Published online Aug 28, 2013. doi: 10.3748/wjg.v19.i32.5340
Revised: June 13, 2013
Accepted: July 4, 2013
Published online: August 28, 2013
Processing time: 155 Days and 20.4 Hours
AIM: To achieve a better understanding of the origination of neuroendocrine (NE) cells in gastric adenocarcinoma.
METHODS: In this study, 120 cases of gastric adenocarcinoma were obtained. First, frozen section-immunohistochemistrical samples were selected from a large quantity of neuroendocrine cells. Second, laser capture microdissection was used to get target cells from gastric adenocarcinoma and whole genome amplification was applied to get a large quantity of DNA for further study. Third, genome-wide microsatellite abnormalities [microsatellite instability (MSI), loss of heterozygosity (LOH)] and p53 mutation were detected by polymerase chain reaction (PCR)-single-strand conformation polymer- phism-silver staining and PCR-sequencing in order to identify the clonality of NE cells.
RESULTS: The total incidence rate of MSI was 27.4%, while LOH was 17.9%. Ten cases had a highest concordance for the two types of cells. The other samples had similar microsatellite changes, except for cases 7 and 10. Concordant p53 mutations exhibited in sample 4, 14, 21 and 27, and there were different mutations between two kinds of cells in case 7. In case 17, mutation took place only in adenocarcinoma cells. p53 mutation was closely related with degree of differentiation, tumor-node-metastasis stage, vessel invasion and lymph node metastasis. In brief, NE and adenocarcinoma cells showed the same MSI, LOH or p53 mutation in most cases (27/30). In the other three cases, different MSI, LOH or p53 mutation occurred.
CONCLUSION: NE and the gastric adenocarcinoma cells may mainly derive from the same stem cells, but the remaining cases showing different origin needs further investigation.
Core tip: There have been only a few studies of neuroendocrine differentiation (NED) in gastric adenocarcinoma. Therefore, we studied the clonality of neuroendocrine (NE) cells in gastric adenocarcinoma using laser capture microdissection, microsatellite instability (MSI), loss of heterozygosity (LOH) and p53 mutation to evaluate the clonality of NED. NE and adenocarcinoma cells showed the same MSI, LOH or p53 mutation in most cases (27/30), they may originate from the same stem cells, but the remaining three cases showed different origins, which warrants further research.
- Citation: Wang LL, Yao GY, Zhao ZS, Wei XL, Xu RJ. Clonality analysis of neuroendocrine cells in gastric adenocarcinoma. World J Gastroenterol 2013; 19(32): 5340-5346
- URL: https://www.wjgnet.com/1007-9327/full/v19/i32/5340.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i32.5340
Although the worldwide incidence and mortality of gastric cancer have been declining steadily, it remains one of the most common cancers and the leading cause of cancer death worldwide[1]. Previous studies have reported that mixed glandular-neuroendocrine (NE) tumors that arise from the gastrointestinal tract, such as the stomach and colon, normally contain both glandular and endocrine cells[2,3]. These studies have suggested that mixed tumors occur as a consequence of multidirectional differentiation of glandular of endocrine stem cells that are derived from the endoderm. However, it remains unclear whether the glandular and endocrine cells expand from two distinct precursors, or arise from a single progenitor cell.
Microsatellite instability (MSI) is a form of genetic instability that is characterized by new alleles that are not present in the normal genotype. This type of mutation occurs in various human carcinomas[4], and is believed to be caused by altered DNA mismatch repair genes. Several genetic alterations have been shown to play a significant role in tumourigenesis. The most frequently observed molecular changes occur in the p53 gene[5]. There is now enough evidence to suggest that the functional inactivation of the p53 gene through allelic loss and point mutation plays an important role[6]. The p53 gene encodes a protein that is involved in control of the cell cycle and acts as a negative regulator in the cell response to damaged DNA. The most widely used molecular approach is single-strand conformation polymorphism (SSCP) analysis of DNA fragments amplified by the polymerase chain reaction (PCR), with subsequent sequence analysis. Functional alteration of p53 protein can occur through several mechanisms: point mutations, deletions, rearrangements in the p53 gene, binding with viral proteins, binding with cellular proteins, and oligomerization[7]. Wild-type p53 protein has a very short half-life, whereas mutated p53 is stable and can accumulate at high concentrations in the nuclei of tumor cells. As a consequence, immunohistochemical staining with specific antibodies can be used to detect mutant p53 protein.
To achieve a better understanding of the origination of NE cells in gastric adenocarcinoma, and provide a clear method of evaluation to clinicians, we performed a prospective study on neuroendocrine differentiation in gastric adenocarcinoma by analyzing MSI, loss of heterozygosity (LOH) and p53 mutation.
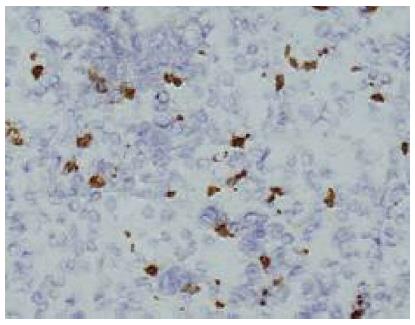
In this study, 120 cases of gastric adenocarcinomas and corresponding non-neoplastic gastric mucosal tissues were obtained from the People’s Hospital of Zhejiang Province, China. The tumors were staged according to the tumor-node-metastasis (TNM) classification and were graded according to the World Health Organization classification. Immunohistochemistry was carried out using the primary antibody against NE marker (chromogranin A, polyclonal, 1:100; Maixin, China). In brief, the tissue sections were incubated in methanol for 5 min. After washing with phosphate-buffered saline (PBS), the sections were incubated in 7.5% hydrogen peroxide for 5 min, followed by further washing with PBS. The sections were then incubated with primary antibodies in the case of chromogranin A at 4 °C overnight. Then these sections were detected using Two-Step Immunohistochemical Detection Reagent (ZSGB-BIO, Beijing, China). Frozen section immunohistochemistry samples were selected from a large quantity of NE cells. The study was approved by the Ethics Committee for Human Study in our institution.
Laser-capture microdissection (LCM) was performed with the use of an Arcturus PixCell II microscope (Arcturus Engineering, Mountain View, CA, United States) to obtain cells from gastric adenocarcinoma. The technology for melting heat of infrared rays was used to melt the polymeride under microscope, followed by molecular biology analysis. Open the instrument, put the complete slice on the objective table, the cell image was exhibited on the computer screen through the microscope. If the cellular morphology was normal, with satisfactory staining, under 10 × 20 lens according to the following conditions: power, 65 mV; duration, 15.5 s; and spots size, 7.5 μm, we attached the transparent Elvax® ethylene vinyl acetate hot plastic film hat by the driving arm to lay aside precisely above the tissue slice. The target cell or the cell group was obtained through the control handle to the slice migration located at the field of vision centre. Press the button according to the target region’s size, and the focusing infrared laser beam carries on the capture. When the laser beam launch ended, move the mechanical arm from the slice to emigrate the cover and the thin film, move the hat into 0.5 mL Eppendorf centrifuge tube (add the Micro-kit extraction reagent box extraction buffer solution beforehand), and proceed with the DNA extraction. A 7.5-mm-diameter laser beam was used to procure NE cells and a 15- or 30-mm-diameter beam for adenocarcinoma cells. LCM cells were pooled from multiple caps, which were stored at -20 °C until dissection was complete. Approximately 15000 laser hits to each specimen gave the necessary cell yield after transfer. LCM was performed with capture of 500 NE cells and thousands of adenocarcinoma cells from each sample. NE and adenocarcinoma cell populations were stored separately. Cell samples were frozen immediately at -20 °C, and were sent on the same day, on caps frozen on dry ice, for DNA extraction and subsequent genetic analysis.
DNA extraction from the captured cells and whole genome amplification (WGA) were performed using DNA Micro-kit and DNA Repli-g Midi kit (QIAGEN, Germany) to obtain a large quantity of DNA. The brief processes were as follows: 15 μL buffer ATL (provided in kit) was added to a 0.5-mL microcentrifuge tube that contained the laser-microdissected cells; 10 μL proteinase K was added and mixed by pulse-vortexing for 15 s; the 0.5-mL tube was then placed in a thermomixer or heated orbital incubator, and incubated at 56 °C for 3 h, with occasional agitation; 25 μL buffer ATL was added with 50 μL buffer AL, and mixed well by pulse-vortexing for 15 s; 50 μL ethanol (96%-100%) was added and mixed thoroughly by pulse-vortexing for 15 s, incubated for 5 min at room temperature. Then, the entire lysate was carefully transferred to the QIAamp MinElute Column, centrifuged at 8000 g for 1 min and placed in a clean 2-mL collection tube; 500 μL buffer AW1 and AW2 (provided in kit) were added, respectively, and centrifuged at 8000 g for 1 min, followed by a full speed centrifugation at 14000 g for 3 min to dry the membrane completely. The QIAamp MinElute Column was placed in a clean 1.5-mL microcentrifuge tube and 20-30 μL buffer AE was added to the centre of the membrane, incubated at room temperature for 1 min, and finally centrifuged at 14000 g for 1 min. The DNA was denatured by adding denaturation buffer and stopped by adding of neutralization buffer that contained DNA polymerase. The isothermal amplification reaction proceeded for at least 8 h at 30 °C. The method was used based on a technology that carries out isothermal genome amplification utilising a unique processive DNA polymerase, which could replicate up to 100 kb without dissociating from the genomic DNA template. The DNA polymerase had a 3’-5’ exonuclease proofreading activity to maintain a high fidelity during replication, and was used with exonuclease-resistant primers to achieve a high yield of DNA product. The final processes were: TE buffer and denaturation solution were added, mixed well and incubated at room temperature for 3 min; neutralization buffer was added, mixed, followed by adding REPLI-g master mix, and incubated for 8-16 h at 30 °C; and REPLI-g Midi DNA polymerase was inactivated by heating the sample at 65 °C for 3 min.
We chose 26 microsatellite markers with genome-wide scope for MSI analysis, and chose p53 exons 5-8 for p53 mutation analysis. The primers for these analyses are listed in Table 1.
| Microsatellite | Sequences |
| D1S104 | ATCCTGCCCTTATGGAGTGCCCCACTCCTCTGTCATTGTA |
| D2S119 | CTTGGGGAACAGAGGTCATTGAGAATCCCTCAATTTCTTTGGA |
| D2S123 | AAACAGGATGCCTGCCTTTAGGACTTTCCACCTATGGGAC |
| D3S1766 | ACCACATGAGCCAATTCTGTACCCAATTATGGTGTTGTTACC |
| D3S2427 | CTCCTCGTCACTGCAGTCTTCTGCCTCATCTGTTCAGGAT |
| D4S174 | AAGAACCATGCGATACGACTCATTCCTAGATGGGTAAAGC |
| D4S402 | CTTACTGTGTTGCCCAAGGTAGCTCTATGATTCATTTCAAGTTTG |
| D5S107 | GATCCACTTTAACCCAAATACGGCATCAACTTGAACAGCAT |
| D5S346 | ACTCACTCTAGTGATAAATCGGGAGCAGATAAGACAGTATTACTAGTT |
| D5S409 | GGGATGAAGTGTGGATAAACTAGGATGGCAGTGCTCTTAG |
| D7S1805 | CCTGCTTTGGCTTACCTGTACCCACTTCTCTGCTATTACATAT |
| D9S157 | AGCAAGGCAAGCCACATTTCTGGGGATGCCCAGATAACTATATC |
| D10S469 | CAACAAGTGTGAGAGTCCATATGTTCTGTCTCTCCACAGT |
| AFMA086WG9 | ATGTACGGTTCATTGACTTGACTGACTACAAATGGGCA |
| D11S861 | CTGAAACCAAGTGAAAAGGAGAAAGCTCCATTGTCTTCTGGC |
| D12S1899 | TTCTTCCTTTCTCTTTCTCTCTTCCGCACAAGTGACACATGGTCC |
| D16S398 | CTTGCTCTTTCTAAACTCCAGAAACCAAGTGGGTTAGGTC |
| D16S496 | GAAAGGCTACTTCATAGATGGCAATATAAGCCACTGCGCCCAT |
| D16S534 | CAACAAAGCAAGACCCTGTCCATCTGCGGTTCTTTCCTC |
| D16S265 | AGCTCTCTGAGTCCTCTGTGCGGAAGCATGGTGTCTCTCG |
| D16S752 | AATTGACGGTATATCTATCTGTCTGGATTGGAGGAGGGTGATTCT |
| D17S250 | GGAAGAATCAAATAGACAATGCTGGCCATATATATATTTAAACC |
| D17S796 | CAATGGAACCAAATGTGGTCAGTCCGATAATGCCAGGATG |
| D19S416 | CCTGTCCCAGAGAGACCCTAAAGAGAGTGTGCCATTTGCT |
| BAT 25 | GTTTCGCCTCCAAGAATGTAAGTGTTTCTGCATTTTAACTATGGCTC |
| BAT 26 | TGACTACTTTTGACTTCAGCCAACCATTCAACATTTTAACCC |
| Exon 5 | GACTTTCAACTCTGTCTCCTCTGGGGACCCTGGGCAAC |
| Exon 6 | GAGACGACAGGGCTGGGTCCACTGACAACCACCCTT |
| Exon 7 | GTGTTGTCTCCTAGGTTGGCAAGTGGCTCCTGACCTGGAG |
| Exon 8 | CCTTACTGCCTCTTGCTTTGAATCTGAGGCATAACTGC |
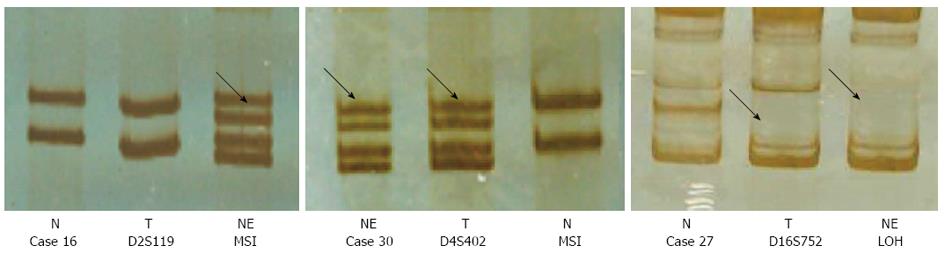
Genome-wide microsatellite abnormalities (MSI and LOH) and p53 mutation were detected by PCR-SSCP silver staining and PCR sequencing to identify the clonality of NE cells. To evaluate microsatellite alterations, extra shadow bands above and below each intense principal allelic band were often visualized in microsatellite studies, and the most intense bands were considered the real alleles.
Statistical analyses were performed using SPSS for Windows version 15.0 (SPSS, Chicago, IL, United States). Survival data were analysed using the χ2 test, Spearman rank correlation analysis, and Kaplan-Meier analysis, and a survival curve was drawn. Differences were analysed using the log rank test and P < 0.05 was considered statistically significant.
Thirty samples from a total of 120 that contained a large number of NE cells were selected for LCM. About 500 NE cells were precisely captured from each sample (Figures 1 and 2).
The total incidence rate of MSI was 27.4%, and LOH rate was 17.9%. The rates in gastric adenocarcinoma cells and NE cells were similar. There was no significant relationship between the MSI or LOH rate and clinicopathological characteristics. According to the coincidence of microsatellite changes, cases 2, 3, 5, 6, 11, 12, 18, 24, 27 and 30 had a highest concordance for the two types of cells. The other samples had similar microsatellite changes, except for cases 7 and 10 (Figure 3).
Most mutations of the p53 gene were detected in exons 7 and 8. Concordant mutations were observed in cases 4, 14, 21 and 27, and there were different mutations in the two types of cells (e.g., NE and gastric adenocarcinoma cells) in case 7. In case 17, the mutation was seen only in the adenocarcinoma cells not in the NE cells. p53 mutation occurred six times in adenocarcinoma cells (20.0%) and five times in NE cells (16.7%). Clinicopathological analysis further showed that p53 mutations were well associated with poor differentiation and TNM stages III or IV tumors, the mutations were also linked to blood vessel invasion and lymph node metastasis (Table 2).
| Case | Cell | Exon | Codon | Mutation | Amino acid | Differentiation | TNM | Metastasis |
| 4 | Cancer | 8 | 273 | GC→AT | Arg→Cys | Poor | IV | + |
| NE | 8 | 273 | GC→AT | Arg→Cys | ||||
| 7 | Cancer | 7 | 244 | GC→AT | Gly→Ser | Poor | IV | + |
| NE | 8 | 287 | GC→AT | Glu→Lys | ||||
| 14 | Cancer | 8 | 287 | GC→AT | Glu→Lys | Poor | IB | + |
| NE | 8 | 287 | GC→AT | Glu→Lys | ||||
| 21 | Cancer | 7 | 244 | GC→AT | Gly→Ser | Poor | IV | + |
| NE | 7 | 244 | GC→AT | Gly→Ser | ||||
| 27 | Cancer | 8 | 282 | GC→AT | Arg→Arg | Moderate | III | + |
| NE | 8 | 282 | GC→AT | Arg→Arg | ||||
| 17 | Cancer | 7 | 244 | GC→AT | Gly→Ser | Poor | III | + |
Our previous studies have demonstrated that NED occurred in 41.5% of colon cancers, 39.6% of gastric cancers, 38.1% of prostate cancers, 21% of breast cancers and 17.9% of pancreatic cancers, and NE in gastric adenocarcinoma was more frequently observed in poorly differentiated cancers than in well-differentiated tumors[8], which was different from other studies that showed that NE was associated with well-differentiated tumors[9,10]. However, it is not clear whether NE is derived from embryogenesis, histogenesis, or genetic changes that are associated with tumor etiology. It has been shown that NED occurs in adenocarcinoma of the prostate, gastrointestinal tract and lungs. These NE cells synthesize and excrete neuropeptides or amines hormones, leading to an increase of plasma hormone levels[11-13]. Hirano et al[14] found that the prognosis for gastric adenocarcinoma with choriocarcinoma and neuroendocrine cell carcinoma is exceedingly poor. Whereas, the biological functions of NED for the development or prognosis of gastric adenocarcinoma are largely unknown. We thus employed LCM to capture NE cells, distinguished from the gastric adenocarcinoma cells, and utilized molecular and genetic approaches to study the origin of NE cells and their association with gastric cancer biology. We found that the NE cells and gastric adenocarcinoma cells shared similar MSI, LOH and p53 mutation, meaning both cell lines may be derived from same stem cells.
It has been well known that the NE cells are derived from multipotent stem cells. NED is initiated by hormonal change, microenvironmental change, and genomic instability. Some subdued genomic codes are randomly depressed and selectively activated by more than two regulatory genes during RNA translation, and as a result, multipotent stem cells generate differentiation or multidifferentiation[15]. Despite the apparently different morphological representation of NE cells in the tumor mass, it is largely unknown whether these cells have chromosomal or genetic alterations. Moreover, it is not clear whether NE cells are present as tumor or stromal components. NE cells from gastric adenocarcinoma were harvested by LCM, which ensured cell purity. Whole genome amplification (WGA) was then employed to compare genomic characteristics of NE cells with adenocarcinoma cells, for the identification of the clonality of the former. The development and prognosis of gastric cancer involves a number of genetic and epigenetic abnormalities[16]. MSI is thought to be an important molecular phenotype in gastric cancer[17]. In gastric cancer, the loss of genomic stability represents a key molecular step that occurs early in carcinogenesis, and creates a permissive environment for the accumulation of genetic and epigenetic alterations in tumor suppresser genes and oncogenes. It is widely accepted that gastric cancer can follow at least two major genomic instability pathways: MSI and chromosome instability[18]. LOH and MSI have strong sensitivity but poor specificity, whereas gene mutation has strong specificity but poor sensitivity. The appropriate combination of the two methods can give more precise results. Huang et al[19] have demonstrated whether different components of combined tumors contain the same or different genetic alterations, thus providing evidence for their clonality. As a result, he has suggested that, in the majority of combined tumors, cells with different phenotypes share similar genotypes and might arise from a single precursor cell. Only in a minority of these tumors are different areas derived from different precursor cells. Our study suggested that concordant microsatellite changes occurred in two types of cells in cases 2, 3, 5, 6, 11, 12, 18, 24, 27 and 30; different microsatellite changes in cases 7 and 10; and in the remaining 18 cases, there were no significant differences in microsatellite changes in the two types of cells. There was no correlation between MSI and degree of differentiation in gastric cancer. Semba et al[20] have suggested that MSI appears at a high frequency in well-differentiated adenocarcinoma, but others have come to the opposite conclusion[21].
Wild-type p53 acts as an anti-oncogene in normal tissues, which is important in DNA repair and cell cycle regulation. Tumourigenesis is closely associated with p53 mutation or loss of function[22]. Genetic changes (such as gain or loss of chromosomal segments, or gene mutation) in allelic genes are induced by unbalanced mitosis during stem cell differentiation. These genetic changes could be used for analysis of cell clonality. They can be detected by microsatellite changes (including LOH and MSI), gene mutation, and comparative genomic hybridisation. The functional inactivation of p53 gene through allelic loss and point mutation plays an important part in the development of gastric cancer. We can detect mutant p53 protein by immunohistochemical staining with specific antibodies[23,24]. Nishikura et al[25] have suggested that NE carcinoma is composed of precursor NE cells that are generated from adenocarcinoma, and p53 promotes this process. While studying gastrointestinal carcinomas, Eren has discovered that p53 mutation might be associated with NED of adenocarcinoma[26]. The rate of p53 positivity in gastric carcinoma with NED was clearly higher than that in gastric carcinoma without NED. Our study showed that the rate of p53 mutation in gastric adenocarcinoma cells was 20%, and it was 16.7% in NE cells. In cases 4, 14, 21 and 27, concordant mutations were seen in exons 7 and 8 in the two types of cells; in case 7, different p53 mutations were observed; and in case 17, p53 mutation was only seen in adenocarcinoma cells and not in the NE cells. The concordance rate of p53 mutation in the two types of cells was 66.7%. Based on the similar microsatellite changes and p53 mutations in both NE cells and adenocarcinoma cells in the 27 of 30 cases, we claimed that the NE and adenocarcinoma cells probably were derived from the same stem cells. Our results provided more evidence to support that the multipotent stem cells could differentiate to NE and adenocarcinoma cells. Whether NE cells can act as parenchyma of carcinoma and secrete hormones to promote carcinoma needs further investigation. We also found that 3 cases showed different MSI, LOH and p53 mutation pattern, suggesting that the NE and gastric adenocarcinoma cells were derived from different stem cells. Further study on the underlying mechanisms is needed.
We would like to thank Professor Wancai Yang (University of Illinois at Chicago, Chicago, United States) for critical discussion and English language revision.
Neuroendocrine differentiation (NED) is a common phenomenon in adenocarcinomas, but there have been only a few studies of NED in gastric adenocarcinoma. It remains unclear whether the glandular and endocrine cells expand from two distinct precursors, or arise from a single progenitor cell.
Authors used laser capture microdissection, microsatellite instability (MSI), loss of heterozygosity (LOH) and p53 mutation to evaluate the clonality of NED.
Authors studied the clonality of neuroendocrine (NE) cells in gastric adenocarcinoma using laser capture microdissection, MSI, LOH and p53 mutation to evaluate the clonality of NED. NE and adenocarcinoma cells showed the same MSI, LOH or p53 mutation in most cases (27/30), which may originate from the same stem cells. In the other three cases, different MSI, LOH or p53 mutation occurred.
The article helps to achieve a better understanding of the origination of NE cells in gastric adenocarcinoma, and provide a clear method of evaluation to clinicians.
Laser-capture microdissection (LCM): LCM was performed to obtain cells from gastric adenocarcinoma. The technology makes use of the melting heat of infrared rays to melt the polymeride under microscope, followed by molecular biology analysis.
The authors discuss the available information on LOH and MSI in view of their findings and published reports. They presented from their and other groups the findings on p53 in gastric cancer and argued that NE and adenocarcinoma cell likely derive from the same stem cell in the majority of the tested tumors. In brief, this is an interesting study that is thoroughly performed and interpreted.
| 1. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [PubMed] |
| 2. | Kim KM, Kim MJ, Cho BK, Choi SW, Rhyu MG. Genetic evidence for the multi-step progression of mixed glandular-neuroendocrine gastric carcinomas. Virchows Arch. 2002;440:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Fukui H, Takada M, Chiba T, Kashiwagi R, Sakane M, Tabata F, Kuroda Y, Ueda Y, Kawamata H, Imura J. Concurrent occurrence of gastric adenocarcinoma and duodenal neuroendocrine cell carcinoma: a composite tumour or collision tumours ? Gut. 2001;48:853-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Semba S, Yokozaki H, Yamamoto S, Yasui W, Tahara E. Microsatellite instability in precancerous lesions and adenocarcinomas of the stomach. Cancer. 1996;77:1620-1627. [PubMed] |
| 5. | Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5532] [Cited by in RCA: 5502] [Article Influence: 157.2] [Reference Citation Analysis (0)] |
| 6. | Renault B, van den Broek M, Fodde R, Wijnen J, Pellegata NS, Amadori D, Khan PM, Ranzani GN. Base transitions are the most frequent genetic changes at P53 in gastric cancer. Cancer Res. 1993;53:2614-2617. [PubMed] |
| 7. | Prokocimer M, Rotter V. Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood. 1994;84:2391-2411. [PubMed] |
| 8. | Yao GY, Zhou JL, Lai MD, Chen XQ, Chen PH. Neuroendocrine markers in adenocarcinomas: an investigation of 356 cases. World J Gastroenterol. 2003;9:858-861. [PubMed] |
| 9. | Atasoy P, Ensari A, Demirci S, Kurşun N. Neuroendocrine differentiation in colorectal carcinomas: assessing its prognostic significance. Tumori. 2003;89:49-53. [PubMed] |
| 10. | Yao GY, Zhou JL, Zhao ZS, Ruan J. Biological characteristics of breast carcinomas with neuroendocrine cell differentiation. Chin Med J (Engl). 2004;117:1536-1540. [PubMed] |
| 11. | Hong SM, Kim MJ, Pi DY, Jo D, Yu E, Ro JY. Neuroendocrine differentiation in extrahepatic bile duct carcinomas and its prognostic significance. Hum Pathol. 2005;36:732-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004;45:586-92; discussion 592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 226] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Waldum HL, Aase S, Kvetnoi I, Brenna E, Sandvik AK, Syversen U, Johnsen G, Vatten L, Polak JM. Neuroendocrine differentiation in human gastric carcinoma. Cancer. 1998;83:435-444. [PubMed] |
| 14. | Hirano Y, Hara T, Nozawa H, Oyama K, Ohta N, Omura K, Watanabe G, Niwa H. Combined choriocarcinoma, neuroendocrine cell carcinoma and tubular adenocarcinoma in the stomach. World J Gastroenterol. 2008;14:3269-3272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Schalken JA, van Leenders G. Cellular and molecular biology of the prostate: stem cell biology. Urology. 2003;62:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Tamura G. [Gastric cancer: histological type, histogenesis, and gene abnormalities]. Gan To Kagaku Ryoho. 2008;35:343-349. [PubMed] |
| 17. | Pedrazzani C, Corso G, Velho S, Leite M, Pascale V, Bettarini F, Marrelli D, Seruca R, Roviello F. Evidence of tumor microsatellite instability in gastric cancer with familial aggregation. Fam Cancer. 2009;8:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Ottini L, Falchetti M, Lupi R, Rizzolo P, Agnese V, Colucci G, Bazan V, Russo A. Patterns of genomic instability in gastric cancer: clinical implications and perspectives. Ann Oncol. 2006;17 Suppl 7:vii97-vi102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Huang J, Behrens C, Wistuba II, Gazdar AF, Jagirdar J. Clonality of combined tumors. Arch Pathol Lab Med. 2002;126:437-441. [PubMed] |
| 20. | Semba S, Yokozaki H, Yamamoto S, Yasui W, Tahara E. Microsatellite instability in precancerous lesions and adenocarcinomas of the stomach. Cancer. 1996;77:1620-1627. [PubMed] |
| 21. | Han HJ, Yanagisawa A, Kato Y, Park JG, Nakamura Y. Genetic instability in pancreatic cancer and poorly differentiated type of gastric cancer. Cancer Res. 1993;53:5087-5089. [PubMed] |
| 22. | Gorgoulis V, Zoumpourlis V, Rassidakis G, Karameris A, Barbatis C, Spandidos DA, Kittas C. Molecular analysis of p53 gene in laryngeal premalignant and malignant lesions. p53 protein immunohistochemical expression is positively related to proliferating cell nuclear antigen labelling index. Virchows Arch. 1995;426:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Karim S, Ali A. Correlation of p53 over-expression and alteration in p53 gene detected by polymerase chain reaction-single strand conformation polymorphism in adenocarcinoma of gastric cancer patients from India. World J Gastroenterol. 2009;15:1381-1387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Soussi T. The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann N Y Acad Sci. 2000;910:121-137; discussion 137-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 228] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Nishikura K, Watanabe H, Iwafuchi M, Fujiwara T, Kojima K, Ajioka Y. Carcinogenesis of gastric endocrine cell carcinoma: analysis of histopathology and p53 gene alteration. Gastric Cancer. 2003;6:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Eren F, Celikel C, Güllüoğlu B. Neuroendocrine differentiation in gastric adenocarcinomas; correlation with tumor stage and expression of VEGF and p53. Pathol Oncol Res. 2004;10:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
P- Reviewers Rodriguez DC, Tarnawski AS, Zoller M S- Editor Gou SX L- Editor Ma JY E- Editor Zhang DN