Published online Aug 14, 2013. doi: 10.3748/wjg.v19.i30.4984
Revised: July 23, 2013
Accepted: July 30, 2013
Published online: August 14, 2013
Processing time: 66 Days and 20.5 Hours
AIM: To investigate the prevalence of minimal hepatic encephalopathy (MHE) and to assess corresponding health-related quality of life (HRQoL) in hospitalized cirrhotic patients in China.
METHODS: This multi-center cross-sectional study included 16 teaching hospitals, which were members of “Hepatobiliary Cooperation Group, Society of Gastroenterology, Chinese Medical Association”, from different areas of China carried out between June and October in 2011. All the eligible hospitalized cirrhotic patients (n = 538) were required to complete triplicate number connection tests combined with one digit symbol test for diagnosing MHE. Patients’ clinical examination data were complemented by a modified questionnaire assessing HRQoL. Written informed consent was obtained from each patient.
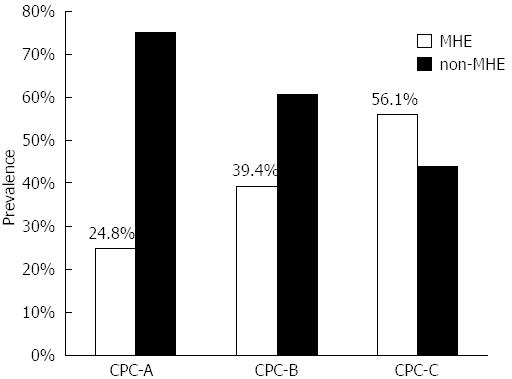
RESULTS: Male was predominant (68.6%) in 519 patients who met the criteria of the study, with a mean age of 49.17 ± 11.02 years. The most common cause of liver cirrhosis was chronic hepatitis B (55.9%). The prevalence of MHE was 39.9% and varied by Child-Pugh-Classification score (CPC-A: 24.8%, CPC-B: 39.4% and CPC-C: 56.1%, P < 0.01). MHE (P < 0.01) and higher CPC scores (P < 0.01) were associated with a high HRQoL scores (reflecting poorer quality of life). The prevalence of MHE was proportionate to CPC (P = 0.01) and high quality of life scores (P = 0.01).
CONCLUSION: Hospitalized cirrhotic patients have a high prevalence of MHE that is proportionate to the degree of liver function and HRQoL impairment.
Core tip: This study showed that 39.9% of hospitalized patients with liver cirrhosis had minimal hepatic encephalopathy (MHE), and patients with Child-Pugh Classification-C had a high prevalence of MHE (56.1%) and increased health-related quality of life scores that reflected poorer life status. Increasing awareness of its adverse impact on life should be emphasized. Recommendations to screen for MHE may be applicable for evaluating the risks of driving and work accidents in patients with cirrhosis.
- Citation: Wang JY, Zhang NP, Chi BR, Mi YQ, Meng LN, Liu YD, Wang JB, Jiang HX, Yang JH, Xu Y, Li X, Xu JM, Zhang G, Zhou XM, Zhuge YZ, Tian DA, Ye J, Liu YL. Prevalence of minimal hepatic encephalopathy and quality of life evaluations in hospitalized cirrhotic patients in China. World J Gastroenterol 2013; 19(30): 4984-4991
- URL: https://www.wjgnet.com/1007-9327/full/v19/i30/4984.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i30.4984
Hepatic encephalopathy (HE) is a serious complication of liver cirrhosis that represents a continuous spectrum of neurologic and neuropsychiatric abnormalities[1,2]. Minimal HE (MHE), the mildest form of HE[1,2], is defined as patients with normal mental and neurological examinations but with a number of neuropsychiatric and neuro-physiological defects identified by psychometric tests[3]. Patients with MHE have various subtle abnormalities in the cognitive functioning that detrimentally affects their fitness to drive[4] and handle complex mechanical machines[5]. In a study by Prasad et al[6], significant impairment was observed in HE patients’ social interactions, alertness, emotional behavior, sleep, work, household management, recreation, and pastimes.
There are several methods of diagnosing MHE, including comprehensive neuropsychological examinations, standard psychometric batteries, neuro-physiological testing, and computerized testing[3]. However, there are no current guidelines for the standardized diagnosis of MHE. The Working Group on HE recommended that at least two of the following neuropsychologic tests should be used for diagnosing MHE: number connection test-A (NCT-A), NCT-B, block-design test (BDT), and the digit-symbol test (DST)[7]. The current definition of MHE is based on psychometric test results that are two SD more than normal on at least two psychometric tests. As there is no gold standard for diagnosis of MHE, the prevalence of MHE in patients with cirrhosis ranges from 30% to 80%[8-11]. Recently, the estimated prevalence of MHE varied from 29.2% to 57.1% in China[12-14]. Some studies only employed one neuropsychologic tests (NCT) for MHE diagnosis. Therefore, the exact prevalence of MHE is unknown in China.
MHE is associated with potential progression to HE, diminished quality of life, driving impairment that increases the risk of traffic accidents, and negative health-related quality of life (HRQoL)[15-17]. Quality of life (QoL) is a multidimensional index that comprehensively addresses all aspects of human well-being, including physical and cognitive capabilities, functional behavior, emotional status, and psychosocial adjustment. As compared with generic measures of impairment, disease-specific measures are more likely to be sensitive to small, yet clinically meaningful, differences in HRQoL. Our group[18] developed and verified a reliable and valid HRQoL instrument that measures the functional and health status of patients with MHE. That study also demonstrated that HRQoL in patients with MHE deteriorates as the disease becomes more severe, although the study had a small sample size and a limited regional scope.
This study investigated the prevalence of MHE in hospitalized cirrhotic patients from different areas of China, and HRQoL evaluations among them.
A multi-center cross-sectional study was initiated by the Hepatobiliary Cooperation Group of Society of Gastroenterology, the Chinese Medical Association. The study was conducted in 16 teaching hospitals representing different areas of China (4 in the East, 3 in the West, 1 in the South, 4 in the North and 4 in the central region). All consecutive cirrhotic hospitalized patients aged between 18 and 70 years and without overt HE (OHE) were screened for MHE between June and October 2011. Cirrhosis was diagnosed based on available clinical data, including laboratory tests, endoscopy, diagnostic imaging, or liver histology. Exclusion criteria included the presence or a history of OHE, a history of taking lactulose or any antibiotics, alcohol intake, gastrointestinal hemorrhage, or spontaneous bacterial peritonitis during the previous 6 wk, significant concurrent diseases such as heart, respiratory, or renal failure, and neurologic abnormalities such as Alzheimer’s disease, Parkinson’s disease, non-hepatic metabolic encephalopathy, electrolyte disorders, inability to perform psychometric tests or complete the questionnaire (caused by either insufficient knowledge of the Chinese language or poor vision). All patients provided written informed consent. Study protocols were approved by the ethics committees of the participating hospitals in accordance with the Principles of Declaration of Helsinki.
Qualified physicians documented routine physical examinations and laboratory assessments that included biochemical tests (alanine aminotransferase, aspartate aminotransferase, bilirubin, albumin, creatinine, prothrombin time, serum potassium, serum sodium, and serum chloride), virological tests [hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), anti-HBe, anti-hepatitis C virus (HCV), hepatitis B virus (HBV) DNA levels, and HCV RNA levels], and diagnostic imaging [ultrasonography, computer tomography (CT), or magnetic resonance imaging], etiology of cirrhosis, a history of medication use and other medical histories. The Child-Pugh-Classification (CPC) scoring system was used to assess the severity of liver disease.
All patients underwent a series of psychometric tests, including triplicate NCT-A and one DST. The NCT-A measures cognitive motor abilities by having patients connect numbers, from 1 to 25 on printed paper, as quickly as possible. DST: Subjects are asked to insert symbols in the blank squares below the numbers using the key provided. The exercise is timed and the number correctly completed in 90 seconds recorded. Bao et al[19] established age-based normal parameters of psychometric measures for NCT-A and DST in China in 2006. Normative values for NCT-A and DST were based on those from healthy volunteers with the same geographical background as liver cirrhosis patients.
According to the normative parameters for NCT-A and DST established by Bao et al, diagnostic criteria for MHE were as follows: time greater than two SD from the mean for the NCT, and score less than two SD from the mean for the DST. For the NCT-A, diagnostic criteria were: > 34.3 s in patients aged < 35 years; > 45.7 s in patients aged 35-44 years; > 52.8 s in patients aged 45-54 years and > 61.9 s in patients aged > 55 years. Diagnostic criteria for the DST were: < 40.5 in patients < 35 years; < 35 in patients aged 35-44 years; < 28.5 in patients aged 45-54 years and < 26 in patients aged > 55 years. Patients with abnormal results from both psychometric tests were diagnosed as having MHE.
A modified Chinese QoL questionnaire with 30 questions verified in Chinese populations in 2009 was used to assess all patients’ HRQoL index[18] The domains included physical functioning (8 questions), psychological well-being (7 questions), symptoms/side effects (7 questions), social functioning (4 questions), and self-evaluation about general health (4 questions). Impact scores for each question ranged from 1 to 5. These scores increase as the QoL declines. The total QoL scores were obtained from the sum of each question’s score.
Continuous variables were expressed as mean ± SD or median (range), where appropriate. Categorical variables were described as the number and proportion of each category. In order to determine relevant risk factors for MHE occurrence, characteristics such as age, gender, pre-existing ascites, variceal bleeding, occupation, driving, alcohol drinking, hepatitis B antigen status, and antiviral therapy for HBV-related cirrhosis were included in the univariate analysis. The χ2 or Fisher’s exact test was used for categorical variables, and the Mann-Whitney U test or analysis of variance (ANOVA), was performed as appropriate to determine associations for continuous data. All tested variables with P values < 0.5 were entered into logistic regression. All statistical testing was two-tailed at the 5% level. Software used for analysis was Statistical Package for Social Science (SPSS, version 14.0, SPSS Inc. Chicago, IL, United States) and Science Analysis Software (SAS, version 9.13; SAS Institute Inc., Cary, NC, United States).
Of the patients screened (n = 538), 519 patients met the study’s criteria for inclusion. Excluded patients included those who were older than 70 years (n = 7) and who did not complete all psychometric tests (n = 12). Liver cirrhosis was diagnosed based on diagnostic imaging (ultrasonography, CT and/or magnetic resonance imaging), histopathology, endoscopy, or clinical and laboratory data. Most patients (n = 356, 68.6%) were male with a mean age of 49.17 ± 11.02 years, and 100 (20.3%) of them had college degrees or higher levels of education. Included patients were diagnosed with liver cirrhosis for 2.54 ± 3.16 years. The most common causes of liver cirrhosis were chronic hepatitis B (CHB) (55.7%), followed by CHB accompanied by alcoholic liver disease (ALD) (11.8%), ALD (8.9%), chronic hepatitis C (CHC) (7.3%), and autoimmune hepatitis (AIH) (7.5%). According to the CPC scoring system, 161 (31.0%) patients were classified as CPC-A, 203 (39.1%) were as CPC-B, and 155 (29.9%) as CPC-C. Of 320 cirrhotic patients with CHB, 41.6% (n = 133) were HBeAg positive, 85.0% (n = 272) were HBV DNA positive, and 202 (60.3%) had received antiviral treatment.
Cirrhotic patients with concurrent positive NCT and DST results (n = 207, 39.9%) were diagnosed with MHE. The prevalence of MHE differed among CPC-A (24.8%), CPC-B (39.4%) and CPC-C (56.1%) patients (CPC-A vs CPC-B, P < 0.05; CPC-A vs CPC-C, P < 0.01; CPC-B vs CPC-C, P < 0.01) (Figure 1). Older patients and patients with lower levels of education, a history of prior ascites had a higher prevalence of MHE (Table 1). Compared to patients without MHE, those with MHE had lower levels of serum albumin (P = 0.01), sodium (P = 0.01), potassium (P = 0.04), and platelet count (P = 0.03); higher levels of serum bilirubin (P < 0.01) and blood ammonia (P = 0.02); and longer prothrombin times (P = 0.01). Many (24.3%) MHE patients were still driving at the time of diagnosis.
| Characteristics | MHE | Non-MHE | P value |
| Gender | |||
| Male | 140 (67.6) | 216 (69.2) | 0.77 |
| Female | 67 (32.4) | 96 (30.8) | |
| Mean age (yr), mean ± SD | 51.56 ± 9.70 | 47.58 ± 11.55 | < 0.01 |
| Level of education | |||
| Grade six or less | 93 (44.9) | 34 (10.9) | < 0.01 |
| Junior high school | 64 (39.9) | 76 (24.4) | |
| Senior high school/vocational school | 32 (15.5) | 94 (30.1) | |
| College degree or more | 12 (5.8) | 88 (28.2) | |
| Unknown | 6 (2.9) | 20 (6.4) | |
| Driving | |||
| Yes | 50 (24.2) | 89 (28.5) | 0.22 |
| No | 144 (69.6) | 193 (61.9) | |
| Unknown | 13 (6.3) | 30 (9.6) | |
| Primary etiology for chronic liver disease | |||
| Hepatitis B virus | 117 (56.5) | 172 (55.5) | 0.18 |
| Hepatitis B virus and alcohol | 21 (10.1) | 40 (12.9) | |
| Alcohol | 30 (14.5) | 19 (6.1) | |
| Hepatitis C virus | 11 (5.3) | 27 (8.7) | |
| Hepatitis B and C virus | 2 (1.0) | 1 (0.3) | |
| Autoimmune hepatitis | 12 (5.8) | 27 (8.7) | |
| Other | 14 (6.8) | 24 (7.7) | |
| History of prior variceal bleeding | |||
| Yes | 43 (20.7) | 82 (26.3) | 0.21 |
| No | 158 (76.3) | 226 (72.4) | |
| Unknown | 6 (2.9) | 4 (1.3) | |
| History of prior ascites | |||
| Yes | 131 (63.3) | 139 (44.6) | 0.00 |
| No | 66 (31.9) | 168 (53.8) | |
| Unknown | 10 (4.8) | 5 (1.6) | |
| Duration of liver cirrhosis (yr), mean ± SD | 2.28 ± 3.06 | 2.73 ± 3.21 | 0.25 |
There were no statistical differences in HBeAg status (P = 0.30) or HBV-DNA levels (P = 0.19), duration of HBV infection (P = 1.00), antiviral therapy (P = 0.17), or duration of antiviral treatment (P = 0.54) between patients with and without MHE.
Compared to cirrhotic patients without MHE, patients diagnosed with MHE had higher scores (more dysfunctions) for physical functioning (20.09 ± 6.26 vs 18.10 ± 6.02, P < 0.01), symptom/side effects (14.98 ± 5.88 vs 13.35 ± 5.61, P < 0.01), and psychological well-being (15.93 ± 6.62 vs 14.80 ± 5.44, P = 0.04) (Table 2). Pooled HRQoL scales were higher in MHE patients than in non-MHE ones (69.12 ± 20.40 vs 63.89 ± 18.85, P < 0.01). Patients with CPC-C had higher HRQoL scores (71.61 ± 21.01) than those with CPC-A ( 61.13 ± 17.24) and CPC-B (65.50 ± 19.31), P < 0.01, which reflect poorer QoL (Table 3).
| MHE | Non-MHE | P value | |
| Physical functioning (8 questions) | 20.09 ± 6.26 | 18.10 ± 6.0 | < 0.01 |
| Psychological well-being (7 questions) | 15.93 ± 6.62 | 14.80 ± 5.44 | 0.04 |
| Symptom/side effects (7 questions) | 14.98 ± 5.88 | 13.35 ± 5.61 | < 0.01 |
| Social functioning (4 questions) | 9.67 ± 2.73 | 9.66 ± 2.65 | 0.95 |
| Self-evaluation regarding general-health (4 questions) | 9.74 ± 2.73 | 9.43 ± 2.57 | 0.21 |
| Total pooled score (30 questions) | 69.12 ± 20.40 | 63.89 ± 18.85 | < 0.01 |
| CPC-A | CPC-B | CPC-C | P value | |
| Physical functioning | 16.77 ± 5.07 | 18.94 ± 6.35 | 20.82 ± 6.33 | < 0.01 |
| Psychological well-being | 14.43 ± 5.46 | 15.03 ± 5.93 | 16.39 ± 6.38 | 0.02 |
| Symptom/side effects | 12.12 ± 5.33 | 13.56 ± 5.21 | 16.45 ± 6.05 | < 0.01 |
| Social functioning | 9.03 ± 2.53 | 9.60 ± 2.75 | 10.37 ± 2.58 | < 0.01 |
| Self evaluation about general-health | 9.20 ± 2.59 | 9.46 ± 2.74 | 10.02 ± 2.52 | 0.02 |
| Total pooled score | 61.13 ± 17.24 | 65.50 ± 19.31 | 71.61 ± 21.01 | < 0.01 |
We employed combined NCT and DST tests as “gold standard” in this study. Consistency of diagnosis between one single psychometric test (NCT or DST) and combined psychometric tests was assessed by Kappa statistics (Table 4). Agreement between DST and combined NCT and DST was good, with a Kappa coefficient around 0.98 (95%CI: 0.97-0.99) for diagnosing MHE. Agreement between NCT and combined tests was fair (Kappa value 0.24, 95%CI: 0.19-0.29).
| NCT and DST | Kappa value (95%CI) | P value | ||
| MHE | Non-MHE | |||
| NCT | ||||
| MHE | 207 | 223 | 0.24 (0.19-0.29) | < 0.01 |
| Non- MHE | 0 | 89 | ||
| DST | ||||
| MHE | 207 | 4 | 0.98 (0.97-0.99) | 0.05 |
| Non- MHE | 0 | 308 | ||
This study was the first nationwide investigation of the prevalence of MHE among hospitalized cirrhotic patients in China. The study locations are dispersed in different parts of China, including east, west, north, south and central regions, covering 16 hospitals located in 10 provinces and 3 municipalities under direct administration of the central government. Because each teaching hospital in the capital city of a province provides service to patients from the entire province, the study population could well represent cirrhotic patients throughout China.
China has the greatest burden of chronic liver disease in the world due to an epidemic of viral B hepatitis. Although the exact nationwide prevalence of liver cirrhosis in China is unknown, a reasonable estimate suggests that up to 1% of the entire population could have histological evidence of cirrhosis[20,21]. The prevalence of MHE in Chinese cirrhotic patients was reported to be 51.3% by Zeng et al[22]. However, their study only included local patients and lacked assessment of cognitive impairments and decreased quality of life. Other studies also reported varying and higher than 50% MHE prevalences among cirrhotic patients[10,23-25]. Our study showed that the nationwide prevalence of MHE in hospitalized cirrhotic patients was 39.9%. These discrepancies were due to the different criteria used to diagnose MHE and inter-population variations. The absence of a gold standard for determining MHE is a major challenge for attaining consistency among studies.
Impairments in visuospatial function, attention, response time, and inhibition are specific to MHE in the absence of other neurocognitive disorders; the psychometric HE score (PHES) was specifically designed to detect these impairments. The PHES comprises 5 different tests: the NCT-A, NCT-B, DST, the line-tracing test, and the serial dotting test. NCT-A and NCT-B evaluate concentration, mental tracking, and visuomotor speed. The DST evaluates psychomotor and visuomotor speed with attention on speed and accuracy. According to the consensus of the Working Group on HE[26], if the entire PHES cannot be completed, at least two of the following tests are recommended for the diagnosis of MHE: NCT-A, NCT-B, block design test, and DST.
In this study, we combined two age-based psychometric tests (NCT-A and DST)[19] as “gold standard” to diagnose MHE. Consistency of MHE diagnosis between one single psychometric test (NCT or DST) and combined psychometric tests was assessed by Kappa statistics. Agreement between DST and combined NCT and DST was good, with a Kappa coefficient around 0.98 for diagnosing MHE. This good agreement indicates that DST is equally good as combined NCT and DST[27-29]. However, single NCT, which showed a higher prevalence of MHE, did not have good agreement with combined test. Therefore, in clinical practice, DST can be used as the first test for screening MEH so as to avoid a high rate of false positive diagnosis.
One of the limitations of neuropsychological test is that the results can be influenced by age, educational level, and learning effects. We used three parallel versions of NCT-A to avoid the effects of education. Yet the limitations of our study were that (1) the normality of the NCT-A and DST scores used was not adjusted by educational level; and (2) half of the patients in our study had lower educational levels, which might have influenced the neuropsychological test results.
Furthermore, the prevalence of MHE reported in other studies was higher in cirrhotic patients with CPC-B, CPC-C, advanced age, alcoholism, a previous episode of overt HE, and portosystemic shunts[30]. None of the patients in our study had previous episodes of OHE or histories of portosystemic shunt surgery. Groeneweg et al[31] found that cirrhotic patients with normal liver function (CPC-A) had a low prevalence (15%) of MHE, while MHE was present in half of the patients with advanced cirrhosis (CPC-B/C). Our study confirmed that the prevalence of MHE in CPC-C patients was the highest (56.5%). The results demonstrated that cirrhotic patients with MHE had impaired liver function, including reduced hepatic biosynthetic, excretory and/or detoxification capacity, hyponatremia, lower platelet count, and high blood ammonia.
Patients with MHE had impaired perception, memory, learning, expression (language, constructive abilities, and voluntary motor control), mental activity (attention and mental speed), and executive function[30,32]. There are many aspects of English-published HRQoL instruments which cannot be adapted well to the Chinese due to the differences in cultures and language. In order to define and assess HRQoL appropriately in the Chinese patients, our group developed a modified Chinese questionnaire of HRQoL that was verified in a Chinese population in 2009[18]. The questionnaire was administered to a cohort of patients with varying types and stages of cirrhosis for assessment of its reliability and discriminant validity. As liver disease becomes more severe, the questionnaire documents deterioration in patients HRQoL[18]. Our study confirmed that severity of liver function impairment, based on CPC scoring system, was associated with HRQoL. Patients with CPC-C had higher HRQoL scores and compromised life status compared to patients with CPC-A/B. Patients with MHE had high health-related QoL scores that reflect poorer QoL.
Patients with MHE have higher physical functioning and symptom/side effects scores than patients without MHE. These two domains of the questionnaire also have a higher test-retest reliability (0.94 and 0.96) than other domains[18]. Our study results retested the discriminant validity of the questionnaire for distinguishing among groups with varying CPC classes. However, the validity of this instrument for evaluating the efficacy of MHE treatment needs to be established.
Physicians formerly agreed that it was unnecessary to screen for and treat MHE in cirrhotic patients without a history of OHE[33]. However, given increased knowledge of the impact of MHE, great emphasis on OHE has recently been shifted towards covert HE[34]. Because of psychomotor defects, patients meeting the criteria for MHE have been shown to have reduced driving skills, who are more likely to suffer from falls and develop episodic HE more frequently[4,35,36]. Our study found that 24.2% of MHE patients were driving at the time of diagnosis. Due to the potential risks, there is a need to assess the presence of MHE in cirrhotic patients who drive. Similarly, recommendations for MHE screening of cirrhotic patients may be valuable in reducing the risk of work-related accidents, especially while handling machinery[30,37].
Compared to OHE, there are fewer randomized clinical trials about the treatment of MHE and these trials have smaller case numbers. Some studies show that treatments using lactulose and/or rifaximin can improve the cognitive abilities, QoL[6,38], and driving ability[39] of patients with MHE. Yet the effects of these drugs on MHE patients’ ability to work or risk of falling remain unproven. The duration of treatment and choice of medication also remain unclear[40]. Therefore, high quality studies are needed to assess whether patients suffering from liver cirrhosis and MHE require a specific treatment.
In conclusion, our study showed that 39.9% of hospitalized patients with liver cirrhosis had MHE, and this was associated with severe liver function and QoL impairment. Cirrhotic patients with CPC-C had a high prevalence of MHE and increased HRQoL scores that reflected poorer life status. The modified Chinese HRQoL questionnaire performed well in this study. The HRQoL scale results indicated that the questionnaire was suitable for evaluating cirrhotic patients in clinical practice in China. Recommendations to screen for MHE using NCT-A combining DST tests may be applicable for evaluating the risks of driving and work accidents in patients with cirrhosis. In clinical practice, DST can be considered as the first screening test of MHE due to the good agreement between DST and combined psychometric tests.
We thank Dr. Bai-Song Wang from Department of Biostatistics, Shanghai Jiao Tong University School of Medicine, for assistance with statistical analyses.
China has the greatest burden of chronic liver disease in the world due to an epidemic of viral B hepatitis. Yet the exact nationwide prevalence of liver cirrhosis in China is unknown. Furthermore, minimal hepatic encephalopathy (MHE) is associated with potential progression to Hepatic encephalopathy (HE), diminished quality of life (QoL), driving impairment that increases the risk of traffic accidents, and negative health-related QoL (HRQoL).
This is a first multicenter nationwide study to investigate the burden of MHE in hospitalized patients with cirrhosis in China. No such data was reported before.
This study was the first nationwide investigation of the prevalence of MHE among hospitalized cirrhotic patients in China. The study locations are dispersed in different parts of China, including east, west, north, south and central regions, covering 16 hospitals located in 10 provinces and 3 municipalities under direct administration of the central government. Because each teaching hospital in the capital city of a province provides service to patients from the entire province, the study population could well represent cirrhotic patients throughout China.
The results showed that 39.9% of hospitalized patients with liver cirrhosis had MHE, and patients with Child-Pugh-Classification score-C had a high prevalence of MHE (56.1%) and increased health-related QoL scores that reflected poorer life status. Increasing awareness of its adverse impact on life quality should be emphasized. Recommendations to screen for MHE may be applicable for evaluating the risks of driving and work accidents in patients with cirrhosis.
HE is a serious complication of liver cirrhosis that represents a continuous spectrum of neurologic and neuropsychiatric abnormalities. MHE, the mildest form of HE, is defined as patients with normal mental and neurological examinations but with a number of neuropsychiatric and neuro-physiological defects identified by psychometric tests.
In this paper, the authors investigated the prevalence of MHE, and assessed corresponding HEQoL in hospitalized cirrhotic patients in China. This is an interesting study regarding the prevalence and clinical features of MHE in China. The article showed a progress in the study of HE in cirrhotic patients. It is an innovative job of HE study and data is reliable.
| 1. | Mullen KD, Prakash RK. New perspectives in hepatic encephalopathy. Clin Liver Dis. 2012;16:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96:2718-2723. [PubMed] |
| 3. | Kharbanda PS, Saraswat VA, Dhiman RK. Minimal hepatic encephalopathy: diagnosis by neuropsychological and neurophysiologic methods. Indian J Gastroenterol. 2003;22 Suppl 2:S37-S41. [PubMed] |
| 4. | Watanabe A, Tuchida T, Yata Y, Kuwabara Y. Evaluation of neuropsychological function in patients with liver cirrhosis with special reference to their driving ability. Metab Brain Dis. 1995;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 68] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Groeneweg M, Quero JC, De Bruijn I, Hartmann IJ, Essink-bot ML, Hop WC, Schalm SW. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 358] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45:549-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 408] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 7. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1435] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 8. | Quero JC, Schalm SW. Subclinical hepatic encephalopathy. Semin Liver Dis. 1996;16:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Quero JC, Hartmann IJ, Meulstee J, Hop WC, Schalm SW. The diagnosis of subclinical hepatic encephalopathy in patients with cirrhosis using neuropsychological tests and automated electroencephalogram analysis. Hepatology. 1996;24:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 134] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Das A, Dhiman RK, Saraswat VA, Verma M, Naik SR. Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. J Gastroenterol Hepatol. 2001;16:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 205] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Amodio P, Quero JC, Del Piccolo F, Gatta A, Schalm SW. Diagnostic tools for the detection of subclinical hepatic encephalopathy: comparison of standard and computerized psychometric tests with spectral-EEG. Metab Brain Dis. 1996;11:315-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Li YY, Nie YQ, Sha WH, Zeng Z, Yang FY, Ping L, Jia L. Prevalence of subclinical hepatic encephalopathy in cirrhotic patients in China. World J Gastroenterol. 2004;10:2397-2401. [PubMed] |
| 13. | Lin Y, Fan YP. [The neuropsychologic tests and the minimal hepatic encephalopathy investigations in liver cirrhotic patients]. Zhonghua Ganzangbing Zazhi. 2011;19:65-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Bao ZJ, Ma X, Qiu DK. [Methods for diagnosis of minimal hepatic encephalopathy and their evaluation]. Zhonghua Ganzangbing Zazhi. 2005;13:878-880. [PubMed] |
| 15. | Marchesini G, Bianchi G, Amodio P, Salerno F, Merli M, Panella C, Loguercio C, Apolone G, Niero M, Abbiati R. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 363] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 16. | Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci. 2003;48:1622-1626. [PubMed] |
| 17. | Schomerus H, Hamster W. Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis. 2001;16:37-41. [PubMed] |
| 18. | Zhou YQ, Chen SY, Jiang LD, Guo CY, Shen ZY, Huang PX, Wang JY. Development and evaluation of the quality of life instrument in chronic liver disease patients with minimal hepatic encephalopathy. J Gastroenterol Hepatol. 2009;24:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Bao ZJ, Qiu DK, Ma X, Zhang GS, Gu T, Yu XF, Fan ZP, Li JQ, Zeng MD. The application of psychometric measures in diagnosis of minimal hepatic encephalopathy. Zhonghua Xiaohua Zazhi. 2006;26:606-609. |
| 20. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1608] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 21. | Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1858] [Article Influence: 92.9] [Reference Citation Analysis (1)] |
| 22. | Zeng Z, Li YY, Nie YQ. [An epidemiological survey of subclinical hepatic encephalopathy]. Zhonghua Ganzangbing Zazhi. 2003;11:680-682. [PubMed] |
| 23. | Dhiman RK, Sawhney MS, Chawla YK, Das G, Ram S, Dilawari JB. Efficacy of lactulose in cirrhotic patients with subclinical hepatic encephalopathy. Dig Dis Sci. 2000;45:1549-1552. [PubMed] |
| 24. | Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 464] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 25. | Senzolo M, Amodio P, D’Aloiso MC, Fagiuoli S, Del Piccolo F, Canova D, Masier A, Bassanello M, Zanus G, Burra P. Neuropsychological and neurophysiological evaluation in cirrhotic patients with minimal hepatic encephalopathy undergoing liver transplantation. Transplant Proc. 2005;37:1104-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Dhiman RK, Saraswat VA, Sharma BK, Sarin SK, Chawla YK, Butterworth R, Duseja A, Aggarwal R, Amarapurkar D, Sharma P. Minimal hepatic encephalopathy: consensus statement of a working party of the Indian National Association for Study of the Liver. J Gastroenterol Hepatol. 2010;25:1029-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | McKenzie DP, Mackinnon AJ, Péladeau N, Onghena P, Bruce PC, Clarke DM, Harrigan S, McGorry PD. Comparing correlated kappas by resampling: is one level of agreement significantly different from another. J Psychiatr Res. 1996;30:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Thompson WD, Walter SD. A reappraisal of the kappa coefficient. J Clin Epidemiol. 1988;41:949-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 294] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 29. | Blackman NJ, Koval JJ. Interval estimation for Cohen’s kappa as a measure of agreement. Stat Med. 2000;19:723-741. [PubMed] |
| 30. | Ortiz M, Jacas C, Córdoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42 Suppl:S45-S53. [PubMed] |
| 31. | Groeneweg M, Moerland W, Quero JC, Hop WC, Krabbe PF, Schalm SW. Screening of subclinical hepatic encephalopathy. J Hepatol. 2000;32:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 144] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis. 2004;19:253-267. [PubMed] |
| 33. | Bajaj JS, Cordoba J, Mullen KD, Amodio P, Shawcross DL, Butterworth RF, Morgan MY. Review article: the design of clinical trials in hepatic encephalopathy--an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33:739-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 34. | Mullen KD, Prakash RK. Future of Hepatic Encephalopathy. Hepatic Encephalopathy. Berlin: Springer 2012; 241-243. |
| 35. | Schomerus H, Hamster W, Blunck H, Reinhard U, Mayer K, Dölle W. Latent portasystemic encephalopathy. I. Nature of cerebral functional defects and their effect on fitness to drive. Dig Dis Sci. 1981;26:622-630. [PubMed] |
| 36. | Román E, Córdoba J, Torrens M, Torras X, Villanueva C, Vargas V, Guarner C, Soriano G. Minimal hepatic encephalopathy is associated with falls. Am J Gastroenterol. 2011;106:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 37. | Córdoba J, Lucke R. Driving under the influence of minimal hepatic encephalopathy. Hepatology. 2004;39:599-601. [PubMed] |
| 38. | Sidhu SS, Goyal O, Mishra BP, Sood A, Chhina RS, Soni RK. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol. 2011;106:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 39. | Bajaj JS, Heuman DM, Wade JB, Gibson DP, Saeian K, Wegelin JA, Hafeezullah M, Bell DE, Sterling RK, Stravitz RT. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology. 2011;140:478-487.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 40. | Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 220] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
P- Reviewers Liu P, Maruyama H, Yang YF S- Editor Zhai HH L- Editor Ma JY E- Editor Ma S