Published online Aug 21, 2013. doi: 10.3748/wjg.v19.i31.5051
Revised: July 7, 2013
Accepted: July 19, 2013
Published online: August 21, 2013
Processing time: 82 Days and 17.7 Hours
Angiogenesis affects both wound healing and malignant cell growth through nutrients and oxygen. Vascular endothelial growth factor (VEGF) is the most important element involved in this complex process. Inhibition of VEGF influences angiogenesis and may restrict tumor growth and metastatic ability. Modern anti-angiogenic therapy is based on this theory. Bevacizumab is a recombinant humanized monoclonal antibody (immunoglobulin G1) which binds with VEGF-A forming a large molecule. It can not be bound with VEGF tyrosine kinase receptors preventing VEGF-A incorporation; thus its activity is inhibited inducing blockage of VEGF-mediated angiogenesis. Bevacizumab, in combination with chemotherapy or other novel targeted therapeutic agents, is currently used more frequently in clinical practice, mainly for managing advanced colorectal cancer. It is also used for managing other malignancies, such as breast cancer, pancreatic cancer, prostate cancer, non small-cell lung cancer, metastatic renal carcinoma and ovarian tumors. Although it is generally considered a safe treatment, there are reports of some rare side effects which should be taken into account. Recent experiments in rats and mice show promising results with a wider therapeutic range.
Core tip: Modern targeted therapy with anti-angiogenic agents is based on inhibition of angiogenesis, as the formation of new vessels is crucial for the growth and metastasis of malignant cells. Recent studies on the biological agent, bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor activity, have shown improved outcome in advanced colorectal cancer. The combination of irinotecan, capecitabine and bevacizumab is currently the most frequently used regime in the treatment of metastatic colorectal cancer with improved response rates. However, the rare side-effects of bevacizumab should always be considered.
- Citation: Pavlidis ET, Pavlidis TE. Role of bevacizumab in colorectal cancer growth and its adverse effects: A review. World J Gastroenterol 2013; 19(31): 5051-5060
- URL: https://www.wjgnet.com/1007-9327/full/v19/i31/5051.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i31.5051
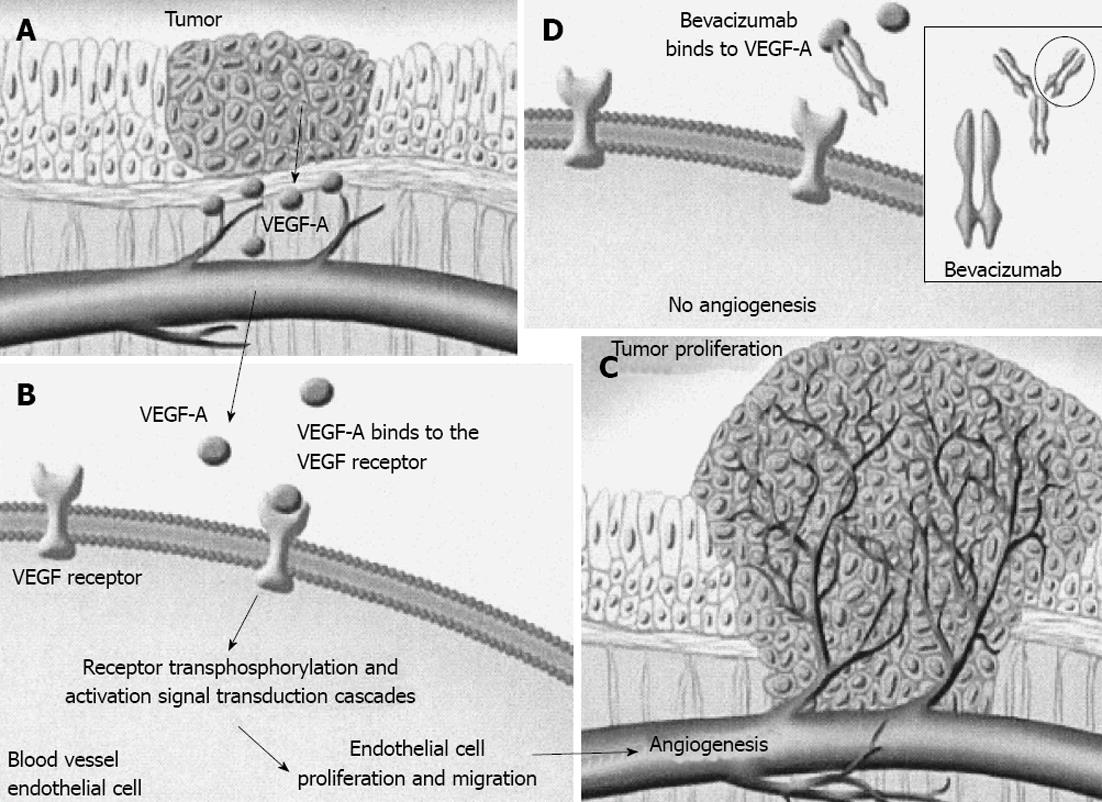
Angiogenesis is a complex process responsible for the formation of new vessels originating from pre-existing vessels. It is necessary for the proliferation and growth of normal cells and tissues during the fetal and neonatal period, but also for the proliferation and growth of cancer cells. Its physiological role in adult life is limited in wound healing and the reproductive cycle of females. The development of such vessel networks, or even collateral circulation, aim to supply the tissues with oxygen and nutrients, remove carbon dioxide and waste products of cell metabolism and transfer hormones. A variety of factors are involved in the regulation of angiogenesis. Vascular endothelial growth factor (VEGF) is one of the main growth factors involved in vessel formation[1,2].
The current targeted therapy of cancer with anti-angiogenic agents is based on angiogenesis inhibition and restriction of tumour spread, as neo-angiogenesis has a crucial effect on the growth and invasion of malignant cells[3-7].
The topic of this study has attracted much interest in clinical oncology and experimental research. VEGF by promoting angiogenesis favours tumor growth, while its inhibition results in tumor limitation. The novel anti-angiogenic agent, bevacizumab, is a recombinant humanized monoclonal antibody against VEGF activity. This targeted therapy is currently combined with chemotherapy and used mainly in the treatment of metastatic colorectal cancer.
VEGF and its receptor (VEGFr) play important roles in the neo-angiogenesis process in physiological growth and healing as well as in pathological states such as malignancy. VEGF levels are known to be increased, particularly in the most malignant tumors, such as colorectal cancer, and are associated with an increased ability of the malignancy to spread and with poorer prognosis. Thus, inhibition of angiogenesis results in growth restriction or even a reduction in malignant cells[8]. A variety of events and factors at the molecular level have been evaluated for application in novel anti-cancer drugs. VEGF is one of these factors. Targeting VEGF with bevacizumab, a humanized monoclonal immunoglobulin G (IgG) antibody, in combination with adjuvant chemotherapy has been proved to effectively manage advanced colorectal cancer[5,6,8].
Malignant tumors require nutrients for growth, and tumors more than 1-2 mm3 in size ensure independent blood flow for continuing growth. These new vessels develop via angiogenesis. Inadequate blood flow leads to hypoxia, the main stimulus for angiogenesis initiation. Proteins such as hypoxia inducible factor are activated resulting in over-expression of pro-angiogenic factors including VEGF and fibroblastic growth factors. The number of cancer cells is reduced in parallel with the expression of anti-angiogenic factors, such as thrombospondin I. Through the over-expression of pro-angiogenic factors, as opposed to anti-angiogenic factors, endothelial cells are activated, thus triggering the initiation of angiogenesis[8].
In spite of the similarities in the angiogenesis process between wound healing and malignancy, there are differences in the structure of new vessels.
Several angiogenic factors derived from platelets and inflammatory cells are involved in the stages of wound healing through various mechanisms. They include phosphorylation of tyrosine kinase receptors, activation and proliferation of epithelial cells, migration and creation of tubular formations and finally new vessel formation. VEGF initiates angiogenesis by abruption of cell walls and protein lysis of vessel walls, proliferation and migration of endothelial cells and formation of new vessels. This vessel network is derived from endothelial tip cells, which have phenotypic and functional differentiation from other endothelial stalk cells[3,4].
Six subtypes of VEGF have been reported, i.e., VEGF-A, VEGF-B, VEGF-C, VEGF-D, virus VEGF-E and placental VEGF (PIGF). VEGF-A increases vascular permeability, degeneration of the extracellular matrix and cell aggravation. VEGF-B and PIGF are involved mainly in the normal angiogenesis process. However, an increase in PIGF levels promotes angiogenesis in pathological conditions, such as tumors and inflammation. VEGF-C and VEGF-D have a predominant role in lymphatic angiogenesis; VEGF (PIGF) regulates placental angiogenesis[8].
Four isomers of VEGF-A have been reported in humans (VEGF121, VEGF165, VEGF184, VEGF206). The isomer VEGF165 is over-expressed in the majority of human malignancies. This over-expression enhances growth, invasiveness and metastatic ability.
VEGF is derived from malignant cells and promotes the growth of colorectal cancer[9]. However, a recent study has shown that the expression of EGFR and VEGF are not prognostic factors in the survival of patients with colorectal cancer and the expression of EGFR does not determine lymphatic metastasis; however, this issue remains controversial[10]. It is the over-expression of VEGF and not the density of microvasculature or vein invasion that plays the important role; it is also responsible for hematogenous dissemination after curative resection for gastric cancer[11].
VEGFr is a receptor of tyrosine kinase and has three forms, VEGFr-1, 2, 3. They are expressed in vessel endothelial cells as well as in cancer cells (VEGFr-1 and 2). VEGFr-1 is also found in monocytes and macrophages. VEGFr-3 is found in endothelial cells of the lymphatic system. VEGF-A correlates with receptors VEGFr-1 and 2, VEGF-B and PIGF with receptor VEGFr-1, and VEGF-C and D correlate with receptor VEGFr-3. VEGFr-2 plays an important role in the angiogenesis process in physiological as well as in pathological conditions. VEGFr-2 stimulation promotes cell growth and migration, the creation of tubular formations (endothelial cells) and the increase in vascular permeability[1,2].
The role of VEGF in other diseases such as allergic and immune-mediated diseases has been well-established[12,13]. The potential positive effect of other biological drugs (specific immunotherapy) such as tumor necrosis factor-α inhibitors on the mechanisms of action of VEGF has also been debated[14].
As mentioned above, angiogenesis plays a pivotal role in cell proliferation and tumor growth. Malignant cells secrete VEGF-A, a growth factor responsible for neo-angiogenesis. This action is accomplished by incorporation of its tyrosine kinase receptors, VEGFrs, which are located on the surface of epithelial cells. An increase in angiogenesis facilitates blood flow to malignant cells permitting their growth and spread by ensuring a supply of oxygen and nutrients. Bevacizumab, a recombinant humanized monoclonal antibody, combines with VEGF-A forming a new molecule that lacks the ability to bind with its receptors, VEGFrs, thus avoiding its incorporation and action. This restriction of VEGF-A receptors activity induces a reduction in small vessel growth, inhibits new vessel formation and restores normal tumor blood supply[15].
Bevacizumab is an IgG1 that inhibits the activity of VEGF and its isomers. This monoclonal antibody has been derived from murine antihuman VEGF and is 93% human and 7% murine[16]. The absence of VEGF influences epithelial cells resulting in destruction of neoplastic capillaries. Although it has been reported that malignant cells continue to grow despite the absence of VEGF, they exhibit reduced invasion ability resulting in reduced metastatic activity. Furthermore, their reduced intracellular pressure makes them more vulnerable to chemotherapy and radiotherapy.
The half-life time of bevacizumab ranges from 11 to 50 d (mean half-life time 20 d). As a result, even small doses of the drug (0.3 mg/kg bw) may be bound with VEGF preventing incorporation with its receptors, and thus inactivating VEGF efficiency. Bearing in mind that the acceptable dose is 5 mg/kg bw every 2 wk, it has been suggested that active levels of the drug may be detected for 12 wk[8] (Figure 1).
The current data on the management of colorectal cancer indicate that angiogenesis and its inhibition are key factors. Bevacizumab remains the most important and well-studied drug among the known anti-angiogenic agents. The use of bevacizumab (Avastin, Roche Pharma AG) has been widely accepted as first-line therapy in the management of advanced colorectal cancer in combination with other classic chemotherapy agents such as 5-fluorouracil (5-FU) or novel agents[17-22]. This combination improves the response rates to treatment, progression-free survival and overall survival, in patients with advanced disease, as opposed to chemotherapy alone[23-25]. Its licence was granted in 2004 in the United States and in 2005 in Europe[26]. Currently, the combination of the novel targeted therapy agents irinotecan, capecitabine and bevacizumab is the most widely used in metastatic colorectal cancer resulting in increased response rates[23,24,27,28].
Bevacizumab is the first agent to affect survival in patients with metastatic colorectal cancer, improving survival by 30%[16]. Furthermore, it has been established as the first- and second-line therapy for this cancer, due to its advantages compared with routine chemotherapy, which include less resistance and toxicity[23]. Its beneficial effect has been proved in phases II and III clinical trials[25].
Conclusions have been drawn from a variety of trials investigating its safety and efficacy. It has been suggested that surgery should be performed at least 6-8 wk after drug cessation to minimize complications; post-operatively, re-initiation should be after 28 d and/or complete wound healing[29].
The usual dose of bevacizumab is 5 mg/kg bw every two weeks in combination with other chemotherapeutic agents such as irinotecan and 5-fluorouracil/leucovorin (LV). It is administered by intravenous (IV) injection which must last 90 min initially and is gradually reduced to 60 min and 30 min; IV bolus injection is contraindicated[16].
Bevacizumab has been used postoperatively 6 wk after colorectal cancer resection for the management of synchronous liver metastasis at a dose of 5 mg/kg bw every 2 wk or 7.5 mg/kg bw every 3 wk[30].
The usual dose of bevacizumab is 5 mg/kg bw every 2 wk for 5 cycles and even the uncommon dose of 10 mg/kg bw has been combined with 5-FU/LV or capecitabine in advanced colorectal cancer[31-34].
Recent trials have confirmed the effectiveness of bevacizumab in combination with other chemotherapeutic agents in metastatic colorectal cancer showing its increasing application in clinical practice. A large randomized multi-center controlled trial showed that the addition of bevacizumab to capecitabine plus or minus mitomycin significantly improved progression-free survival (PFS) without inducing further major toxicity; only expected modest adverse events including proteinuria, hypertension, arterial thromboembolism and hemolytic uremic syndrome were observed. However, it did not improve response rate or overall survival (OS), and overall quality of life was similar. Furthermore, there were 11 treatment-related deaths: one in the capecitabine group (sepsis); seven in the capecitabine-bevacizumab group (hemorrhage, myocarditis, bowel perforation, sepsis); and three in the capecitabine-bevacizumab-mitomycin group (hemorrhage, pulmonary embolism, neutropenic colitis)[35]. A meta-analysis of 5 randomized controlled trials showed that the addition of bevacizumab to first-line chemotherapy significantly increased both the PFS and OS. Females and patients with primary rectal tumors seemed to benefit most[36].
Based on a pivotal study, the United States Food and Drug Administration (FDA) in February 2004 approved bevacizumab for the first-line treatment of patients with metastatic carcinoma of the colon and rectum. In this study, 833 patients were randomly allocated to irinotecan, 5-FU, and LV either alone (the IFL regimen) or with bevacizumab (5 mg/kg every 2 wk). In the group treated with bevacizumab, OS was significantly longer (median, 20.3 mo vs 15.6 mo) as were PFS and response rate[24]. Subsequently on June 20, 2006, the FDA approved bevacizumab administered in combination with 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) as a second-line treatment for metastatic carcinoma of the colon or rectum. This was based on the Eastern Cooperative Oncology Group open-label, multicenter, randomized, three-arm, active-controlled trial. In this study, 829 patients with recurrence following prior chemotherapy were randomly allocated to bevacizumab (10 mg/kg, as a 90-min iv infusion on day 1, every 2 wk) with FOLFOX4, or FOLFOX4 alone. In the group treated with bevacizumab, there was a statistically significant and clinically meaningful improvement in OS (13.0 mo vs 10.8 mo) in patients whose disease had progressed after adjuvant chemotherapy with 5-FU and irinotecan and in patients with advanced or metastatic disease who had received prior 5-FU and irinotecan. The administration of bevacizumab was beneficial in these sub groups, well tolerated and with no impact on quality of life[37].
In a recent phase II study, bevacizumab was added to capecitabine plus irinotecan (XELIRI) as first-line treatment for metastatic colorectal cancer and acceptable tolerability and improved outcome were observed[38].
An updated meta-analysis and systematic review of 10 randomized controlled trials including 1366 patients with metastatic colorectal cancer identified the additional benefits of bevacizumab to cytotoxic chemotherapy regarding OS and PFS[39].
However, there was controversy regarding the aforementioned findings in a large phase III trial of 2672 patients with stage II to III colon cancer. The addition of bevacizumab to modified FOLFOX6 (mFOLFOX6; i.e., infusional/bolus fluorouracil, leucovorin, and oxaliplatin) as adjuvant treatment for 1 year, did not significantly prolong disease free survival[40].
The development of bevacizumab-induced hypertension as a biomarker did not predict radiological response or survival in patients with poor-risk colorectal liver-only metastases unsuitable for upfront resection[41].
Overall survival, disease-free survival, and local control showed favourable trends in patients with stage II/III rectal cancer treated with neo-adjuvant bevacizumab with chemoradiotherapy followed by surgery[42]. Another study of neo-adjuvant oxaliplatin, bevacizumab, continuous infusion 5-FU, and radiation in rectal cancer was terminated early because of significant gastrointestinal toxicity[43].
Bevacizumab has been used as first-line treatment early in advanced cancer and in patients with stage III unresectable or stage IV adenocarcinoma of the colon or rectum[44,45].
A retrospective analysis of a large United States managed database estimated that the cost of treatment containing bevacizumab was lower than that containing cetuximab[46].
Several clinical trials have confirmed the effectiveness of bevacizumab in other malignancies, i.e., breast cancer, pancreatic cancer, prostate cancer, non-small cell lung cancer, metastatic renal carcinoma, and ovarian tumors.
Recently, targeted therapy with various anti-angiogenic agents including sunitinib, sorafenib, temsirolimus, everolimus and bevacizumab has been used as first-line systemic therapy with impressive success in patients with metastatic renal cell carcinoma, which otherwise has a poor prognosis[47].
The combination with another anti-angiogenic agent enhances activity and decreases toxicity[48].
Bevacizumab has been accepted in combination with taxanes for the treatment of metastatic breast cancer in unselected patients[49]. Its combination with paclitaxel showed a statistically significant difference in outcome compared to treatment with paclitaxel alone.
Results of trial E2100 led to the initial approval of bevacizumab as first-line therapy for patients with metastatic breast cancer in the United States in February 2008. However, based on results from subsequent trials, the United States FDA Oncologic Drugs Advisory Committee revoked its approval in July 2010[50-52]. The drug costs about $90000 (£58000; €68000). Bevacizumab has not been shown to be safe and effective in metastatic breast cancer, as several studies showed no influence on overall survival or benefits in overcoming the drug’s serious and potentially life-threatening side effects.
Despite the FDA decision, it was not withdrawn in Europe by the European Medicines Agency, however, the prescribing practice has been reduced[50]. A recent survey highlighted the discord between the opinion of oncologists and the FDA’s recent decision[53]; similarly there is controversy over the FDA decision[54].
Bevacizumab has also been used in primary and metastatic brain tumors, mainly in glioblastomas[55]. It has been extensively studied in patients with primary malignant gliomas and has been approved as second-line chemotherapy alone or in combination with irinotecan following first or second recurrence after radiotherapy and temozolomide[56-58]. Furthermore, the efficacy and safety of combining bevacizumab with standard-of-care therapy in patients with newly diagnosed glioblastoma multiforme is currently being studied by the AVAGLIO phase III randomized trial[59].
Bevacizumab has also been proved to be effective as mono-therapy in recurrent ovarian stromal tumors[60].
Chemotherapy plus targeted therapy with bevacizumab had better efficacy than chemotherapy alone in patients with non-small cell lung cancer, which otherwise has a poor prognosis[61]. The combination of paclitaxel/carboplatin with bevacizumab showed increased efficacy (27% vs 10% with chemotherapy alone) and raised overall survival to 12.5 mo vs 10.2 mo, respectively.
Bevacizumab is currently being used more frequently in the management of breast, ovarian and cervical cancer[62-64]. It has also been used in advanced pancreatic cancer in phase II clinical trials alone or combined with other therapeutic agents, but without improved outcome[21,65,66].
Despite the documented benefits of bevacizumab use in the treatment of colorectal cancer, there have been reports of rare side effects, i.e., thrombosis, arterial hypertension, proteinuria, perforation of the gastrointestinal tract or nasal septum, wound healing abnormalities, irreversible leuco-encephalopathy syndrome, allergic skin rash and hypersensitivity reactions[15,67,68]. Wound healing abnormalities include wound dehiscence, ecchymosis, bleeding and wound infection. Hypersensitivity reactions include flashing, pruritus, arterial hypertension, rigors, broncho-constriction, chest pain, and sweats. The risk of postoperative bleeding is statistically significant[25,29] as well as the risk of thromboembolic events, i.e., deep vein thrombosis, pulmonary embolism, transient ischemic attack, and acute mesenteric ischemia[69-71]. Due to the aforementioned side effects, continuous monitoring of patients receiving bevacizumab treatment is mandatory to achieve the best outcome[72].
The contraindications of bevacizumab use include hypersensitivity to its active components or to recombinant monoclonal antibodies, pregnancy, lactation, brain metastasis without treatment due to bleeding risk, gastrointestinal tract perforation, wound healing complications, persistent arterial hypertension, proteinuria, arterial thromboembolic episodes, hemorrhage and congestive heart failure or cardiomyopathy[16].
The reported wound healing complications include bowel perforation, external abdominal fistula, anastomotic dehiscence, intraperitoneal bleeding, gastrointestinal hemorrhage and cellulitis. In oncoplastic surgery for advanced breast cancer, failure of free flaps due to increased thrombotic risk as well as bleeding episodes increase the morbidity and mortality rate[67].
The risks of GI-tract perforation including free perforation, fistula formation and intra-abdominal abscess are rare, but these are serious complications, which may be fatal[73,74]. These risks depend on the drug dose and increases in cancer patients. The use of non-steroidal or other anti-inflammatory drugs, peptic ulcer and colon diverticular disease are also risk factors. It should be stressed that there have been isolated reports of spontaneous delayed (several months or even one year after operation) leakage from previous colon or rectal anastomosis after treatment with bevacizumab[75-78].
An interesting case reported skin flap necrosis in a female undergoing preoperative bevacizumab and paclitaxel plus 5-FU, epirubicin, and cyclophosphamide treatment for locally advanced breast cancer[78]; we should also mention the case of Fournier’s gangrene in a male during bevacizumab treatment 4 mo after chemotherapy with 5-FU/LV/oxaliplatin for advanced colorectal cancer[79].
However, in a recent study of 57 cancer patients who received bevacizumab and underwent immediate insertion of a central venous access port, there were no side-effects such as delayed wound healing, bleeding, infection or ulceration[80].
The reported long-term anastomotic complications attributed to the use of the anti-angiogenic agent refer to 18 cases[81]. They occurred more than a year or even 78 mo following bevacizumab treatment. The risk factors included low anterior recto-sigmoid resection for rectal cancer, perioperative radiotherapy and healed early anastomotic leakage.
For the aforementioned reasons, it has been recommended that a period of 6 wk should elapse following drug cessation before hepatectomy; post-operatively, a 4-wk period is required before therapy is re-initiated[82]. However, there has recently been a debate based on experimental findings[83,84] and clinical data. The safety and effectiveness of bevacizumab were proved in a large meta-analysis of randomized controlled trials, which found no statistically significant difference in wound healing[85].
Bevacizumab at an IV dose of 5 mg/kg bw has been used in combination with irinotecan in an experimental model of implanted colon cancer cells in rats[86].
Intraperitoneal administration of bevacizumab in combination with other novel targeted agents has been proven to be effective in reducing tumor size in an experimental cancer model (colon, renal) in mice[87].
Bevacizumab at an IV dose of 10 mg/kg bw per week was effective in reducing tumor size and vasculature in an experimental model of breast cancer with bone metastasis in rats using volumetric computed tomography and magnetic resonance imaging (MRI)[88]. Also, the effectiveness of intraperitoneal administration of bevacizumab at different doses has been documented in an experimental model of implanted breast cancer cells in rats using MRI[89,90].
Intraperitoneal administration of bevacizumab has been used with encouraging results in several experimental tumor models in mice, i.e., tuberous sclerosis[91], glioblastoma[92], medullary thyroid carcinoma[93], gastric cancer[94-96], malignant fibrous histiocytoma[97], ovarian cancer[98], and endometrial cancer[99].
Furthermore, it has been used in lung cancer xenografts[100], immune-mediated vascular remodeling[101], tumor angiogenesis assessment using positron emission tomography (PET) imaging in experimental models of colorectal and ovarian cancer in mice[102], an experimental model of schwannoma[103] and in a mouse model of hepatocellular carcinoma with promising results[104]. PET imaging and VEGF bio-distribution with radio-labeled bevacizumab in colorectal cancer xenografts has been performed[105].
These experimental data on the use of bevacizumab or other novel anti-angiogenic agents in cancer models using rats or mice open new horizons broadening its targeted therapeutic application with promising results.
The promotion of angiogenesis by VEGF favors tumor growth. Bevacizumab, which is a recombinant humanized monoclonal antibody against VEGF activity, inhibits angiogenesis restricting the growth of malignant cells and thus prevents tumor spread. It has recently been used as targeted therapy in combination with chemotherapy, mainly in advanced colorectal cancer with hepatic or other metastasis, and in breast cancer despite the debate surroundings its use for this disease, and occasionally in pancreatic cancer (but without proven efficiency), ovarian tumors, small-cell lung cancer, renal cancer and prostate cancer. A number of experimental studies have also attracted great interest on its use in other advanced malignancies. This novel biological agent is generally safe and well-tolerated. However, there are rare, although serious side effects and complications that should be considered.
| 1. | Kajdaniuk D, Marek B, Foltyn W, Kos-Kudła B. Vascular endothelial growth factor (VEGF) - part 1: in physiology and pathophysiology. Endokrynol Pol. 2011;62:444-455. [PubMed] |
| 2. | Kajdaniuk D, Marek B, Foltyn W, Kos-Kudła B. Vascular endothelial growth factor (VEGF) - part 2: in endocrinology and oncology. Endokrynol Pol. 2011;62:456-464. [PubMed] |
| 3. | Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34:1785-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Ichihara E, Kiura K, Tanimoto M. Targeting angiogenesis in cancer therapy. Acta Med Okayama. 2011;65:353-362. [PubMed] |
| 5. | Bruce D, Tan PH. Vascular endothelial growth factor receptors and the therapeutic targeting of angiogenesis in cancer: where do we go from here? Cell Commun Adhes. 2011;18:85-103. [PubMed] |
| 6. | Linkous AG, Yazlovitskaya EM. Novel therapeutic approaches for targeting tumor angiogenesis. Anticancer Res. 2012;32:1-12. [PubMed] |
| 7. | Sakurai T, Kudo M. Signaling pathways governing tumor angiogenesis. Oncology. 2011;81 Suppl 1:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Thornton AD, Ravn P, Winslet M, Chester K. Angiogenesis inhibition with bevacizumab and the surgical management of colorectal cancer. Br J Surg. 2006;93:1456-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Konno H, Tanaka T, Baba M, Kanai T, Matsumoto K, Kamiya K, Nakamura S, Baba S. Quantitative analysis of vascular endothelial growth factor in colon cancer. Clinical and experimental. Eur Surg Res. 1998;30:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Doger FK, Meteoglu I, Tuncyurek P, Okyay P, Cevikel H. Does the EGFR and VEGF expression predict the prognosis in colon cancer? Eur Surg Res. 2006;38:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Konno H, Baba M, Tanaka T, Kamiya K, Ota M, Oba K, Shoji A, Kaneko T, Nakamura S. Overexpression of vascular endothelial growth factor is responsible for the hematogenous recurrence of early-stage gastric carcinoma. Eur Surg Res. 2000;32:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 12. | Ciprandi G, Murdaca G, Colombo BM, De Amici M, Marseglia GL. Serum vascular endothelial growth factor in allergic rhinitis and systemic lupus erythematosus. Hum Immunol. 2008;69:510-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Ciprandi G, Colombo BM, Murdaca G, De Amici M. Serum vascular endothelial growth factor and sublingual immunotherapy. Allergy. 2008;63:945-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Murdaca G, Spanò F, Miglino M, Puppo F. Effects of TNF-α inhibitors upon the mechanisms of action of VEGF. Immunotherapy. 2013;5:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Shord SS, Bressler LR, Tierney LA, Cuellar S, George A. Understanding and managing the possible adverse effects associated with bevacizumab. Am J Health Syst Pharm. 2009;66:999-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 16. | Krämer I, Lipp HP. Bevacizumab, a humanized anti-angiogenic monoclonal antibody for the treatment of colorectal cancer. J Clin Pharm Ther. 2007;32:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | McWilliams RR, Erlichman C. Novel therapeutics in colorectal cancer. Dis Colon Rectum. 2005;48:1632-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | McCormack PL, Keam SJ. Bevacizumab: a review of its use in metastatic colorectal cancer. Drugs. 2008;68:487-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Kocáková I, Kocák I, Svoboda M, Nemecek R, Rehák Z, Standara M. Bevacizumab in combination with capecitabine and irinotecan (XELIRI) in treatment of metastatic colorectal cancer. Klin Onkol. 2009;22:73-76. [PubMed] |
| 20. | Lee JM, Sarosy GA, Annunziata CM, Azad N, Minasian L, Kotz H, Squires J, Houston N, Kohn EC. Combination therapy: intermittent sorafenib with bevacizumab yields activity and decreased toxicity. Br J Cancer. 2010;102:495-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Starling N, Watkins D, Cunningham D, Thomas J, Webb J, Brown G, Thomas K, Oates J, Chau I. Dose finding and early efficacy study of gemcitabine plus capecitabine in combination with bevacizumab plus erlotinib in advanced pancreatic cancer. J Clin Oncol. 2009;27:5499-5505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Okines A, Puerto OD, Cunningham D, Chau I, Van Cutsem E, Saltz L, Cassidy J. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br J Cancer. 2009;101:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Degirmenci M, Karaca B, Gorumlu G, Durusoy R, Demir Piskin G, Bozkurt MT, Cirak Y, Tunali D, Karabulut B, Sanli UA. Efficacy and safety of bevacizumab plus capecitabine and irinotecan regimen for metastatic colorectal cancer. Med Oncol. 2010;27:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7788] [Article Influence: 354.0] [Reference Citation Analysis (8)] |
| 25. | Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1209] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 26. | Magdelaine-Beuzelin C, Kaas Q, Wehbi V, Ohresser M, Jefferis R, Lefranc MP, Watier H. Structure-function relationships of the variable domains of monoclonal antibodies approved for cancer treatment. Crit Rev Oncol Hematol. 2007;64:210-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Lee JJ, Chu E. An update on treatment advances for the first-line therapy of metastatic colorectal cancer. Cancer J. 2007;13:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Moehler M, Sprinzl MF, Abdelfattah M, Schimanski CC, Adami B, Godderz W, Majer K, Flieger D, Teufel A, Siebler J. Capecitabine and irinotecan with and without bevacizumab for advanced colorectal cancer patients. World J Gastroenterol. 2009;15:449-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Scappaticci FA, Fehrenbacher L, Cartwright T, Hainsworth JD, Heim W, Berlin J, Kabbinavar F, Novotny W, Sarkar S, Hurwitz H. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173-180. [PubMed] |
| 30. | Bège T, Lelong B, Viret F, Turrini O, Guiramand J, Topart D, Moureau-Zabotto L, Giovannini M, Gonçalves A, Delpero JR. Bevacizumab-related surgical site complication despite primary tumor resection in colorectal cancer patients. Ann Surg Oncol. 2009;16:856-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Li J, Saif MW. Current use and potential role of bevacizumab in the treatment of gastrointestinal cancers. Biologics. 2009;3:429-441. [PubMed] |
| 32. | Welch S, Spithoff K, Rumble RB, Maroun J. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol. 2010;21:1152-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Sharma S, Abhyankar V, Burgess RE, Infante J, Trowbridge RC, Tarazi J, Kim S, Tortorici M, Chen Y, Robles RL. A phase I study of axitinib (AG-013736) in combination with bevacizumab plus chemotherapy or chemotherapy alone in patients with metastatic colorectal cancer and other solid tumors. Ann Oncol. 2010;21:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Koukourakis MI, Giatromanolaki A, Sheldon H, Buffa FM, Kouklakis G, Ragoussis I, Sivridis E, Harris AL. Phase I/II trial of bevacizumab and radiotherapy for locally advanced inoperable colorectal cancer: vasculature-independent radiosensitizing effect of bevacizumab. Clin Cancer Res. 2009;15:7069-7076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, van Hazel GA, Robinson B, Broad A, Ganju V, Ackland SP. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28:3191-3198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 327] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 36. | Loupakis F, Bria E, Vaccaro V, Cuppone F, Milella M, Carlini P, Cremolini C, Salvatore L, Falcone A, Muti P. Magnitude of benefit of the addition of bevacizumab to first-line chemotherapy for metastatic colorectal cancer: meta-analysis of randomized clinical trials. J Exp Clin Cancer Res. 2010;29:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist. 2007;12:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 38. | García Alfonso P, Muñoz Martin A, Alvarez Suarez S, Blanco Codeidido M, Mondejar Solis R, Tapia Rico G, López Martín P, Martin M. Bevacizumab in Combination with Capecitabine plus Irinotecan as First-Line Therapy in Metastatic Colorectal Cancer: A Pooled Analysis of 2 Phase II Trials. Onkologie. 2013;36:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Lv C, Wu S, Zheng D, Wu Y, Yao D, Yu X. The Efficacy of Additional Bevacizumab to Cytotoxic Chemotherapy Regimens for the Treatment of Colorectal Cancer: An Updated Meta-Analysis for Randomized Trials. Cancer Biother Radiopharm. 2013;28:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, Atkins JN, Seay TE, Fehrenbacher L, Goldberg RM. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 447] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 41. | Dewdney A, Cunningham D, Barbachano Y, Chau I. Correlation of bevacizumab-induced hypertension and outcome in the BOXER study, a phase II study of capecitabine, oxaliplatin (CAPOX) plus bevacizumab as peri-operative treatment in 45 patients with poor-risk colorectal liver-only metastases unsuitable for upfront resection. Br J Cancer. 2012;106:1718-1721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Willett CG, Duda DG, Ancukiewicz M, Shah M, Czito BG, Bentley R, Poleski M, Fujita H, Lauwers GY, Carroll M. A safety and survival analysis of neoadjuvant bevacizumab with standard chemoradiation in a phase I/II study compared with standard chemoradiation in locally advanced rectal cancer. Oncologist. 2010;15:845-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Dipetrillo T, Pricolo V, Lagares-Garcia J, Vrees M, Klipfel A, Cataldo T, Sikov W, McNulty B, Shipley J, Anderson E. Neoadjuvant bevacizumab, oxaliplatin, 5-fluorouracil, and radiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Zafar SY, Malin JL, Grambow SC, Abbott DH, Schrag D, Kolimaga JT, Zullig LL, Weeks JC, Fouad MN, Ayanian JZ. Early dissemination of bevacizumab for advanced colorectal cancer: a prospective cohort study. BMC Cancer. 2011;11:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Madajewicz S, Waterhouse DM, Ritch PS, Khan MQ, Higby DJ, Leichman CG, Malik SK, Hentschel P, Gill JF, Zhao L. Multicenter, randomized phase II trial of bevacizumab plus folinic acid, fluorouracil, gemcitabine (FFG) versus bevacizumab plus folinic acid, fluorouracil, oxaliplatin (FOLFOX4) as first-line therapy for patients with advanced colorectal cancer. Invest New Drugs. 2012;30:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Dacosta Byfield S, Yu E, Morlock R, Evans D, Teitelbaum A. Corroboration of claims algorithm for second-line costs of metastatic colorectal cancer treatment with targeted agents. J Med Econ. 2013;16:1071-1081. [PubMed] |
| 47. | Abel EJ, Wood CG. Cytoreductive nephrectomy for metastatic RCC in the era of targeted therapy. Nat Rev Urol. 2009;6:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Azad NS, Posadas EM, Kwitkowski VE, Steinberg SM, Jain L, Annunziata CM, Minasian L, Sarosy G, Kotz HL, Premkumar A. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709-3714. [PubMed] |
| 49. | Normanno N, Morabito A, De Luca A, Piccirillo MC, Gallo M, Maiello MR, Perrone F. Target-based therapies in breast cancer: current status and future perspectives. Endocr Relat Cancer. 2009;16:675-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Preusser M, Fülöp G, Berghoff AS, Heinzl H, Steger GG, Greil R, Zielinski CC, Bartsch R. Influence of the American ODAC statement on Austrian bevacizumab prescribing practice for metastatic breast cancer. Oncologist. 2012;17:e13-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Tanne JH. FDA cancels approval for bevacizumab in advanced breast cancer. BMJ. 2011;343:d7684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Rose S. FDA pulls approval for avastin in breast cancer. Cancer Discov. 2011;1:OF1-OF2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Dawood S, Shaikh AJ, Buchholz TA, Cortes J, Cristofanilli M, Gupta S, Gonzalez-Angulo AM. The use of bevacizumab among women with metastatic breast cancer: a survey on clinical practice and the ongoing controversy. Cancer. 2012;118:2780-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Shamloo BK, Chhabra P, Freedman AN, Potosky A, Malin J, Weiss Smith S. Novel adverse events of bevacizumab in the US FDA adverse event reporting system database: a disproportionality analysis. Drug Saf. 2012;35:507-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Rinne ML, Lee EQ, Nayak L, Norden AD, Beroukhim R, Wen PY, Reardon DA. Update on bevacizumab and other angiogenesis inhibitors for brain cancer. Expert Opin Emerg Drugs. 2013;18:137-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722-4729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 1030] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 57. | Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1213] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 58. | Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733-4740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1951] [Cited by in RCA: 1894] [Article Influence: 111.4] [Reference Citation Analysis (4)] |
| 59. | Chinot OL, de La Motte Rouge T, Moore N, Zeaiter A, Das A, Phillips H, Modrusan Z, Cloughesy T. AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther. 2011;28:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 60. | Tao X, Sood AK, Deavers MT, Schmeler KM, Nick AM, Coleman RL, Milojevic L, Gershenson DM, Brown J. Anti-angiogenesis therapy with bevacizumab for patients with ovarian granulosa cell tumors. Gynecol Oncol. 2009;114:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Rossi A, Maione P, Colantuoni G, Ferrara C, Rossi E, Guerriero C, Nicolella D, Falanga M, Palazzolo G, Gridelli C. Recent developments of targeted therapies in the treatment of non-small cell lung cancer. Curr Drug Discov Technol. 2009;6:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Samaranayake H, Määttä AM, Pikkarainen J, Ylä-Herttuala S. Future prospects and challenges of antiangiogenic cancer gene therapy. Hum Gene Ther. 2010;21:381-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Mabuchi S, Terai Y, Morishige K, Tanabe-Kimura A, Sasaki H, Kanemura M, Tsunetoh S, Tanaka Y, Sakata M, Burger RA. Maintenance treatment with bevacizumab prolongs survival in an in vivo ovarian cancer model. Clin Cancer Res. 2008;14:7781-7789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Delli Carpini J, Karam AK, Montgomery L. Vascular endothelial growth factor and its relationship to the prognosis and treatment of breast, ovarian, and cervical cancer. Angiogenesis. 2010;13:43-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 65. | Ko AH, Youssoufian H, Gurtler J, Dicke K, Kayaleh O, Lenz HJ, Keaton M, Katz T, Ballal S, Rowinsky EK. A phase II randomized study of cetuximab and bevacizumab alone or in combination with gemcitabine as first-line therapy for metastatic pancreatic adenocarcinoma. Invest New Drugs. 2012;30:1597-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Crane CH, Winter K, Regine WF, Safran H, Rich TA, Curran W, Wolff RA, Willett CG. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol. 2009;27:4096-4102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 67. | Gordon CR, Rojavin Y, Patel M, Zins JE, Grana G, Kann B, Simons R, Atabek U. A review on bevacizumab and surgical wound healing: an important warning to all surgeons. Ann Plast Surg. 2009;62:707-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 68. | Okines A, Cunningham D. Current perspective: bevacizumab in colorectal cancer--a time for reappraisal? Eur J Cancer. 2009;45:2452-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, Bergsland E, Ngai J, Holmgren E, Wang J. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 699] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 70. | Zangari M, Fink LM, Elice F, Zhan F, Adcock DM, Tricot GJ. Thrombotic events in patients with cancer receiving antiangiogenesis agents. J Clin Oncol. 2009;27:4865-4873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 71. | Suenaga M, Mizunuma N, Kobayashi K, Shinozaki E, Matsusaka S, Chin K, Kuboki Y, Ichimura T, Ozaka M, Ogura M. Management of venous thromboembolism in colorectal cancer patients treated with bevacizumab. Med Oncol. 2010;27:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Lemmens L, Claes V, Uzzell M. Managing patients with metastatic colorectal cancer on bevacizumab. Br J Nurs. 2008;17:944-949. [PubMed] |
| 73. | Smith MS, Browne JD. The effect of endothelial cell growth factor on peripheral nerve regeneration. Otolaryngol Head Neck Surg. 1998;118:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 74. | Collins D, Ridgway PF, Winter DC, Fennelly D, Evoy D. Gastrointestinal perforation in metastatic carcinoma: a complication of bevacizumab therapy. Eur J Surg Oncol. 2009;35:444-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | August DA, Serrano D, Poplin E. “Spontaneous,” delayed colon and rectal anastomotic complications associated with bevacizumab therapy. J Surg Oncol. 2008;97:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Akkouche A, Sidéris L, Leblanc G, Leclerc YE, Vafiadis P, Dubé P. Complications after colorectal anastomosis in a patient with metastatic rectal cancer treated with systemic chemotherapy and bevacizumab. Can J Surg. 2008;51:E52-E53. [PubMed] |
| 77. | Abbrederis K, Kremer M, Schuhmacher C. Ischemic anastomotic bowel perforation during treatment with bevacizumab 10 months after surgery. Chirurg. 2008;79:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Lazzati V, Zygoń J, Lohsiriwat V, Veronesi P, Petit JY. Impaired wound healing and bilateral mastectomy flap necrosis in a patient with locally advanced breast cancer after neoadjuvant Paclitaxel with bevacizumab. Aesthetic Plast Surg. 2010;34:796-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 79. | Gamboa EO, Rehmus EH, Haller N. Fournier’s gangrene as a possible side effect of bevacizumab therapy for resected colorectal cancer. Clin Colorectal Cancer. 2010;9:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Grenader T, Goldberg A, Verstandig A, Shavit L. Indwelling central venous access port insertion during bevacizumab-based therapy. Anticancer Drugs. 2010;21:704-707. [PubMed] |
| 81. | Deshaies I, Malka D, Soria JC, Massard C, Bahleda R, Elias D. Antiangiogenic agents and late anastomotic complications. J Surg Oncol. 2010;101:180-183. [PubMed] |
| 82. | Mariani P. The safety of perioperative bevacizumab use. J Chir (Paris). 2010;147 Suppl 1:S12-S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 83. | Pavlidis ET, Ballas KD, Symeonidis NG, Psarras K, Koliakos G, Kouzi-Koliakos K, Topouridou K, Rafailidis SF, Pavlidis TE, Marakis GN. The effect of bevacizumab on colon anastomotic healing in rats. Int J Colorectal Dis. 2010;25:1465-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Pavlidis ET, Ballas KD, Psarras K, Symeonidis NG, Koliakos G, Kouzi-Koliakos K, Rafailidis SF, Pavlidis TE, Marakis GN, Sakantamis AK. Intraperitoneal administration of bevacizumab intraoperatively does not affect abdominal wound healing in rats. Eur Surg Res. 2011;47:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | Geiger-Gritsch S, Stollenwerk B, Miksad R, Guba B, Wild C, Siebert U. Safety of bevacizumab in patients with advanced cancer: a meta-analysis of randomized controlled trials. Oncologist. 2010;15:1179-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Mizobe T, Ogata Y, Murakami H, Akagi Y, Ishibashi N, Mori S, Sasatomi T, Shirouzu K. Efficacy of the combined use of bevacizumab and irinotecan as a postoperative adjuvant chemotherapy in colon carcinoma. Oncol Rep. 2008;20:517-523. [PubMed] |
| 87. | Ayral-Kaloustian S, Gu J, Lucas J, Cinque M, Gaydos C, Zask A, Chaudhary I, Wang J, Di L, Young M. Hybrid inhibitors of phosphatidylinositol 3-kinase (PI3K) and the mammalian target of rapamycin (mTOR): design, synthesis, and superior antitumor activity of novel wortmannin-rapamycin conjugates. J Med Chem. 2010;53:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 88. | Bäuerle T, Hilbig H, Bartling S, Kiessling F, Kersten A, Schmitt-Gräff A, Kauczor HU, Delorme S, Berger MR. Bevacizumab inhibits breast cancer-induced osteolysis, surrounding soft tissue metastasis, and angiogenesis in rats as visualized by VCT and MRI. Neoplasia. 2008;10:511-520. [PubMed] |
| 89. | Raatschen HJ, Simon GH, Fu Y, Sennino B, Shames DM, Wendland MF, McDonald DM, Brasch RC. Vascular permeability during antiangiogenesis treatment: MR imaging assay results as biomarker for subsequent tumor growth in rats. Radiology. 2008;247:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 90. | Raatschen HJ, Fu Y, Rogut V, Simon GH, Sennino B, Wolf KJ, Brasch RC. Effects of MRI-assayed microvascular permeability on the accumulation of vinorelbine in xenograft tumors. Rofo. 2010;182:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 91. | Woodrum C, Nobil A, Dabora SL. Comparison of three rapamycin dosing schedules in A/J Tsc2+/- mice and improved survival with angiogenesis inhibitor or asparaginase treatment in mice with subcutaneous tuberous sclerosis related tumors. J Transl Med. 2010;8:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12:233-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 419] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 93. | Salaun PY, Bodet-Milin C, Frampas E, Oudoux A, Saï-Maurel C, Faivre-Chauvet A, Barbet J, Paris F, Kraeber-Bodéré F. Toxicity and efficacy of combined radioimmunotherapy and bevacizumab in a mouse model of medullary thyroid carcinoma. Cancer. 2010;116:1053-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Yagi Y, Fushida S, Harada S, Tsukada T, Kinoshita J, Oyama K, Fujita H, Ninomiya I, Fujimura T, Kayahara M. Biodistribution of humanized anti-VEGF monoclonal antibody/bevacizumab on peritoneal metastatic models with subcutaneous xenograft of gastric cancer in mice. Cancer Chemother Pharmacol. 2010;66:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Ninomiya S, Inomata M, Tajima M, Ali AT, Ueda Y, Shiraishi N, Kitano S. Effect of bevacizumab, a humanized monoclonal antibody to vascular endothelial growth factor, on peritoneal metastasis of MNK-45P human gastric cancer in mice. J Surg Res. 2009;154:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 96. | Imaizumi T, Aoyagi K, Miyagi M, Shirouzu K. Suppressive effect of bevacizumab on peritoneal dissemination from gastric cancer in a peritoneal metastasis model. Surg Today. 2010;40:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 97. | Okada Y, Akisue T, Hara H, Kishimoto K, Kawamoto T, Imabori M, Kishimoto S, Fukase N, Onishi Y, Kurosaka M. The effect of bevacizumab on tumour growth of malignant fibrous histiocytoma in an animal model. Anticancer Res. 2010;30:3391-3395. [PubMed] |
| 98. | Shah DK, Veith J, Bernacki RJ, Balthasar JP. Evaluation of combined bevacizumab and intraperitoneal carboplatin or paclitaxel therapy in a mouse model of ovarian cancer. Cancer Chemother Pharmacol. 2011;68:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 99. | Davies S, Dai D, Pickett G, Thiel KW, Korovkina VP, Leslie KK. Effects of bevacizumab in mouse model of endometrial cancer: Defining the molecular basis for resistance. Oncol Rep. 2011;25:855-862. [PubMed] |
| 100. | Kenmotsu H, Yasunaga M, Goto K, Nagano T, Kuroda J, Koga Y, Takahashi A, Nishiwaki Y, Matsumura Y. The antitumor activity of NK012, an SN-38-incorporating micelle, in combination with bevacizumab against lung cancer xenografts. Cancer. 2010;116:4597-4604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 101. | Zhang J, Silva T, Yarovinsky T, Manes TD, Tavakoli S, Nie L, Tellides G, Pober JS, Bender JR, Sadeghi MM. VEGF blockade inhibits lymphocyte recruitment and ameliorates immune-mediated vascular remodeling. Circ Res. 2010;107:408-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 102. | Nayak TK, Garmestani K, Baidoo KE, Milenic DE, Brechbiel MW. PET imaging of tumor angiogenesis in mice with VEGF-A-targeted (86)Y-CHX-A″-DTPA-bevacizumab. Int J Cancer. 2011;128:920-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Wong HK, Lahdenranta J, Kamoun WS, Chan AW, McClatchey AI, Plotkin SR, Jain RK, di Tomaso E. Anti-vascular endothelial growth factor therapies as a novel therapeutic approach to treating neurofibromatosis-related tumors. Cancer Res. 2010;70:3483-3493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 104. | Finn RS, Bentley G, Britten CD, Amado R, Busuttil RW. Targeting vascular endothelial growth factor with the monoclonal antibody bevacizumab inhibits human hepatocellular carcinoma cells growing in an orthotopic mouse model. Liver Int. 2009;29:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Paudyal B, Paudyal P, Oriuchi N, Hanaoka H, Tominaga H, Endo K. Positron emission tomography imaging and biodistribution of vascular endothelial growth factor with 64Cu-labeled bevacizumab in colorectal cancer xenografts. Cancer Sci. 2011;102:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
P- Reviewers de Mello RA, Koukourakis GV, Murdaca G, Navea-Tejerina A, Slomiany BL, Yao Y S- Editor Gou SX L- Editor Webster JR E- Editor Li JY