Published online Aug 28, 2013. doi: 10.3748/wjg.v19.i32.5278
Revised: June 11, 2013
Accepted: July 4, 2013
Published online: August 28, 2013
Processing time: 205 Days and 16.3 Hours
AIM: To evaluate the effect of long-term treatment with leukocyte natural α-interferon (ln-α-IFN) plus ribavirin (RBV).
METHODS: Forty-six patients with hepatitis C virus (HCV) recurrence received 3 MU three times a week of ln-α-IFN plus RBV for 1 mo; then, patients with good tolerability (n = 30) were switched to daily IFN administration, while the remaining were treated with the same schedule. Patients have been treated for 12 mo after viral clearance while non-responders (NR) entered in the long-term treatment group. Liver biopsies were planned at baseline, 1 year after sustained virological response (SVR) and at 36 mo after start of therapy in NR. MedCalc software package was used for statistical analysis.
RESULTS: About 16.7% of genotype 1-4 and 70% of genotype 2-3 patients achieved SVR. Nine patients withdrew therapy because of non-tolerance or non-compliance. A significant improvement in serum biochemistry and histological activity was observed in all SVR patients and long-term treated; 100% of patients with SVR achieved a histological response (fibrosis stabilization or improvement) with a significant reduction in mean staging value (from 2.1 to 1.0; P = 0.0031); histological response was observed in 84% of long-term treated patients compared to 57% of drop-out. Six patients died during the entire study period (follow-up 40.6 ± 7.7 mo); of them, 5 presented with severe HCV recurrence on enrollment. Diabetes (OR = 0.38, 95%CI: 0.08-0.59, P = 0.01), leukopenia (OR = 0.54, 95%CI: 0.03-0.57, P = 0.03) and severe HCV recurrence (OR = 0.51, 95%CI: 0.25-0.69, P = 0.0003) were variables associated to survival. Long-term treatment was well tolerated; no patients developed rejection or autoimmune disease.
CONCLUSION: Long-term treatment improves histology in SVR patients and slows disease progression also in NR, leading to a reduction in liver decompensation, graft failure and liver-related death.
Core tip: Recurrent hepatitis C virus hepatitis is associated with a significant increase in morbidity and mortality of transplanted patients; biochemical and necro-inflammatory improves in transplanted patients who achieved a virological response after a course of antiviral treatment. Although the relative small sample size of our study, we demonstrated the efficacy of long-term antiviral treatment on disease progression despite the virological response, without significant side effects.
- Citation: Tamè M, Buonfiglioli F, Del Gaudio M, Lisotti A, Cecinato P, Colecchia A, Azzaroli F, D’Errico A, Arena R, Calvanese C, Quarneti C, Ballardini G, Pinna AD, Mazzella G. Long-term leukocyte natural α-interferon and ribavirin treatment in hepatitis C virus recurrence after liver transplantation. World J Gastroenterol 2013; 19(32): 5278-5285
- URL: https://www.wjgnet.com/1007-9327/full/v19/i32/5278.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i32.5278
Hepatitis C virus (HCV) -related end-stage liver disease is the main indication for liver transplantation (LT) in Western countries[1]. However, graft re-infection is almost universal, leading to accelerated, severe liver disease with a 30% rate of graft cirrhosis after 5 years[2,3]. Antiviral treatment is indicated for all patients with evidence of recurrent HCV hepatitis[4]; patients with signs of severe HCV recurrence, such as fibrosing cholestatic hepatitis (FCH), must be treated because of the aggressive disease course. The combination of interferon (IFN)-α (both standard and pegylated) plus ribavirin (RBV) is the treatment of choice; however, in the transplant setting, antiviral therapy is less effective. Indeed, IFN plus RBV combination therapy leads to a sustained virological response (SVR) rate of 17%-30%[5,6]. PEG-IFN plus RBV treatment has an SVR rate of approximately 30%[7-9], while in immunocompetent patients, the SVR rate ranges from 40%-82% according to the viral genotype[10]. The decreased efficacy of antiviral treatment in post-transplant patients may be explained by the low tolerability and the high rate of dose reduction and therapy discontinuation due to adverse events[5]. As previously reported, PEG-IFN-based treatment appears to be associated with more hematological and autoimmune adverse events than natural IFN-based therapy[11-13].
Previous studies[14,15] have reported that daily IFN administration leads to good virological and histological outcomes with an acceptable tolerability profile; we hypothesized that daily IFN administration could induce an higher, stable serum IFN concentration, similar to PEG-IFN therapy.
To the best of our knowledge, patients with a SVR to antiviral treatment have improved biochemical and necro-inflammatory activity, while the effect of antiviral treatment on disease progression in non-responders is still controversial[16]. Kornberg et al[17] first described the effect of long-term IFN and ribavirin treatment in transplant patients; the authors reported that antiviral maintenance treatment could prevent disease progression, leading to improved long-term survival. Walter et al[18] in their retrospective analysis, confirmed the previous results and reported that even in non-responders, long-term antiviral treatment significantly slowed the progression of fibrosis.
Our study aimed to evaluate the virological and histological effects of long-term leukocyte natural α IFN (ln-α-IFN) plus RBV treatment in patients with recurrent HCV hepatitis.
From January 2003 to January 2008, 46 patients with recurrent HCV after liver transplantation were prospectively enrolled in our study. The diagnosis of recurrent hepatitis C was made using a combination of biochemical [increase in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) of at least 2x the ULN], virological (positive serum HCV-RNA) and histological findings. Patients with evidence of decompensated liver disease, histological evidence of rejection or drug-related injury, HBsAg positivity, HIV positivity, moderate to severe anemia (Hb < 10 g/dL), leukopenia (WBC < 1500/μL), thrombocytopenia (platelet count < 50000), impaired renal function (creatinine clearance < 50 mL/min), significant history of cardiovascular and psychiatric diseases or ongoing alcohol abuse were excluded.
After the diagnosis of recurrent hepatitis C, all of the patients received a standard dose of leuckocyte natural α-IFN (Alfaferone, Alfawasserman, Bologna, Italy), 3 MU three times a week (tiw) and ribavirin. After one month of treatment, patients with good tolerance received an increased ln-α-IFN dose of 3 MU daily (Group A), while patients with poor tolerance to the antiviral treatment were maintained on tiw dosing (Group B). Tolerance to antiviral treatment was evaluated based on hematological side effects and patient compliance.
Patients who achieved undetectable HCV-RNA levels continued treatment for 12 mo after viral clearance. Non-responders and relapsers entered the long-term treatment group and were treated with ln-α-IFN plus RBV. The S. Orsola-Malpighi internal review board performed a case-by case evaluation for the use of off-label, daily IFN treatment and long-term antiviral therapy. The patients gave informed consent.
Standard immunosuppressive treatment was prescribed to all of the patients at the S. Orsola-Malpighi Hospital; 7 patients received a cyclosporine-based regimen (CyA), while 39 received a tacrolimus-based one (FK).
Patients who presented with anemia or neutropenia received the scheduled IFN and RBV doses; then, erythropoietin was prescribed when the hemoglobin level fell below 10 g/dL, while granulocyte-colony stimulating factor was administered when the neutrophil count was < 700 mmc. When the anemia or neutropenia did not improve with growth factors, the IFN or RBV dose was reduced.
Quantitative and qualitative HCV-RNA (Versant HCV-RNA 3.0 bDNA, and Versant TMA; Bayer Diagnostics) were measured before starting treatment, after 1 mo and every 3 mo for the first year; then, serum HCV-RNA was checked every 6 mo. Routine blood tests (blood cell counts and liver and renal function tests) were performed at baseline and weekly for the first 4 wk and then monthly.
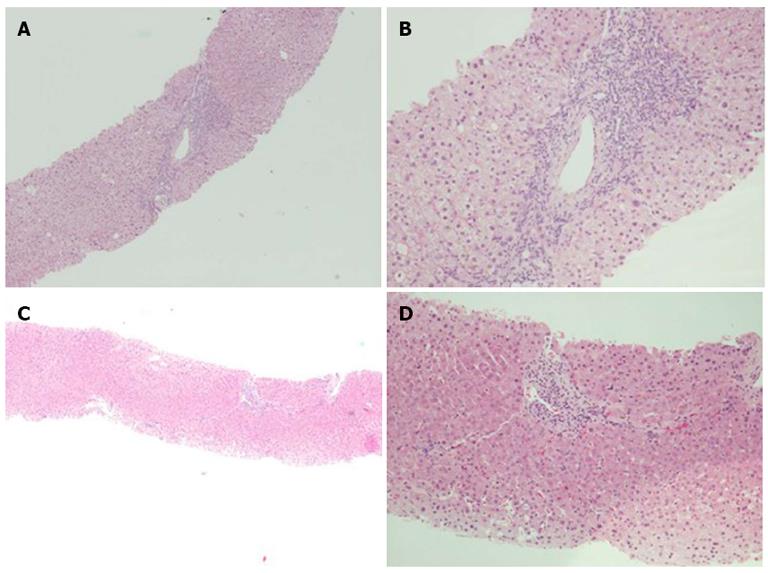
A liver biopsy was performed for all of the patients before enrollment. For patients who achieved a virological response, a liver biopsy was repeated 1 year after the end of treatment; the non-responders and relapsers had a repeat liver biopsy after 30 mo of treatment. The histological staging and grading of chronic, recurrent HCV were evaluated according to the Knodell score[19]. The diagnosis and grading of liver allograft rejection were made according to the Banff international consensus[20].
Five-micron-thick sections of liver tissue were obtained and stored at -80 °C. HCV immunohistochemistry (IHC) was performed as previously described[21-23]. Reaction positivity was graded according to the percentage of positive cells divided by the total number of hepatocytes (at least 200 cells/high magnification field)[21-23].
A rapid virological response was defined as HCV-RNA decrease of at least 2 log UI/mL or an undetectable level after 1 mo of treatment. A SVR was defined as a persistently undetectable serum HCV-RNA 6 mo after the end of treatment. The presence of fibrosing cholestatic hepatitis (FCH) or F4 fibrosis on enrollment was considered to be severe, recurrent HCV. A histological response was defined as an improvement or stabilization of liver fibrosis.
The data are expressed as the mean ± SE. Group comparisons were calculated using the χ2 test, the Mann-Whitney test, the Wilcoxon test, t tests (both independent sample t-test and paired t-test), and an ANOVA when appropriate. Clinical events (SVR, and death) were analyzed using Kaplan Meier curves. Logistic regression was used to detect variables that were independently related to the clinical events. Statistical analysis was performed using the MedCalc package v.11.5 for Windows.
Forty-six patients (30 males, 57.9 ± 1.28 years old) with post-transplant HCV recurrence were prospectively enrolled; the patients’ baseline characteristics are described in Table 1. Eleven patients presented with severe liver disease; 3 patients had FHC, and 8 patients had F4 fibrosis. Thirty-five patients presented with chronic HCV hepatitis with F1-3 fibrosis. Thirty-six patients had HCV genotype 1 or 4, while 10 patients had HCV genotype 2 or 3.
| Characteristic | Patients (n = 46) |
| Sex (M/F) | 30/16 |
| Age (yr) | 57.9 ± 1.28 |
| Time from OLT (mo) | 26.3 ± 5.1 |
| ALT (IU/L) | 152.3 ± 17.8 |
| AST (IU/L) | 105.9 ± 11.5 |
| Gamma-GT (IU/L) | 188.7 ± 39.3 |
| Alkaline phosphatases (IU/L) | 364.7 ± 39.9 |
| Bilirubin (mg/L) | 1.7 ± 0.37 |
| Viral load (log10) | 6.25 ± 0.09 |
| Genotypes 1/4 vs 2/3 | 36/10 |
| F1/F2/F3/F4 | 13/16/9/8 |
| Fibrosing cholestatic hepatitis | 3 |
| Cyclosporine A vs tacrolimus | 7/39 |
The delay between LT and the initiation of treatment was 26.3 ± 5.1 mo. All of the patients (n = 46) received tiw IFN plus RBV treatment for the first month; then, 30 patients received 3 MU IFN daily plus RBV (Group A), while 16 continued tiw IFN + RBV treatment (Group B). The mean ribavirin dose during the treatment period was 8.4 ± 0.7 mg/kg per day; there was no difference in the RBV dose between Groups A and B.
Among the entire population, seventeen patients (37%) achieved undetectable HCV-RNA levels during therapy and continued IFN+RBV treatment for 12 mo after viral clearance (mean 20.7 ± 2.5 mo); 4 of them relapsed after discontinuing treatment and were included in the long-term treatment group. Thirteen patients achieved an SVR: 8 of 30 patients (26.7%) in Group A and 5 of 16 (31.2%) in Group B. No difference between the groups was observed (P > 0.05).
The SVR rate was significantly higher for those with HCV genotype 2 or 3 than genotype 1 or 4 (70.0% vs 16.7%, respectively P = 0.0007); the overall SVR rate was 28.3%. Nine patients were rapid virological responders; among them, 7 had HCV genotype 2 or 3, and 2 had genotype 1 or 4. The variables from the univariate analysis associated with an SVR are shown in Table 2; in the multivariate analysis, a rapid virological response (OR = 99.6, 95%CI: 3.1-3190.0, P = 0.0093), a cyclosporine-based immunosuppressive regimen (OR = 685.4, 95%CI: 1.5-314392.9, P = 0.036) and the presence of severe, recurrent HCV (OR = 0.91 95%CI: 0.82-0.99, P = 0.04) were independently associated with a SVR. Eight patients withdrew therapy after 15.2 ± 2.0 mo, one because of moderate-severe anemia and seven because of non-compliance to therapy. Finally, 25 HCV-RNA-positive patients (21 non-responders and 4 relapsers) entered the long-term treatment group and were treated for a mean of 32.4 ± 2.8 mo.
| Variable | r | 95%CI | P-value |
| BMI > 25 kg/m2 | 0.30 | 0.01455-0.5458 | 0.0400 |
| Genotypes 2-3 vs 1-4 | 0.37 | 0.09103-0.5974 | 0.0100 |
| RVR | 0.54 | 0.2992-0.7194 | 0.0001 |
| HCV-RNA clearance during treatment | 0.82 | 0.6948-0.8967 | 0.0001 |
| CyA vs FK immunosuppression | 0.41 | 0.09597-0.6519 | 0.0120 |
A significant improvement in ALT was observed in the 13 patients who achieved an SVR (186.1 ± 40.4 IU/L before enrollment vs 21.4 ± 2.2 IU/L after treatment, P = 0.0028) and in the 25 long-term treatment patients (154.0 ± 26.6-37.2 ± 4.7 U/L, P = 0.0003). Also, those patients who discontinued therapy (n = 8) showed a biochemical improvement (ALT 169.5 ± 42.7-58.1 ± 3.6 U/L, P = 0.0389). At the end of follow-up, the patients who achieved an SVR had a significantly lower ALT than the other patients (P < 0.05). We also observed that the ALT levels were lower in long-term treatment patients compared to the patients who had to stop treatment (P < 0.05).
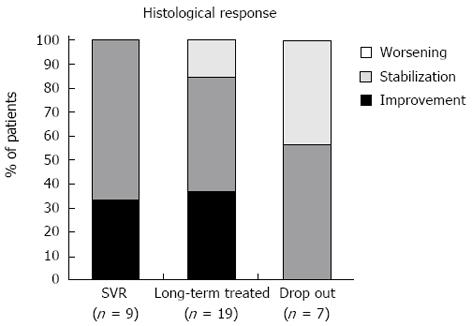
Among the entire population, 35 patients (9 sustained virological responders, 19 long-term treatment patients and 7 who stopped treatment) underwent a second liver biopsy after 30.3 ± 2.7 mo; seven patients refused the paired biopsy, while four died before the scheduled follow-up. The mean grade and stage are shown in Table 3. The post-treatment grade was significantly lower for the patients who achieved a SVR and received long-term treatment (P = 0.0039 and 0.0001, respectively), while the grade was unchanged for the patients who discontinued therapy.
| Grading | Staging | |||||
| Before | After | P-value | Before | After | P-value | |
| Sustained virological response (n = 9) | 7.2 ± 0.8 | 2.6 ± 0.6 | 0.0039 | 2.1 ± 0.3 | 1.0 ± 0.1 | 0.0031 |
| Long-term treated (n = 19) | 7.9 ± 0.7 | 4.7 ± 0.6 | 0.0001 | 2.7 ± 0.3 | 2.5 ± 0.3 | 0.0001 |
| Drop out (n = 7) | 7.4 ± 1.1 | 6.0 ± 0.8 | NS | 2.6 ± 0.6 | 3.0 ± 0.6 | NS |
Liver fibrosis improved (at least 1 stage) in ten of the 35 (28.6%) patients, remained stable in 19 patients (54.3%) and worsened in six patients (17.1%). The histological response in the three groups (responders, long-term treatment and discontinued treatment) is shown in Figures 1 and 2. Liver fibrosis remained stable or improved (histological response) in all of the patients who achieved a SVR (9 of 9, 100%); in this group, the mean post-treatment fibrosis appeared to be significantly lower (P = 0.0031). Interestingly, in the non-sustained virological responders, the histological response was higher in long-term treatment patients (16 of 19) than in the patients who stopped treatment (4 of 7) (84% vs 57%, P > 0.05). In the long-term treatment patients, the mean post-treatment fibrosis values were unchanged (2.7 ± 0.3 vs 2.5 ± 0.3).
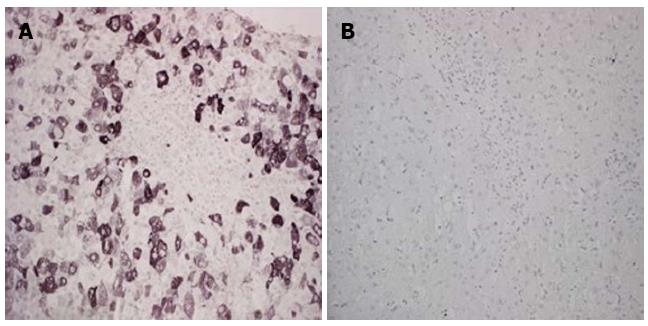
HCV IHC (Figure 3) was performed for all of the liver biopsy (46/46) and paired liver biopsy samples (35/35). The median number of immunoreactive hepatocytes before treatment was 50% (95%CI: 38.9-60.0), and there was no significant difference among the responders, long-term treatment patients and patients who stopped treatment (median 60.0%, from 35.3% to 70.0%; median 45.0%, from 17.6% to 68.3%; median 40.0%, from 4.4% to 80.0%, respectively; P > 0.05).
After treatment, all of the patients who achieved a SVR (9 of 9) had no (0%) immunoreactive hepatocytes in the liver samples (P = 0.0002). Interestingly, the non-responders who received long-term treatment had a significant reduction (P = 0.001) in immunoreactive hepatocytes (before treatment: median 45%, from 17.6% to 68.3%; after treatment: median 0.0%, from 0.0% to 14.1%). No significant difference was observed before and after treatment in the patients who stopped treatment (before: median 40.0%, from 4.4% to 80.0%; after: median 20.0%, from 10.0% to 33.1%; P > 0.05).
Treatment was generally well tolerated; 29 (63%) patients, during combination therapy, required growth factors with 28 (61%) patients receiving erythropoietin for anemia and thirteen (28%) receiving G-CSF for neutropenia. Despite the use of grow factors, one patient withdrew from treatment due to moderate-severe anemia (no need for blood transfusion or hospitalization). No patient developed autoimmune disease or graft rejection.
Six patients died during the study period (follow-up 40.6 ± 7.7 mo). Two patients with a severe HCV recurrence (FCH), one who was a non-responder and another who stopped therapy, died because of a severe infection (encephalitis and cholangitis). Two patients (basal fibrosis F4), who were non-responders (1 long-term treatment and 1 who stopped treatment), died from liver decompensation. One patient with FCH on enrollment received long-term treatment for 40 mo and died 44 mo after enrollment due to liver decompensation. One patient with a mild HCV recurrence died due to a myocardial infarction.
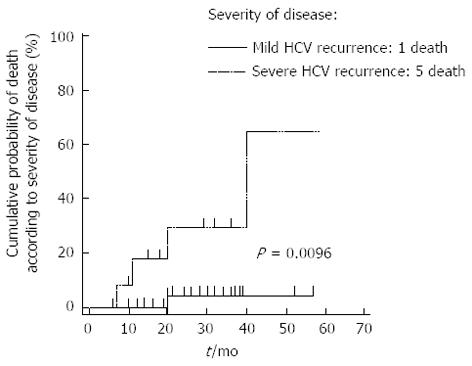
The presence of diabetes (OR = 0.38, 95%CI: 0.08-0.59; P = 0.01), leukopenia (OR = 0.54, 95%CI: 0.03-0.57; P = 0.03) or severe HCV recurrence (OR = 0.51, 95%CI: 0.25-0.69; P = 0.0003) were associated with survival; in a multivariate analysis, the presence of a severe HCV recurrence (OR = 29.6, 95%CI: 2.4-371.2; P = 0.0086) was the only variable to be independently associated with death. Kaplan-Maier analysis (curve shown in Figure 4) demonstrated an increased risk of death (P = 0.0096) for patients with a severe, recurrent HCV compared to patients with a mild recurrence. No difference between the survival of patients undergoing long-term treatment and those who discontinued treatment was observed.
Recurrent HCV hepatitis is associated with a significant increase in the morbidity and mortality of transplanted patients due to the early development of graft cirrhosis. In the post-transplant setting, the goals of antiviral treatment are to induce viral eradication and to slow disease progression.
Previous studies[5,24-27] have reported biochemical and necro-inflammatory improvement in transplanted patients who achieved a virological response after a course of antiviral treatment; however, the response rate is still unsatisfactory (17%-30% with IFN plus RBV; 18%-45% with PEG-IFN plus RBV)[5-9]. Among the non-responders, a significant number of patients will develop of graft cirrhosis and liver-related death in a few years[2,3]. To slow disease progression, the efficacy of antiviral maintenance therapy was evaluated in two studies[17,18], which showed preliminary evidence of benefit. Our study aimed to evaluate the efficacy of long-term treatment with ln-α-IFN plus ribavirin.
We reported an overall SVR rate of 28.3%; our response rate was similar to those observed with IFN-based and PEG-IFN-based regimens[5-9]. We did not observe any different virological outcomes between patients receiving daily and tiw IFN; this result could be due to variation in the HCV genotype distribution. Six patients of 16 (37.5%) in the Group B had a favorable genotype (2 or 3).
The role of cyclosporine in patients with HCV recurrence is still controversial. Our previous experience has demonstrated that the type of immunosuppression during antiviral treatment may predict the SVR[28], but a meta-analysis failed to demonstrate a significant difference in clinical outcome (graft survival and mortality)[29]. Our results showed that a cyclosporine-based immunosuppressive regimen correlated with an increased SVR rate, although there was a small number of CyA-treated patients.
The potential efficacy of pegylated IFN-based antiviral treatments in the transplant setting is limited by poor tolerability and a high rate of adverse events (hematological, autoimmune and rejection) leading to dose reduction and/or therapy discontinuation. Moreover, in our center, we previously experienced several (9 of 44 patients) de novo cases of autoimmune hepatitis during PEG-IFN plus ribavirin treatment[12]; therefore, in this study, to reduce adverse events and increase patient tolerability, we used a natural IFN-based regimen. As expected, we observed a good safety and tolerability profile; no patient developed autoimmune disease or graft rejection, while only one (2.2%) stopped treatment due to anemia.
Histological analysis from paired liver biopsy samples showed a reduction in necro-inflammatory activity in the patients who cleared HCV-RNA and those who received a long-term course of therapy. As in the non-transplant setting, achievement of complete viral eradication led to an improvement in liver inflammation, biochemically and histologically. Also, we observed a significant decrease in activity scores (from 7.9 ± 0.7 to 4.7 ± 0.6; P = 0.0001) in the non-responders who received long-term treatment with IFN plus RBV.
To evaluate the anti-viral and anti-inflammatory effects of IFN plus RBV treatment, we tested, on paired liver tissue samples, hepatocyte expression of viral proteins using IHC analysis as previously described by Ballardini et al[21,22]. As expected, our results showed that patients who cleared HCV did not have HCV-positive hepatocytes in their liver biopsies, while the non-responders who had interrupted antiviral treatment did not have reduced HCV protein expression. Interestingly, the non-responders who received a long-term course of therapy had a significantly reduced percentage of HCV-positive hepatocytes (median: 45%-0%), leading to a significant reduction in liver inflammation. To exclude sampling error, liver HCV-RNA was quantified in those cases; in all of the liver biopsies, HCV-RNA was detected. We hypothesized that the effect of IFN treatment, even in the non-responders, could reduce liver tissue inflammation by reducing the degree of hepatitis C viral antigen staining[30].
The role of antiviral treatment on disease progression is still debated. Patients who achieve an SVR have been shown to have delayed fibrosis progression[24], while some authors have also observed fibrosis regression[16]. A pivotal role for antiviral therapy was demonstrated by a previous study that reported treatment was the only variable to be independently associated with histological improvement or stabilization among patients with HCV recurrence[16].
In our experience, nine patients who cleared HCV had a significantly reduced staging score on liver biopsy; moreover, a significant percentage (84%) of long-term treatment patients had a histological response despite the lack of viral clearance. Although there was a small number of patients, these results suggest the efficacy of long-term antiviral treatment on disease progression independent of a virological response.
The presence of severe HCV recurrence (histological cirrhosis, cholestatic hepatitis or FCH) is associated with a worse clinical outcome; as in the non-transplant setting[31,32], patients with advanced disease have a reduced SVR rate, due to increased averse events and therapy discontinuation. Moreover, we found that severe HCV recurrence is the only variable to be independently related to the risk of death. These findings suggest that recurrent HCV hepatitis should be treated at the onset of biochemical and histological signs to improve virological and clinical outcomes.
In conclusion, long-term treatment with ln-α-IFN plus ribavirin was able to improve histological staging in SVR patients, slow disease progression in non-responders, and demonstrate a good safety and tolerability profile. These findings suggest the importance of long-term treatment for HCV recurrence; this treatment seems to be able to reduce liver decompensation, graft failure and liver-related death.
We thank Bologna Liver Transplant Group: Pietro Andreone, Giampaolo Bianchi, Sonia Berardi, Maurizio Biselli, Stefano Brillanti, Matteo Cescon, Alessandro Cucchetti, Giorgio Ercolani, Gian Luca Grazi, Marco Lenzi, Simona Leoni, Francesca Lodato, Maria Crisitina Morelli, Fabio Piscaglia, Matteo Ravaioli, Claudia Sama, Gabriella Verucchi, Marco Vivarelli.
Hepatitis C virus (HCV) graft re-infection after liver transplantation is almost universal, leading to accelerated, severe liver disease; moreover, antiviral therapy in this setting is less effective. Only patients who achieve a virological response have an improvement in biochemical and necro-inflammatory activity, while the effect of antiviral treatment on disease progression in non-responders is still controversial.
The use of long-term maintenance therapy in transplanted patients who do not achieve a virological response is still debated. Some authors have reported that antiviral maintenance treatment could prevent disease progression, leading to an improvement in long-term survival.
The results support the concept that long-term antiviral treatment leads to a better histological outcome even in patients who do not achieve viral clearance; therefore, long-term antiviral treatment improves disease progression, leading to a better clinical outcome.
New direct antiviral agents are changing the approach to HCV treatment, including in transplanted patients; however, the management of patients who do not achieve a viral response will be a future clinical challenge. The results supported the safety and tolerability of long-term treatment with interferon and ribavirin in patients who did not respond to therapy. We demonstrated that, even in non-responders, long-term treatment improves clinical and histological outcomes.
As a retrospective clinical study on patients with HCV recurrence after liver transplantation, the results proved the efficacy of long-term antiviral treatment with leukocyte natural α-interferon and ribavirin on disease progression and showed a good safety and tolerability profile.
| 1. | Berenguer M, López-Labrador FX, Wright TL. Hepatitis C and liver transplantation. J Hepatol. 2001;35:666-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, Maertens G, Williams R. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 737] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 3. | Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, Córdoba J, Herola A, Ascher N, Mir J. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000;32:673-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 597] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 4. | Roche B, Samuel D. Hepatitis C virus treatment pre- and post-liver transplantation. Liver Int. 2012;32 Suppl 1:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 5. | Samuel D, Bizollon T, Feray C, Roche B, Ahmed SN, Lemonnier C, Cohard M, Reynes M, Chevallier M, Ducerf C. Interferon-α 2b plus ribavirin in patients with chronic hepatitis C after liver transplantation: a randomized study. Gastroenterology. 2003;124:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Wang CS, Ko HH, Yoshida EM, Marra CA, Richardson K. Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: a review and quantitative analysis. Am J Transplant. 2006;6:1586-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008;49:274-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 8. | Xirouchakis E, Triantos C, Manousou P, Sigalas A, Calvaruso V, Corbani A, Leandro G, Patch D, Burroughs A. Pegylated-interferon and ribavirin in liver transplant candidates and recipients with HCV cirrhosis: systematic review and meta-analysis of prospective controlled studies. J Viral Hepat. 2008;15:699-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Lodato F, Berardi S, Gramenzi A, Mazzella G, Lenzi M, Morelli MC, Tame MR, Piscaglia F, Andreone P, Ballardini G. Clinical trial: peg-interferon alfa-2b and ribavirin for the treatment of genotype-1 hepatitis C recurrence after liver transplantation. Aliment Pharmacol Ther. 2008;28:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 922] [Article Influence: 61.5] [Reference Citation Analysis (1)] |
| 11. | Toniutto P, Fabris C, Fumo E, Apollonio L, Caldato M, Avellini C, Minisini R, Pirisi M. Pegylated versus standard interferon-α in antiviral regimens for post-transplant recurrent hepatitis C: Comparison of tolerability and efficacy. J Gastroenterol Hepatol. 2005;20:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Berardi S, Lodato F, Gramenzi A, D’Errico A, Lenzi M, Bontadini A, Morelli MC, Tamè MR, Piscaglia F, Biselli M. High incidence of allograft dysfunction in liver transplanted patients treated with pegylated-interferon α-2b and ribavirin for hepatitis C recurrence: possible de novo autoimmune hepatitis? Gut. 2007;56:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Guido M, Burra P. De novo autoimmune hepatitis after liver transplantation. Semin Liver Dis. 2011;31:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Cotler SJ, Ganger DR, Kaur S, Rosenblate H, Jakate S, Sullivan DG, Ng KW, Gretch DR, Jensen DM. Daily interferon therapy for hepatitis C virus infection in liver transplant recipients. Transplantation. 2001;71:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Beckebaum S, Cicinnati VR, Karliova M, Dirsch O, Erim Y, Frilling A, Malago M, Broelsch CE, Treichel U, Gerken G. Daily interferon α-2B and ribavirin combination therapy for liver transplant patients with chronic hepatitis C infection. Transplant Proc. 2003;35:2080-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Carrión JA, Navasa M, García-Retortillo M, García-Pagan JC, Crespo G, Bruguera M, Bosch J, Forns X. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132:1746-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 265] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 17. | Kornberg A, Küpper B, Tannapfel A, Bärthel E, Thrum K, Settmacher U. Antiviral maintenance treatment with interferon and ribavirin for recurrent hepatitis C after liver transplantation: pilot study. J Gastroenterol Hepatol. 2007;22:2135-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Walter T, Scoazec JY, Guillaud O, Hervieu V, Chevallier P, Boillot O, Dumortier J. Long-term antiviral therapy for recurrent hepatitis C after liver transplantation in nonresponders: biochemical, virological, and histological impact. Liver Transpl. 2009;15:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2558] [Cited by in RCA: 2519] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 20. | Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1014] [Article Influence: 35.0] [Reference Citation Analysis (1)] |
| 21. | Ballardini G, Groff P, Giostra F, Francesconi R, Miniero R, Ghetti S, Zauli D, Bianchi FB. Hepatocellular codistribution of c100, c33, c22, and NS5 hepatitis C virus antigens detected by using immunopurified polyclonal spontaneous human antibodies. Hepatology. 1995;21:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Ballardini G, Groff P, Pontisso P, Giostra F, Francesconi R, Lenzi M, Zauli D, Alberti A, Bianchi FB. Hepatitis C virus (HCV) genotype, tissue HCV antigens, hepatocellular expression of HLA-A,B,C, and intercellular adhesion-1 molecules. Clues to pathogenesis of hepatocellular damage and response to interferon treatment in patients with chronic hepatitis C. J Clin Invest. 1995;95:2067-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Grassi A, Quarneti C, Ravaioli M, Bianchini F, Susca M, D’Errico A, Piscaglia F, Tamè MR, Andreone P, Grazi G. Detection of HCV antigens in liver graft: relevance to the management of recurrent post-liver transplant hepatitis C. Liver Transpl. 2006;12:1673-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Bizollon T, Ahmed SN, Radenne S, Chevallier M, Chevallier P, Parvaz P, Guichard S, Ducerf C, Baulieux J, Zoulim F. Long term histological improvement and clearance of intrahepatic hepatitis C virus RNA following sustained response to interferon-ribavirin combination therapy in liver transplanted patients with hepatitis C virus recurrence. Gut. 2003;52:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Roche B, Sebagh M, Canfora ML, Antonini T, Roque-Afonso AM, Delvart V, Saliba F, Duclos-Vallee JC, Castaing D, Samuel D. Hepatitis C virus therapy in liver transplant recipients: response predictors, effect on fibrosis progression, and importance of the initial stage of fibrosis. Liver Transpl. 2008;14:1766-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (2)] |
| 26. | Bizollon T, Pradat P, Mabrut JY, Chevallier M, Adham M, Radenne S, Souquet JC, Ducerf C, Baulieux J, Zoulim F. Benefit of sustained virological response to combination therapy on graft survival of liver transplanted patients with recurrent chronic hepatitis C. Am J Transplant. 2005;5:1909-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Abdelmalek MF, Firpi RJ, Soldevila-Pico C, Reed AI, Hemming AW, Liu C, Crawford JM, Davis GL, Nelson DR. Sustained viral response to interferon and ribavirin in liver transplant recipients with recurrent hepatitis C. Liver Transpl. 2004;10:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Cescon M, Grazi GL, Cucchetti A, Vetrone G, Ravaioli M, Ercolani G, Morelli MC, Piscaglia F, Tamè M, Pinna AD. Predictors of sustained virological response after antiviral treatment for hepatitis C recurrence following liver transplantation. Liver Transpl. 2009;15:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 29. | Berenguer M, Royuela A, Zamora J. Immunosuppression with calcineurin inhibitors with respect to the outcome of HCV recurrence after liver transplantation: results of a meta-analysis. Liver Transpl. 2007;13:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Di Bisceglie AM, Hoofnagle JH, Krawczynski K. Changes in hepatitis C virus antigen in liver with antiviral therapy. Gastroenterology. 1993;105:858-862. [PubMed] |
| 31. | Bruno S, Shiffman ML, Roberts SK, Gane EJ, Messinger D, Hadziyannis SJ, Marcellin P. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology. 2010;51:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 32. | Cheng WS, Roberts SK, McCaughan G, Sievert W, Weltman M, Crawford D, Rawlinson W, Marks PS, Thommes J, Rizkalla B. Low virological response and high relapse rates in hepatitis C genotype 1 patients with advanced fibrosis despite adequate therapeutic dosing. J Hepatol. 2010;53:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
P- Reviewers Lu XM, Yu B S- Editor Zhai HH L- Editor A E- Editor Zhang DN