] |
| 6. | Lichtenstein G. Bowel preparations for colonoscopy: a review. Am J Health Syst Pharm. 2009;66:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378-384. [PubMed] |
| 8. | Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76-79. [PubMed] |
| 9. | Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 278] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 10. | Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 379] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 11. | Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22-30. [PubMed] |
| 12. | Halphen M, Heresbach D, Gruss HJ, Belsey J. Validation of the Harefield Cleansing Scale: a tool for the evaluation of bowel cleansing quality in both research and clinical practice. Gastrointest Endosc. 2013;78:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Nyberg C, Hendel J, Nielsen OH. The safety of osmotically acting cathartics in colonic cleansing. Nat Rev Gastroenterol Hepatol. 2010;7:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Valiante F, Pontone S, Hassan C, Bellumat A, De Bona M, Zullo A, de Francesco V, De Boni M. A randomized controlled trial evaluating a new 2-L PEG solution plus ascorbic acid vs 4-L PEG for bowel cleansing prior to colonoscopy. Dig Liver Dis. 2012;44:224-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 15. | DiPalma JA, Wolff BG, Meagher A, Cleveland Mv. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol. 2003;98:2187-2191. [PubMed] |
| 16. | Parra-Blanco A, Nicolas-Perez D, Gimeno-Garcia A, Grosso B, Jimenez A, Ortega J, Quintero E. The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: a randomized study. World J Gastroenterol. 2006;12:6161-6166. [PubMed] |
| 17. | Siddiqui AA, Yang K, Spechler SJ, Cryer B, Davila R, Cipher D, Harford WV. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Marmo R, Rotondano G, Riccio G, Marone A, Bianco MA, Stroppa I, Caruso A, Pandolfo N, Sansone S, Gregorio E. Effective bowel cleansing before colonoscopy: a randomized study of split-dosage versus non-split dosage regimens of high-volume versus low-volume polyethylene glycol solutions. Gastrointest Endosc. 2010;72:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Cohen LB. Split dosing of bowel preparations for colonoscopy: an analysis of its efficacy, safety, and tolerability. Gastrointest Endosc. 2010;72:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | El Sayed AM, Kanafani ZA, Mourad FH, Soweid AM, Barada KA, Adorian CS, Nasreddine WA, Sharara AI. A randomized single-blind trial of whole versus split-dose polyethylene glycol-electrolyte solution for colonoscopy preparation. Gastrointest Endosc. 2003;58:36-40. [PubMed] |
| 21. | Aoun E, Abdul-Baki H, Azar C, Mourad F, Barada K, Berro Z, Tarchichi M, Sharara AI. A randomized single-blind trial of split-dose PEG-electrolyte solution without dietary restriction compared with whole dose PEG-electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest Endosc. 2005;62:213-218. [PubMed] |
| 22. | Repici A, Cestari R, Annese V, Biscaglia G, Vitetta E, Minelli L, Trallori G, Orselli S, Andriulli A, Hassan C. Randomised clinical trial: low-volume bowel preparation for colonoscopy - a comparison between two different PEG-based formulations. Aliment Pharmacol Ther. 2012;36:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Cesaro P, Hassan C, Spada C, Petruzziello L, Vitale G, Costamagna G. A new low-volume isosmotic polyethylene glycol solution plus bisacodyl versus split-dose 4 L polyethylene glycol for bowel cleansing prior to colonoscopy: a randomised controlled trial. Dig Liver Dis. 2013;45:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Wu L, Cao Y, Liao C, Huang J, Gao F. Systematic review and meta-analysis of randomized controlled trials of Simethicone for gastrointestinal endoscopic visibility. Scand J Gastroenterol. 2011;46:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (4)] |
| 25. | Park JJ, Lee SK, Jang JY, Kim HJ, Kim NH. The effectiveness of simethicone in improving visibility during colonoscopy. Hepatogastroenterology. 2009;56:1321-1325. [PubMed] |
| 26. | Lazzaroni M, Petrillo M, Desideri S, Bianchi Porro G. Efficacy and tolerability of polyethylene glycol-electrolyte lavage solution with and without simethicone in the preparation of patients with inflammatory bowel disease for colonoscopy. Aliment Pharmacol Ther. 1993;7:655-659. [PubMed] |
| 27. | McNally PR, Maydonovitch CL, Wong RK. The effectiveness of simethicone in improving visibility during colonoscopy: a double-blind randomized study. Gastrointest Endosc. 1988;34:255-258. [PubMed] |
| 28. | Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006;63:894-909. [PubMed] |
| 29. | Manno M, Pigò F, Manta R, Barbera C, Bertani H, Mirante VG, Dabizzi E, Caruso A, Olivetti G, Hassan C. Bowel preparation with polyethylene glycol electrolyte solution: optimizing the splitting regimen. Dig Liver Dis. 2012;44:576-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
P- Reviewers Belsey J, Bordas JM S- Editor Gou SX L- Editor A E- Editor Li JY