Published online Aug 14, 2013. doi: 10.3748/wjg.v19.i30.4907
Revised: June 13, 2013
Accepted: June 28, 2013
Published online: August 14, 2013
Processing time: 112 Days and 10.3 Hours
AIM: To determine the magnetic resonance cholangiopancreatography (MRCP) and magnetic resonance imaging (MRI) features of pancreatitis with pancreas divisum (PD) and the differences vs pancreatitis without divisum.
METHODS: Institutional review board approval was obtained and the informed consent requirement was waived for this HIPAA-compliant study. During one year period, 1439 consecutive patients underwent successful MRCP without injection of secretin and abdominal MRI studies for a variety of clinical indications using a 1.5 T magnetic resonance scanner. Two experienced radiologists retrospectively reviewed all the studies in consensus. Disputes were resolved via consultation with a third experienced radiologist. The assessment included presence and the imaging findings of PD, pancreatitis, and distribution of abnormalities. The pancreatitis with divisum constituted the study group while the pancreatitis without divisum served as the control group. MRCP and MRI findings were correlated with final diagnosis. Fisher exact tests and Pearson × 2 tests were performed.
RESULTS: Pancreatitis was demonstrated at MRCP and MRI in 173 cases (38 cases with and 135 cases without divisum) among the 1439 consecutive cases. The recurrent acute pancreatitis accounted for 55.26% (21 of 38) in pancreatitis patients associated with PD, which was higher than 6.67% (9 of 135) in the control group, whereas the chronic pancreatitis was a dominant type in the control group (85.19%, 115 of 135) when compared to the study group (42.11%, 16 of 38) (χ2 = 40.494, P < 0.0001). In cases of pancreatitis with PD, the dorsal pancreatitis accounted for a much higher percentage than that in pancreatitis without PD (17 of 38, 44.74% vs 30 of 135, 22.22%) (χ2 = 7.257, P < 0.05).
CONCLUSION: MRCP and MRI can depict the features of pancreatitis associated with divisum. Recurrent acute pancreatitis and isolated dorsal involvement are more common in patients with divisum.
Core tip: We reviewed 1439 cases of abdominal magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP). There were 122 cases of pancreas divisum (PD) and 38 of them were diagnosed as pancreatitis. The pancreatitis associated with PD was usually distributed in dorsal pancreas and presented as recurrent acute type. MRCP in combination with MRI can accurately detect ductal and parenchymal abnormalities of pancreas. Therefore, MRCP and MRI should be referred to as primary diagnostic tools for pancreatitis with PD whereas endoscopic retrograde cholangiopancreatography can be reserved for those who require therapeutic interventions.
- Citation: Wang DB, Yu J, Fulcher AS, Turner MA. Pancreatitis in patients with pancreas divisum: Imaging features at MRI and MRCP. World J Gastroenterol 2013; 19(30): 4907-4916
- URL: https://www.wjgnet.com/1007-9327/full/v19/i30/4907.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i30.4907
Pancreas divisum (PD) is the most common developmental anatomic variant of pancreatic duct with a reported incidence of 4%-14% in the population at autopsy series, 3%-8% at endoscopic retrograde cholangiopancreatography (ERCP), and 9% at magnetic resonance cholangiopancreatography (MRCP)[1-5]. This abnormality occurs when the dorsal and ventral pancreas anlage fails to fuse during the 6th-8th week of gestation. PD is characterized not only by the anatomical morphology but also by the physiology in which the majority of pancreatic juice drains through the duct of Santorini into the duodenum at orifice of minor papilla while the minority (about 10%) drains through the (ventral) duct of Wirsung into the duodenum at major papilla[1]. Although the clinical significance still remains controversial, there seems to be an association between PD and chronic abdominal pain and recurrent acute pancreatitis. Moreover, the timely and appropriate therapeutic interventions such as minor papillotomy or stent placement in the dorsal pancreatic duct or surgical procedures can benefit the patients with symptomatic PD remarkably from reducing the pressure in the main pancreatic duct[6].
The manifestations of acute and chronic pancreatitis at magnetic resonance imaging (MRI) and magnetic resonance (MR) cholangiopancreatography (MRCP) have been well described in previous studies[7-10], however, to our knowledge, there is no published literature on imaging features of pancreatitis in patients with PD using MRI together with MRCP without secretin injection. Although ERCP is considered as a gold standard of diagnosis, prior studies have shown that there is a great correlation between MRCP and ERCP in detecting PD[11,12]. Currently, the multidetector computed tomography (MDCT) has been reported to be valuable in the detection of PD[13]. As a noninvasive approach, MRCP can be used much more extensively than ERCP when radiation is in consideration and can always be performed together with MRI, which can depict the morphologic changes in detail[9,14]. Therefore, since MRI and MRCP can be employed to establish a diagnosis non-invasively, including for patients who are unable to undergo diagnostic ERCP, the ERCP can be reserved for those who require therapeutic intervention.
Therefore, the purpose of this study was to retrospectively evaluate the imaging features of pancreatitis in patients with PD at MRI and MRCP without injection of secretin and to describe the differences of MR imaging between pancreatitis with and without divisum.
During one year period, a total of 1439 consecutive patients (age range, 16-95 years; 698 men and 741 women) consecutively underwent successful abdominal MRI and MRCP without injection of secretin in our institution for a variety of clinical indications. Among them, 173 cases were finally diagnosed as pancreatitis based on clinical presentations, laboratory values, and imaging findings. Of the 173 cases, 38 cases associated with PD constituted the study group in this study. A total of 42 times of ERCP examination and interventional therapy were performed in 21 cases in the study group. Among them, minor papillotomy and temporary transpapillary stent placement in main pancreatic duct (n = 15) through minor papilla, stent placement in common bile duct (CBD) (n = 3), and surgery of Puestow procedure (n = 1) were performed. Eighteen patients were male and 20 were female, with a mean age of 43.6 years (range, 20-79 years). The remaining 135 cases of pancreatitis without PD (66 male and 69 female) served as control group, aged from 19 to 85 years with a mean age of 53.4 years. We obtained the institutional review board approval and waiver of informed consent for this retrospective HIPPA-compliant study.
The recurrent acute pancreatitis was defined in this study as a clinical setting in which the clinical or/and serologic features were characteristic of acute pancreatitis with a history of recurrence at least 2 times. The clinical data, which included symptoms at presentation, history of previous episodes of pancreatitis, associated symptoms involving other systems, and laboratory findings, were reviewed for all of the 173 cases of pancreatitis in this study.
All the MR studies including coronal MRCP and axial MRI were performed with a 1.5-T MR imager (Magneton Vision; Siemens, Erlangen, Germany) using a phase array body coil. The MRCP images were initially obtained and axial MR imaging followed. The pancreaticobiliary tract was localized with a thick-slab (40 mm) half-Fourier RARE image in coronal-oblique (25 degrees) and axial planes, which necessitated an acquisition time of 7 s. The thin-slab MRCP acquisitions were obtained at various angles that allowed optimal visualization of the bile and pancreatic ducts; the number of thin-slab acquisitions per patient ranged from 3 to 15 (mean, 7 acquisitions). Both the thick-slab and thin slab images were obtained during breath hold. The half-Fourier RARE parameters included repetition time ms/echo time ms (effective) of ∞/95.0; echo train length, 128; flip angle, 150 degrees; section thickness, 3.0 mm with no gap; field of view, 270 mm × 270 mm; number of signals acquired, 1; matrix, 240 × 256 and acquisition time, 20 s. Fat saturation and shim adjustments were used in all cases.
After MRCP, conventional axial MR imaging was conducted to examine the abdomen. MR imaging sequences included unenhanced T1-weighted breath-hold spoiled gradient echo (148/5 ms; flip angle, 70 degrees; section thickness, 10 mm; gap, 30%), unenhanced T2-weighted breath-hold fast SE (3500/138 ms; section thickness, 8 mm; gap, 25%), unenhnaced in phase and out-of-phase T1-weighted gradient recalled echo, unenhanced and double-phased dynamic contrast-enhanced T1-weighted fat suppression (200/4.4 ms; flip angle, 70 degrees; section thickness, 8 mm; gap, 20%) 30 and 60 s after beginning of intravenous administration of the contrast materials. Gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ, United States) was administered intravenously using an automatic injector at a dose of 0.1 mmol per kilogram of body weight as a bolus followed by a normal-saline flush.
All the images in this study were reviewed retrospectively using interactive picture archiving and communicating system (PACS) workstations by two experienced (10-15 years of practice) abdominal radiologists (Wang D and Yu J) in consensus. The disputes were resolved via consultation with a third experienced abdominal radiologist (Fulcher AS). During the reading, the following items were taken into account: (1) classification of PD, complete PD or incomplete PD; (2) distribution of pancreatitis in the pancreas (ventral, dorsal or ventral plus dorsal pancreas); (3) morphologic changes including pancreatic parenchyma (enlargement or atrophy of pancreas), and pancreatic duct changes (side branch ectasia, pancreatic ductal dilatation and strictures, and intraductal calculi); (4) signal intensity abnormalities on unenhanced or enhanced images, including necrosis or cystic changes in pancreas; (5) changes outside of the pancreas, i.e., peripancreatic stranding, fluid collections, and involvement of the adjacent organs and vessels, etc; and (6) abscess inside pancreas and outside of pancreas. The classifications and distributions of pancreatitis were compared between the study group and control group. After careful analysis of the abovementioned findings, the imaging features of pancreatitis associated with PD were established.
Pearson χ2 and Fisher exact probability test were introduced for classification and distribution of pancreatitis in patients with PD in the study group compared with those in the control group. A P value less than 0.05 was considered to indicate a statistically significant difference.
The classifications of pancreatitis in the 38 cases with PD in the study group included: recurrent acute pancreatitis in 21 cases (all cases with abdominal pain, 5 with gallstones, 1 with jaundice, and 16 with hyperlipasemia and hyperamylasemia), chronic pancreatitis in 8 cases (all cases with abdominal pain, 3 with mild serologic enzymes elevation, and 1 with gallstones) and the other 8 cases revealed with chronic pancreatitis incidentally at MRI and MRCP primarily for detecting hepatic lesions or biliary abnormalities, and acute pancreatitis in 1 case with worsening abdominal pain and serum lipase elevation of more than 1000 U/LH (normal, 23-300 U/LH). The gallstone pancreatitis was the dominant type in the control group (75.6%, 102/135). The other etiologies included intrapancreatic calculi (11.1%, 15/135), pancreatic ductal strictures (5.9%, 8/135), and autoimmune pancreatitis (4.4%, 6/135); and no distinct etiologic factors were found in 4 of the 135 cases (3%). The recurrent acute pancreatitis accounted for 55.26% (21 of 38) in pancreatitis with PD, which was higher in percentage than 6.67% (9 of 135) in the control group, whereas the chronic pancreatitis was a dominant type in the control group (85.19%, 115 of 135) when compared to the study group (42.11%, 16 of 38) (χ2 = 40.494, P < 0.0001) (Table 1). The pancreatitis in patients with PD accounted for 21.96% (38 of 173) in the total population of pancreatitis in this study.
| Acute pancreatitis | Chronic pancreatitis | Recurrent acute pancreatitis | Total | |
| Pancreatitis with PD | 1 (2.63) | 16 (42.11) | 21(55.26) | 38 |
| Pancreatitis without PD | 11 (8.14) | 115 (85.19) | 9 (6.67) | 135 |
| Total | 12 (6.93) | 131 (75.72) | 30(17.34) | 173 |
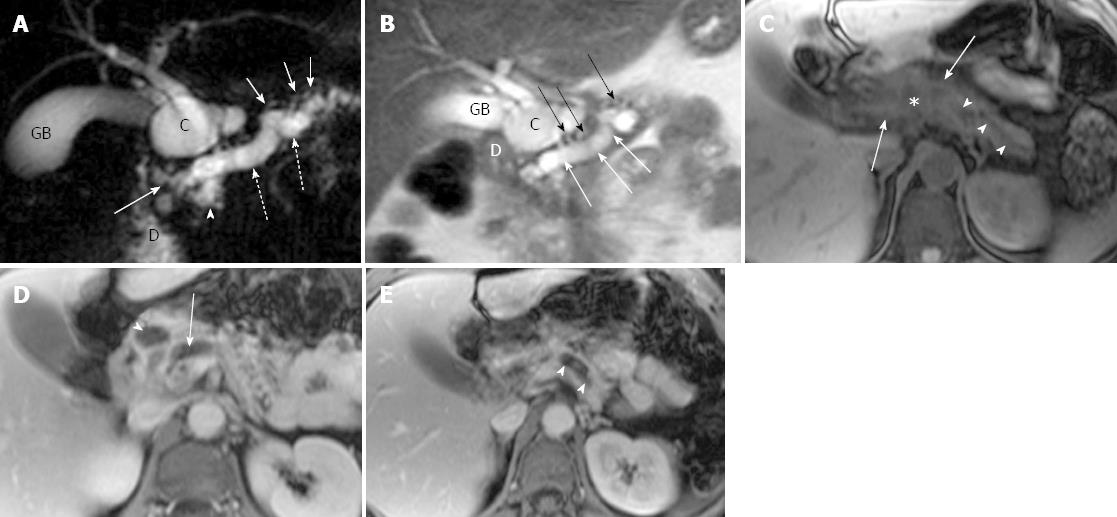
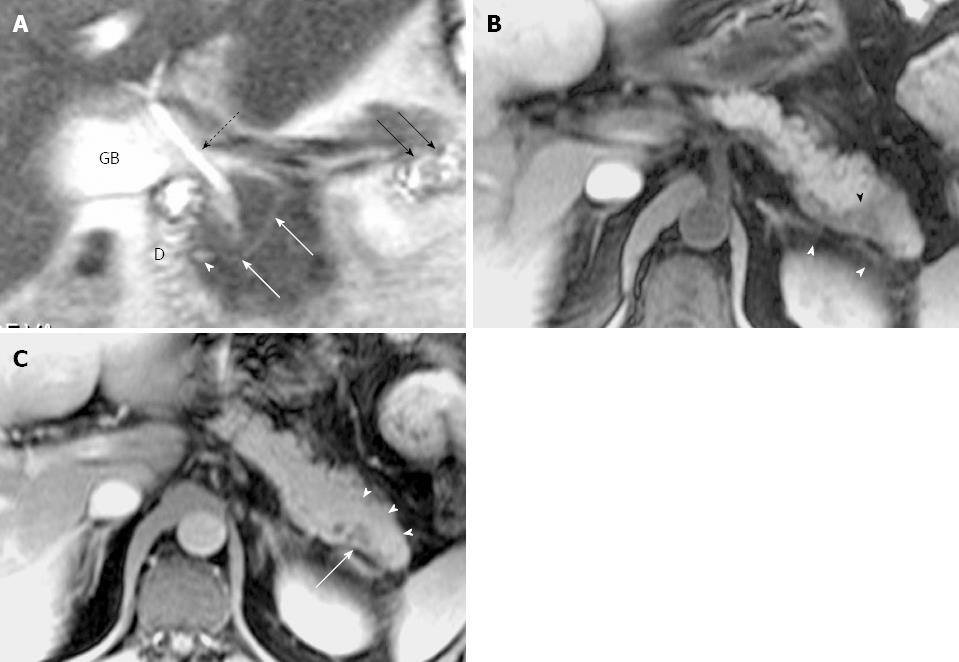
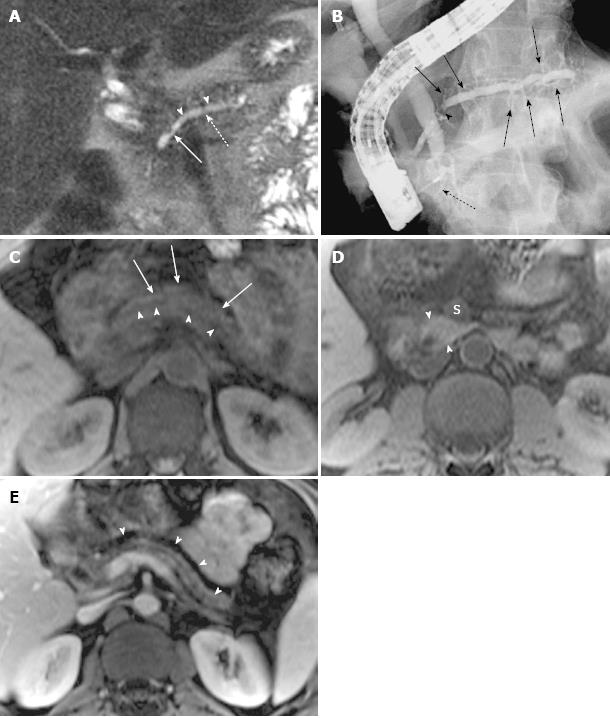
Ductal and parenchymal changes of pancreas: Pancreatic duct in patients with PD and without PD both showed dilatation (6 vs 65), irregularity (16 vs 86), dilatation with irregularity (8 vs 80), focal stricture (6 vs 31), intrapancreatic duct calculi ( 2 vs 15), and side branch ectasia (36 vs 116) (Figure 1). In the study group, 7 cases of the isolated dorsal pancreatitis showed dilatation of main pancreatic duct all the way proximally to minor papilla. Two of recurrent cases each had a santorinicele of 5 mm (Figure 2) and 15 mm, respectively. Totally, 38 segments of duct of Santorini, 32 of duct of Wirsung, and 36 of duct in the body and in the tail as well were visualized clearly enough to be measured. The mean duct diameter was 3.00 ± 1.17 mm (SD) for the duct of Santorini, 2.22 ± 0.906 mm for the duct of Wirsung, 4.11 ± 2.72 mm for the body, and 3.27 ± 2.14 mm in the tail segments (Table 2). There were 3 severe chronic cases and 3 recurrent cases of pancreatitis showing the maximum of dorsal pancreatic ductal dilatation measured from 5 mm to 13.5 mm.
| Santorini | Wirsung | Body | Tail | |
| Acute pancreatitis (mm) | 4.5 | 2.4 | 3.5 | 2.2 |
| Recurrent pancreatitis (mm) | 3.81 ± 1.02 | 2.40 ± 1.360 | 4.62 ± 3.49 | 3.37 ± 1.99 |
| Chronic pancreatitis (mm) | 4.06 ± 1.26 | 2.11 ± 0.597 | 3.74 ± 2.01 | 3.24 ± 2.35 |
| Mean (mm) | 4.00 ± 1.17 | 2.22 ± 0.906 | 4.11 ± 2.72 | 3.27 ± 2.14 |
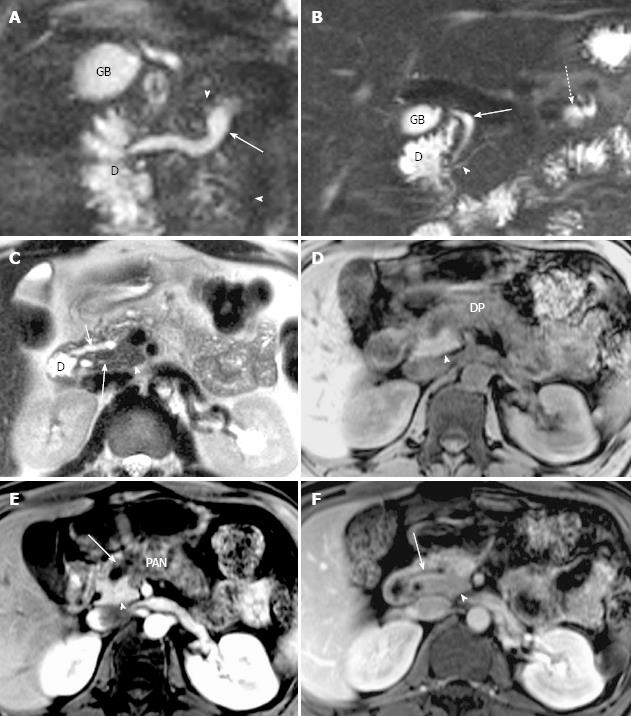
In the study group, pancreatic edematous enlargement (n = 3), peripancreatic stranding (n = 13), atrophy with T1 signal intensity decrease (n = 12), atrophy with normal signal (n = 12), only T1 signal decrease (n = 3), and small intrapancreatic necrosis (n = 4) were detected in pancreatitis patients with PD. Six intrapancreatic pseudocysts were detected in 6 cases with a size ranging from 5 to 20 mm while 4 extrapancreatic fluid collections and pseudocysts were formed in the other 3 cases sized 35 to 76 mm. Thirty-two cases (recurrent acute, n = 21; chronic pancreatitis, n = 8) demonstrated delayed enhancement of pancreas (Figure 3). There were no adjacent organ and vessel involvement, and no hemorrhage or abscess formation due to pancreatitis in patients with PD.
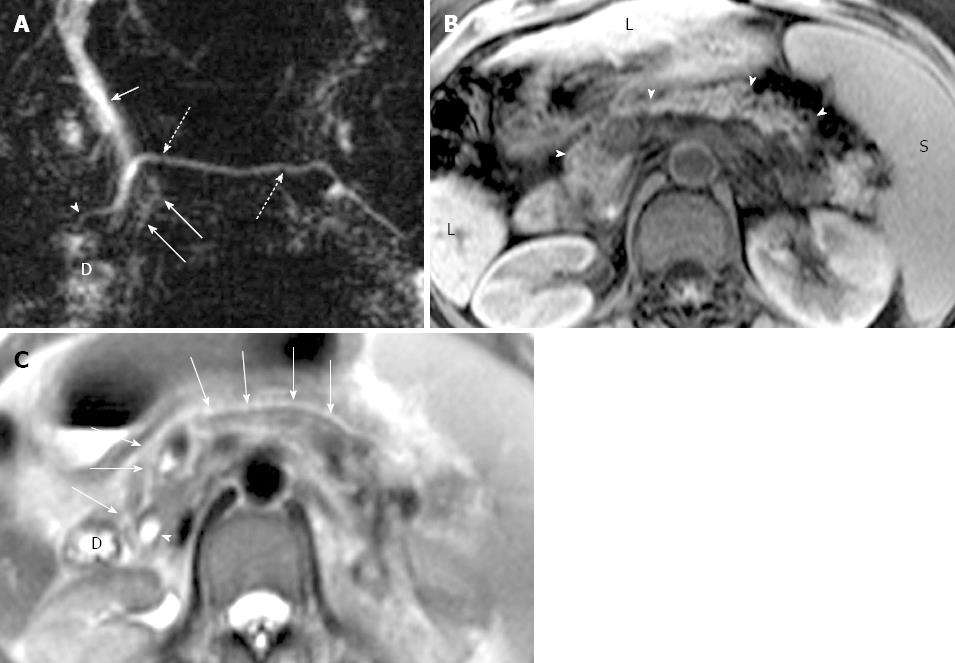
Classification of pancreas divisum and distribution of pancreatitis: In the study group, 35 cases were classified as complete PD and 3 cases as incomplete PD. Totally, 16 cases with complete divisum and 1 with incomplete divisum comprised dorsal pancreatitis in patients with PD. In cases of pancreatitis with PD, the dorsal pancreatitis accounted for a much higher percentage than in pancreatitis without PD (17 of 38, 44.74% vs 30 of 135, 22.22%) (χ2 =7.257, P < 0.05) (Table 3, Figures 4 and 5). Fifteen cases of dorsal pancreatitis were classified as recurrent acute pancreatitis. According to the anatomical involvement of lesion, the 17 cases of dorsal pancreatitis could be classified as complete dorsal involvement (n = 7), suproanterior portion of head and neck involvement (n = 3), dominant body involvement (n = 2), and dominant tail involvement (n = 5) with ductal stricture at body-tail junction resulting in upstream ductal dilatation in the tail.
| Dorsal | Ventral | Entire | Total | |
| Pancreatitis with PD | 17 (44.74) | 0 (0) | 21 (55.26) | 38 |
| Pancreatitis without PD | 30 (22.22) | 4 (2.96) | 101 (74.81) | 135 |
| Total | 47 (27.17) | 4 (2.31) | 122 (70.52) | 173 |
Classic PD (type 1) is defined as complete failure of fusion of the ducts of Santorini and Wirsung; other fusion anomalies with dominant dorsal drainage include absence of duct of Wirsung (type 2) and the presence of a filamentous or tiny caliber communication between the dominant dorsal duct of Santorini and the duct of Wirsung (type 3, incomplete PD)[15]. In patient with complete PD, a larger amount secretion from the dorsal pancreas could exert a significant burden on the relatively smaller orifice of minor duodenal papilla causing elevation of endoluminal pressure in pancreatic duct, resulting in subsequent pancreatitis. It has been reported that the clinical implications of incomplete PD may be similar to those of complete PD though the precise physiology may differ from each other[16,17].
A prior study with ERCP showed that the highest incidence of PD associated with idiopathic acute pancreatitis reached 50% in a total of 58 cases, which is significantly higher than in both controls and the whole population[18]. The recurrent acute pancreatitis in our study accounted for 55.26% (21 of 38) of pancreatitis in patients with PD, which is higher than in control group (6.67%, 9 of 135) (P < 0.0001, Table 1). The recurrent acute pancreatitis is a clinical entity that is characterized by repeated episodes of pancreatitis, which evolves over time with recurrent attacks of acute pancreatitis in otherwise normal pancreas until interventional procedures were performed in this clinical setting[19]. The pancreatitis in patients with PD accounted for 21.97% (38 of 173) in total population of pancreatitis in this study, which is higher than in the results based on ERCP by Bernard et al[18] and Kamisava et al[20].
In Western countries, the incomplete PD is uncommon with a reported incidence of 0.13%-0.9%. However, there was a much higher prevalence of incomplete PD in the recent reports from Japan and Korea, indicating 48% and 52% of PD[16,17]. In the present study, the incomplete PD occurred in 7.9% (3 of 38) among the pancreatitis patients with PD. Partially, the fluctuation of the frequency of incomplete PD could result from the different techniques employed for the detection of PD, i.e., the ERCP or MRCP, even for the same imaging modality, the techniques may be different with time due to intrinsic advances resulting in improved resolution[16,21-24].
In our study, 94.1% (16 of 17) of dorsal pancreatitis were detected in cases with complete PD. According to the pathophysiology of PD, the majority of pancreatic juice drained through minor papilla can result in endoluminal pressure elevation or obstruction with subsequent pancreatitis likely involving the dorsal pancreas and sparing the ventral pancreas instead.
Although the incidence of dorsal pancreatitis (44.74%, 17 of 38) in patients with PD was significantly higher than in patients without PD (30 of 135, 22.22%) (P < 0.05, Table 3) in this study and in the study with ERCP conducted by Kamisava et al[20], it is lower than the prior study performed with ERCP by Morgan et al[4]. The presence of pancreas divisum may reduce the severity of acute gallstone pancreatitis, as stone impaction at the major papilla only affects the ventral pancreas, a smaller portion (about 10%) of pancreas compared to the dorsal pancreas[25].
Among the isolated dorsal pancreatitis cases, severe strictures at the duct junction of body and tail were responsible for upstream ductal dilatation in tail with atrophy of the affected pancreas, which presented with recurrent acute pancreatitis clinically. The pancreatic parenchyma in pancreatitis associated with PD demonstrated a spectrum of abnormalities including low-signal-intensity of pancreas on T1-weighted fat-suppressed images due to edema or fibrosis, decreased and delayed enhancement after intravenous contrast administration, parenchymal atrophy or enlargement, and pseudocysts. Inflammation and fibrosis can diminish the proteinaceous fluid content in the pancreas, leading to the loss of the usual high signal intensity on T1-weighted fat-suppressed images; therefore, the pancreatitis in patients with PD can have some findings similar to the pancreatitis without PD as indicated in literature[8].
Although the ERCP is still considered as a gold standard for diagnosing PD, it is an invasive technique and expensive, particularly it has several drawbacks including failure to cannulate minor papilla[12,26], a high rate of complications such as ERCP-induced pancreatitis[26], radiation, and use of iodinated contrast medium. It was reported that as high as 35% of patients with pancreatitis had no abnormalities on ERCP[27]. A recent article reported that the MDCT could detect the PD via visualization of the Santorini duct[13]. However, MRCP together with MRI is a non-invasive technique without radiation; MRCP can always be done together with MRI in a single study, which can delineate the parenchymal morphology in detail[28]. Comparing to ERCP and MDCT, MRCP and MRI can be repeated more safely in the follow-up of the patients of pancreatitis with PD since patients in this subgroup are likely younger and more sensitive to radiation. MRCP with secretin stimulation can provide better visualization of pancreatic duct, resulting in higher sensitivity and specificity for diagnosis of the pancreatic abnormalities[29,30]. MRI can have the same accuracy as CT for pancreatitis at present. Additionally, MRCP is thought to depict the pancreatic duct in more physiologic states than under exogenous pressure such as ERCP. Therefore, MRCP and MRI can serve as a comprehensive diagnostic tool without radiation for the pancreatitis associated with PD whereas the ERCP can be reserved for those who require interventional procedures for therapeutic purpose.
This study had several limitations. One limitation was that not all cases (21 of 38) underwent ERCP procedure for reference or interventional management. Owing to the resolution of MRCP without secretin stimulation at present, there could be some compromises resulting in possible false-negative consequences in detection and characterization of PD and pancreatitis associated with PD in some cases. Finally, the subjects enrolled into the study was based on the referring criteria for MRCP and MRI studies; the severity of pancreatitis or the classifications of pancreatitis might not exactly reflect the real profile of pancreatitis in patients with PD since most of severe and acute patients prefer CT because it is quicker than MRI in examination.
In conclusion, recurrent acute pancreatitis is more common in patients with divisum than in patients without divisum (21 of 38, 55.26% vs 9 of 135, 6.67%). In divisum patients, the dorsal pancreatitis accounts for a much higher percentage than in patients without PD (17 of 38, 44.74% vs 30 of 135, 22.22%). Therefore, MRCP and MRI could be a comprehensive diagnostic tool without radiation for the pancreatitis associated with PD whereas the ERCP can be reserved for those who require therapeutic interventions.
Pancreas divisum (PD) is the most common developmental anatomic variant of pancreatic duct. Elevation of the intraluminal pressure of the pancreatic duct could result in pancreatitis. Magnetic resonance-cholangiopancreatography (MRCP) can always be performed together with magnetic resonance imaging (MRI), which can accurately detect both the ductal and the parenchymal abnormalities of the pancreas in detail.
The pancreatitis in patients with PD could be different from the cases without PD both in clinical presentations and distribution of the abnormalities in pancreas since the congenital anomaly, however, the clinical significance remains controversial. To their knowledge, there is no published literature on imaging features of pancreatitis in patients with PD using MRI together with MRCP without secretin injection.
The pancreatitis associated with PD was usually distributed in dorsal pancreas and presented as recurrent acute type. MRCP in combination with MRI can accurately detect ductal and parenchymal abnormalities of pancreatitis in patients with PD.
The results of the present study indicated that repeated attacks of acute pancreatitis or isolated dorsal involvement of pancreas could imply a congenital PD in pancreas. MRCP and MRI should be referred to as a primary diagnostic tool for pancreatitis patients associated with PD whereas ERCP can be reserved for those who require therapeutic interventions.
Recurrent acute pancreatitis was defined in this study as a condition in which the clinical or/and serologic features were characteristic of acute pancreatitis with a history of recurrence at least 2 times.
Pancreas divisum is a common congenital anomaly of the pancreatic duct and possible cause of recurrent pancreatitis and chronic pancreatitis. But, there are still controversies in clinical significance as a cause of pancreatitis. This study revealed that recurrent acute pancreatitis is more common in divisum patients compared with those without divisum. The sample size was large to reach a conclusion and those results can help clinicians understand clinical significance of pancreas divisum. The imaging quality of presented figures is excellent and representative.
| 1. | Kozu T, Suda K, Toki F. Pancreatic development and anatomical variation. Gastrointest Endosc Clin N Am. 1995;5:1-30. [PubMed] |
| 2. | Lehman GA, Sherman S. Diagnosis and therapy of pancreas divisum. Gastrointest Endosc Clin N Am. 1998;8:55-77. [PubMed] |
| 3. | Bret PM, Reinhold C, Taourel P, Guibaud L, Atri M, Barkun AN. Pancreas divisum: evaluation with MR cholangiopancreatography. Radiology. 1996;199:99-103. [PubMed] |
| 4. | Morgan DE, Logan K, Baron TH, Koehler RE, Smith JK. Pancreas divisum: implications for diagnostic and therapeutic pancreatography. AJR Am J Roentgenol. 1999;173:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Agha FP, Williams KD. Pancreas divisum: incidence, detection, and clinical significance. Am J Gastroenterol. 1987;82:315-320. [PubMed] |
| 6. | Gerke H, Byrne MF, Stiffler HL, Obando JV, Mitchell RM, Jowell PS, Branch MS, Baillie J. Outcome of endoscopic minor papillotomy in patients with symptomatic pancreas divisum. JOP. 2004;5:122-131. [PubMed] |
| 7. | Lecesne R, Taourel P, Bret PM, Atri M, Reinhold C. Acute pancreatitis: interobserver agreement and correlation of CT and MR cholangiopancreatography with outcome. Radiology. 1999;211:727-735. [PubMed] |
| 8. | Miller FH, Keppke AL, Wadhwa A, Ly JN, Dalal K, Kamler VA. MRI of pancreatitis and its complications: part 2, chronic pancreatitis. AJR Am J Roentgenol. 2004;183:1645-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Sica GT, Miller FH, Rodriguez G, McTavish J, Banks PA. Magnetic resonance imaging in patients with pancreatitis: evaluation of signal intensity and enhancement changes. J Magn Reson Imaging. 2002;15:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Varghese JC, Masterson A, Lee MJ. Value of MR pancreatography in the evaluation of patients with chronic pancreatitis. Clin Radiol. 2002;57:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Mortelé KJ, Rocha TC, Streeter JL, Taylor AJ. Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics. 2006;26:715-731. [PubMed] |
| 12. | Sica GT, Braver J, Cooney MJ, Miller FH, Chai JL, Adams DF. Comparison of endoscopic retrograde cholangiopancreatography with MR cholangiopancreatography in patients with pancreatitis. Radiology. 1999;210:605-610. [PubMed] |
| 13. | Asayama Y, Fang W, Stolpen A, Kuehn D. Detectability of pancreas divisum in patients with acute pancreatitis on multi-detector row computed tomography. Emerg Radiol. 2012;19:121-125. [PubMed] |
| 14. | Barish MA, Yucel EK, Ferrucci JT. Magnetic resonance cholangiopancreatography. N Engl J Med. 1999;341:258-264. [PubMed] |
| 15. | Warshaw AL, Simeone JF, Schapiro RH, Flavin-Warshaw B. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined). Am J Surg. 1990;159:59-64; discussion 64-66. [PubMed] |
| 16. | Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A. Clinical implications of incomplete pancreas divisum. JOP. 2006;7:625-630. [PubMed] |
| 17. | Kim MH, Lee SS, Kim CD, Lee SK, Kim HJ, Park HJ, Joo YH, Kim DI, Yoo KS, Seo DW. Incomplete pancreas divisum: is it merely a normal anatomic variant without clinical implications. Endoscopy. 2001;33:778-785. [PubMed] |
| 18. | Bernard JP, Sahel J, Giovannini M, Sarles H. Pancreas divisum is a probable cause of acute pancreatitis: a report of 137 cases. Pancreas. 1990;5:248-254. [PubMed] |
| 20. | Kamisawa T, Egawa N, Tsuruta K, Okamoto A, Mtsukawa M. Pancreatitis associated with congenital abnormalities of the pancreaticobiliary system. Hepatogastroenterology. 2005;52:223-229. [PubMed] |
| 21. | Fulcher AS, Turner MA, Capps GW, Zfass AM, Baker KM. Half-Fourier RARE MR cholangiopancreatography: experience in 300 subjects. Radiology. 1998;207:21-32. [PubMed] |
| 22. | Leyendecker JR, Elsayes KM, Gratz BI, Brown JJ. MR cholangiopancreatography: spectrum of pancreatic duct abnormalities. AJR Am J Roentgenol. 2002;179:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 23. | Fulcher AS, Turner MA, Capps GW. MR cholangiography: technical advances and clinical applications. Radiographics. 1999;19:25-41; discussion 41-44. [PubMed] |
| 24. | Soto JA, Yucel EK, Barish MA, Chuttani R, Ferrucci JT. MR cholangiopancreatography after unsuccessful or incomplete ERCP. Radiology. 1996;199:91-98. [PubMed] |
| 25. | Boon N, Delhaye M, Le Moine O, De Maertelaer V, Devière J. Severity of acute gallstone pancreatitis in patients with pancreas divisum. Endoscopy. 2003;35:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 852] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 27. | Gold RP, Berman H, Fakhry J, Heier S, Rosenthal W, DelGuercio L. Pancreas divisum with pancreatitis and pseudocyst. AJR Am J Roentgenol. 1984;143:1343-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Yu J, Turner MA, Fulcher AS, Halvorsen RA. Congenital anomalies and normal variants of the pancreaticobiliary tract and the pancreas in adults: part 2, Pancreatic duct and pancreas. AJR Am J Roentgenol. 2006;187:1544-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Matos C, Metens T, Devière J, Delhaye M, Le Moine O, Cremer M. Pancreas divisum: evaluation with secretin-enhanced magnetic resonance cholangiopancreatography. Gastrointest Endosc. 2001;53:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
P- Reviewers Lee KT, Motoo Y S- Editor Wen LL L- Editor Ma JY E- Editor Ma S