Published online Aug 14, 2013. doi: 10.3748/wjg.v19.i30.4841
Revised: April 15, 2013
Accepted: July 17, 2013
Published online: August 14, 2013
Processing time: 216 Days and 15.5 Hours
Acoustic radiation force impulse (ARFI) imaging is a new and promising ultrasound-based diagnostic technique that, evaluating the wave propagation speed, allows the assessment of the tissue stiffness. ARFI is implemented in the ultrasound scanner. By short-duration acoustic radiation forces (less than 1 ms), localized displacements are generated in a selected region of interest not requiring any external compression so reducing the operator dependency. The generated wave scan provides qualitative or quantitative (wave velocity values) responses. Several non-invasive methods for assessing the staging of fibrosis are used, in order to avoid liver biopsy. Liver function tests and transient elastography are non-invasive, sensitive and accurate tools for the assessment of liver fibrosis and for the discrimination between cirrhotic and non-cirrhotic liver. Many published studies analyse ARFI performance and feasibility in studying diffuse liver diseases and compare them to other diagnostic imaging modalities such as conventional ultrasonography and transient elastography. Solid focal liver lesions, both benign and malignant, are common findings during abdominal examinations. The accurate characterization and differential diagnosis are important aims of all the imaging modalities available today. Only few papers describe the application of ARFI technology in the study of solid focal liver lesions, with different results. In the present study, the existing literature, to the best of our knowledge, about ARFI application on diffuse and focal liver pathology has been evaluated and results and statistical analyses have been compared, bringing to the conclusion that ARFI can be used in the study of the liver with similar accuracy as transient elastography in diagnosing significant fibrosis or cirrhosis and has got some advantages in respect to transient elastography since it does not require separate equipment, better displays anatomical structures and measurements can be successfully carried out almost in every patient.
Core tip: In the present study, the existing literature, to the best of our knowledge, about acoustic radiation force impulse (ARFI) application on diffuse and focal liver pathology has been evaluated and results and statistical analyses have been compared, bringing to the conclusion that ARFI can be used in the study of the liver with similar accuracy than transient elastography in diagnosing significant fibrosis or cirrhosis and has got some advantages in respect to transient elastography since it does not require separate equipment, better displays anatomical structures and measurements can be successfully carried out almost in every patient.
- Citation: D’Onofrio M, Crosara S, De Robertis R, Canestrini S, Demozzi E, Gallotti A, Mucelli RP. Acoustic radiation force impulse of the liver. World J Gastroenterol 2013; 19(30): 4841-4849
- URL: https://www.wjgnet.com/1007-9327/full/v19/i30/4841.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i30.4841
Acoustic radiation force impulse (ARFI) imaging is a new and promising ultrasound-based diagnostic technique that, evaluating the wave propagation speed, allows the assessment of the tissue stiffness[1-3]. ARFI is implemented in the ultrasound scanner and by using a conventional probe, without any need for external compression so reducing the operator dependency, it evaluates deep tissues stiffness providing complementary informations potentially useful for the diagnosis[1-6]. By short-duration acoustic radiation forces (less than 1 ms), it generates localized displacements in a selected region of interest (ROI; a box with dimension of 1 cm × 0.5 cm), identified on a conventional B-mode (Figure 1) image[7,8]. Depending on the interactions with the transducer[8,9], the generated wave scan provides qualitative (imaging) or quantitative (wave velocity values, measured in m/s) responses, by Virtual Touch Tissue Imaging and Virtual Touch Tissue Quantification, respectively (Siemens, Erlangen, Germany).
Biopsy provides an extremely valuable contribution to the assessment of liver status in the case of chronic disease, offering information both on fibrosis and necro-inflammatory activity. However, not only the risk of complications, which have been reported with a frequency of 5%-20% for minor complications and 0.3%-0.5% for major complications[10] including also exceptional cases of death, but also contraindications, such as coagulopathy, poor patients cooperation or lack of consent, tend to limit its use, especially for repeated use over time. Furthermore, insufficient sampling and inter-observer variability may occur[11].
Considerable efforts have been made to develop non-invasive methods for assessing the staging of fibrosis, in order to avoid liver biopsy. In this setting the ideal method should be simple, inexpensive, easily available, repeatable and accurate.
Liver function tests [alanine aminotransferase (ALT), aspartate aminotransferase (AST), total proteins, serum albumin, gamma-globulins, gamma glutamyl transpeptidase (GGT), total bilirubin, alkaline phosphatase, prothrombin time/international normalized ratio] can be performed prior to the liver biopsy. The 2 main scoring systems used to predict and evaluate liver fibrosis are AST-platelet ratio index (AST level and platelet count) and FIBROMAX (Biopredictive, France) that combines the measurement of 10 indirect parameters adjusted for age, sex, weight and height: α2-macroglobulin, haptoglobin, apolipoprotein A1, total bilirubin, GGT, ALT, AST, fasting glucose, triglycerides and total cholesterol.
Transient elastography (TE) (Fibroscan, Echosense, Paris, France) has proved to be a non-invasive, sensitive and accurate tool for the assessment of liver fibrosis and particularly for the discrimination between cirrhotic and non-cirrhotic liver and its use is rapidly spreading. However, since it requires separate equipment, it means that at least one other examination is necessary in addition to conventional ultrasonography (US) of the liver, requiring additional time and costs after B-mode ultrasonography. Moreover, during TE examination, only A-mode imaging is displayed on the screen in order to select the area of scanning and, consequently, ligaments, vascular structures or even lesions, may inadvertently be included in the ROI, possibly affecting the final results.
ARFI imaging offers the possibility of performing a quantitative measurement of the elasticity of the hepatic parenchyma during conventional US evaluations, without requiring additional transducers or other equipment[7].
Many studies analyse ARFI performance in studying diffuse liver diseases. Piscaglia et al[12] show that Virtual Touch Tissue Quantification is able to identify the presence of cirrhosis with good accuracy and produces results correlated with those obtained by transient elastography with Fibroscan. They performed measurement in the right lobe, by means of an intercostal scan, a condition which offers high inter-observer reproducibility (r = 0.874 in their series[12]).
The great advantage of fibrosis assessment using Virtual Touch Tissue Quantification is the fact that it can be performed at the same time as conventional US investigation. US is routinely used worldwide in the management of patients with chronic liver disease and is the first imaging technique employed when liver disease is suspected. With conventional US, certain features are highly specific for predicting severe fibrosis or cirrhosis (surface nodularity: specificity 95%; caudate lobe hypertrophy: 91%), but are not very sensitive (sensitivity of 54% and 41% respectively)[13]. Piscaglia et al[12] affirm that results in performing ARFI imaging have found to be similar to those of other works, all of which showed an area under the receiver operating characteristic curve (AUROC) above 0.9 for the diagnosis of cirrhosis with a cut-off value of 1.77 m/s (sensitivity 93%, specificity 85.1%). This cut-off value is very similar to those reported by Friedrich-Rust et al[7] (1.75 m/s), Sporea et al[14] and Takahashi et al[15], but differs from the ones obtained in other studies[16,17] that reported slightly higher thresholds. In their series the good performance of the ARFI technique with the previously reported cut-off value was also confirmed by the testing in a population with cirrhosis proven by biopsy as the reference standard.
Other works compare ARFI imaging to TE by means of Fibroscan, such as the one from Colombo et al[18]. They similarly found that TE and ARFI are both highly effective in diagnosing cirrhosis, but they came to the conclusion that TE is probably more accurate in predicting significant fibrosis (AUROC of TE 0.897, AUROC of ARFI 0.815), although they could not demonstrate a statistically significant difference between the two curves. Their results were consistent with Boursier et al[19] and Lupsor et al[16] who found the same diagnostic accuracy for cirrhosis, but better performance of TE in predicting significant fibrosis (F2 or higher), but were at variance with three studies which instead found similar accuracies of TE and ARFI in diagnosing significant fibrosis[7,20,21].
Another interesting finding was that Virtual Touch Tissue Quantification measurements could be successfully carried out in all patients enrolled[12], while TE was unsuccessful in 7% of cases (e.g., in patients with narrow intercostal spaces and in those with morbid obesity), as reported also in literature[22-26]. A possible explanation for this is that Virtual Touch Tissue Quantification may not be limited by narrow intercostal spaces or even by moderate excess weight, as it only requires the visible liver is not deeper than a fixed distance from the skin surface (in order to put the ROI in the parenchyma), while with TE the liver must not be more than 25 mm from the skin.
Regarding steatosis and inflammatory changes in diffuse liver disease, there is no agreement in the possible use of ARFI in diagnosing these parenchymal changes and in the effects of these changes themselves on ARFI measurements[20,27,28]. It seems unlikely that changes that minimally affect the parenchymal stiffness will be at this moment accurately depicted and diagnosed by using this non invasive technique.
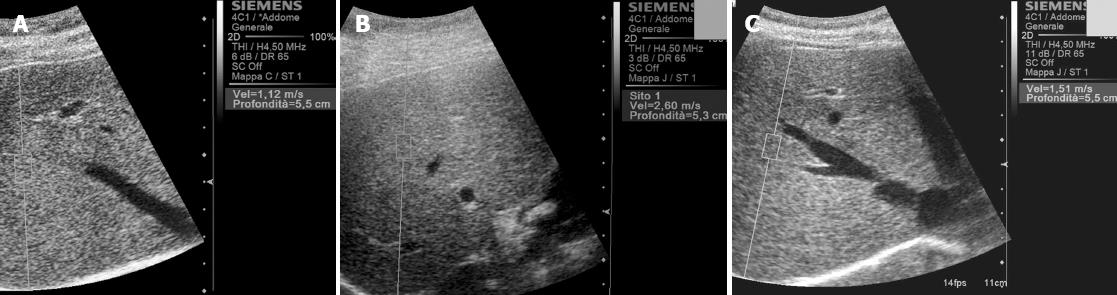
Mean normal values and mean values indicating severe fibrosis (Table 1) range about 0.8-1.7 m/s (Figure 2A) and about 1-3.4 m/s (Figure 2B) respectively.
| Ref. | Value (m/s) |
| The right lobe of healthy liver | |
| Eiler et al[33] | 1.16 |
| Sporea et al[48] | 1.19 |
| Karlas et al[49] | 1.19 |
| Jaffer et al[50] | 1.12 |
| Marginean et al[51] | 1.18 ± 0.27 |
| Crespo et al[52] | 1.06 |
| Kircheis et al[53] | 1.09 ± 0.13 |
| Yoon et al[54] | 1.06 |
| Colombo et al[18] | 1.40 |
| Sporea et al[55] | 1.28 ± 0.43 |
| Noruegas et al[56] | 1.11 |
| Rizzo et al[57] | 0.99 |
| Karlas et al[58] | 1.15 ± 0.17 |
| Sporea et al[59] | 0.97 ± 0.19 |
| Son et al[60] | 1.07 ± 0.11 |
| Rifai et al[61] | 1.10 ± 0.17 |
| Kuroda et al[62] | 0.99 ± 0.21 |
| Popescu et al[63] | 1.15 ± 0.21 |
| Piscaglia et al[12] | 1.13 |
| Toshima et al[64] | 1.15 |
| Horster et al[65] | 1.19 |
| Goertz et al[66] | 1.16 ± 0.11 |
| Goertz et al[35] | 1.09 |
| D'Onofrio et al[32] | 1.56 |
| Fierbinteanu-Braticevici et al[17] | < 1.185 (cut-off) |
| Friedrich-Rust et al[7] | 1.10 |
| A liver with severe fibrosis (> F3) | |
| Ye et al[67] | 1.69 |
| Sporea et al[48] | 1.43 |
| Karlas et al[49] | 1.43 |
| Chen et al[68] | 2.43 ± 0.13 |
| Sporea et al[69] | 1.60 ± 0.49 HBV; 1.55 ± 0.63 HCV |
| Crespo et al[52] | 1.77 |
| Kircheis et al[53] | 1.44 + 0.26 |
| Yoon et al[54] | 1.89 |
| Friedrich-Rust et al[70] | 1.55 |
| Colombo et al[18] | 1.44 |
| Sporea et al[55] | 1.64 ± 0.51 |
| Noruegas et al[56] | 1.48 |
| Rizzo et al[57] | 1.70 |
| Karlas et al[58] | 1.70 if non-viral |
| Sporea et al[59] | 1.71 ± 0.52 |
| Kuroda et al[62] | 1.61 ± 0.79 F3; 2.35 ± 1.11 F4 |
| Toshima et al[64] | 1.88 |
| Sporea et al[21] | 1.78 ± 0.77 |
| Fierbinteanu-Braticevici et al[17] | > 1.54 (cut-off) |
| Takahashi et al[15] | 2.57 ± 0.52 mean value for F4 (cut-off > F3 = 1.44) |
| Lupsor et al[16] | 1.520 ± 0.575 |
| Friedrich-Rust et al[7] | 1.64 |
Fierbinteanu-Braticevici et al[17] demonstrated that ARFI elastography could predict fibrosis of F2 METAVIR stage or higher with a validity of 90.2%. They calculated the optimal cut-off point between stages F1 and F2 to be 1.21 m/s. At this value, ARFI elastography had a sensitivity of 89.4% and a specificity of 100%. In their work, they also demonstrated that ARFI can predict even better F3 and F4 stage fibrosis. The optimal cut-off value to identify fibrosis stage F3 or higher was 1.54 m/s, with sensitivity and specificity of 97% and 100% respectively. The optimal cut-off value in predicting cirrhosis (stage F4) was 1.94 m/s with a sensitivity of 100% and a specificity of 98.1%.
In chronic viral hepatitis, the knowledge of the stage of liver fibrosis is important for prognosis and for decisions about antiviral treatment[29]. Fibrosis staged higher than F2 is an indicator for antiviral treatment, hence the great therapeutic value of a highly accurate diagnostic test. Moreover, early detection of significant fibrosis (F3 or higher) is essential since patients with significant fibrosis are at high risk of developing complications, such as portal hypertension or hepatocellular carcinoma, and consequently need specific follow-up[17].
At present, it’s difficult to determine the real impact of ARFI in the early diagnosis of hepatic fibrosis[17,27]. According to Fierbinteanu-Braticevici et al[17], in fact, there is a values overlap between F0-F1 and F2 fibrosis stages. The increase in liver propagation velocity has been demonstrated to be more important between stages F2 and F3 than between F1 and F2. This is consistent with the fact that the increase in fibrous tissue is more important between stages F2 and F3 than between F1 and F2. This limit of ARFI was overcome by the fact that fibrosis staged F2 or higher is considered a hallmark of progressive liver disease, therefore these are the patients in which there is a stronger indication for treatment as compared with patients with no or mild fibrosis[30,31].
There is in fact a range of variability of normal and pathological values in the Literature (Table 1). So what is important is to give the correct task to this new technique at present. The correct use of this technique has to be based on the true possibility of this system to detect changes in liver stiffness related to the development of different amount of fibrosis. The risk that absolutely should be avoided is to overestimate pathology and to look for inconsistent diseases. Therefore, in conclusion, the normal cut-off values must not be too strict but perhaps they also have to be adapted from time to time in relation to clinical and technical setting and from measurement to measurement.
In example, variability in the normal value is reported in literature[32] with a mean value of about 1.5 m/s in healthy subjects. This result can be considered an outlier[33] but however possible (Figure 2C). Moreover higher values can be obtained measuring in the left lobe[12,32] and in the superficial part of the right lobe[32]. This last aspect can be contrarily absent in child[33] due to a lower age-related fibrosis in the superficial liver parenchyma. Also in other published series Virtual Touch Tissue Quantification results in the right and left liver lobes did not appear to be strictly similar and, on average, the stiffness values were found to be higher in the left lobe than in the right lobe, at least in patients with chronic hepatitis (68% of patients had higher values in the left lobe than in the right lobe). Furthermore the diagnostic capacity to establish the histological degree of liver fibrosis (with a reference biopsy taken in the right lobe) was lower in Virtual Touch Tissue Quantification measurements from the left lobe than from the right lobe. These data, however, are not to be considered as a limitation of Virtual Touch Tissue Quantification to date, since they may perhaps more correctly reflect real differences and heterogeneity in the disease progression rates between the two lobes. It was in fact demonstrated that when two biopsies were taken in the two lobes during laparoscopy, a difference in one fibrosis stage between the two lobes occurred in up to 33% of cases[34]. However since our reference standard for the assessment of fibrosis in chronic liver disease is biopsy of the right lobe of the liver, it is recommended to measure liver stiffness by Virtual Touch Tissue Quantification in this lobe. Moreover, an approach with multiple measurements in various liver sites is worthy of further investigation as it may lead to interesting and original diagnostic results. In addition, Goertz et al[35] suggest to perform Virtual Touch Tissue Quantification via intercostal access in order to minimize invalid measurements and standard deviation. In their series, in fact, values taken subcostally were slightly higher than those measured through an intercostal approach.
Also some technical aspects need to be taken into account because they may explain some variability among published data. In example, the new release of the system is based on two acoustic pulses laterally to the ROI one by one at both sides and the maximum depth of the system nowadays achievable is 8 cm. Based on these considerations, the data published in the more recent papers should be more indicative of what can be obtainable with the new systems.
A recent study by Han et al[36], compares ARFI performance to Doppler parameters and describes a weak but significant relationship between liver stiffness, measured by ARFI, and the parameters related to the portal pressure, as measured by Doppler US in patients with liver cirrhosis at different Child-Pugh stages, but having no oesophageal varices. The study demonstrates a positive correlation between the median ARFI sonoelastographic velocity, which reveals liver stiffness, and the flow parameters of Doppler US, which reflects portal hypertension. All these features, however, appear in advanced stage of disease.
Regarding the possible role of ARFI, it can be for sure employed in the follow up of cirrhotic patients in order to avoid multiple biopsies comparing the result before and after treatment. Liver biopsy is not suitable for repeated evaluations because it is invasive and can cause major complications (0.3%-0.5%)[37]. Moreover liver fibrosis is a sequential and continuous process, and the staging of liver fibrosis should be evaluated frequently (Table 2). In contrast to liver biopsy, ARFI imaging is not invasive and can be repeated many times in the same patient[27].
| AUROC | Ref. | |
| Laboratory test (APRI score) | 0.80 | Lin et al[71] |
| 0.76 | Friedrich-Rust et al[7] | |
| 0.84 | Leroy et al[72] | |
| Transient elastography | 0.87 | Boursier et al[73] |
| 0.96 | Ferraioli et al[74] | |
| 0.90 | Friedrich-Rust et al[7] | |
| Acoustic radiation force impulse | 0.91 | Friedrich-Rust et al[7] |
| 0.90 | Lupsor et al[16] | |
| 0.99 | Fierbinteanu-Braticevici et al[17] |
Solid focal liver lesions are common findings during abdominal examinations. The accurate characterization and differential diagnosis are important aims of all the imaging modalities available today[38-43]. Only three papers describe the application of ARFI technology in the study of solid focal liver lesions, with different results[44-46].
The first human images of hepatic malignancies acquired in vivo using the ARFI technique or any other elasticity imaging technique appeared in the work of Fahey et al[44]. His group compared B-mode and ARFI images both qualitatively (assessing the lesion margins definition by B-mode ultrasonography and ARFI imaging) and quantitatively (comparing the images contrast for both the techniques). They came to the conclusion that lesions margins definition at ARFI imaging was superior to that seen at B-mode US imaging (qualitative analysis). They also calculated that ARFI imaging can provide improvements in defining the contrast of tissue masses demonstrating, in fact, that the mean contrast for suspected hepatocellular carcinoma (HCC) in B-mode imaging was 2.9 dB (range 1.5-4.2 dB) vs 7.5 dB (range 3.1-11.9 dB) in ARFI images, with all HCCs appearing less stiff (brighter) than regional cirrhotic non-tumorous liver parenchyma. Moreover, the mean contrast for hepatic metastases in B-mode images was 3.1 dB (range 1.2-5.2 dB) vs 9.3 dB (range 5.7-13.9 dB) in ARFI images. Metastatic lesions in fact are stiffer (darker) than regional non-cirrhotic, non-neoplastic liver parenchyma. Fahey et al[44] also stated that combined US/ARFI could find application in tumor screening, lesion characterization and early detection of disease. Since HCC screening is not considered cost-effective in regions with low prevalence, due to the low sensitivity of both sonography and serum AFP sampling[47], ARFI imaging can improve sensitivity and cost-efficiency given its low cost, its capability of improving tumor contrast in comparison to US alone. If ARFI imaging had been proven to be a feasible alternative to contrast-enhanced ultrasound for liver applications, it could hold potential advantages related to the cost and complexity of the imaging protocol used for HCC screening. The authors, however, did not take into account the mechanical response of benign abdominal masses to applied radiation forces, thus they couldn’t evaluate the ability of ARFI in differentiating benign from malignant liver masses.
More recent works about ARFI imaging applied to solid focal liver lesions are the ones from Cho et al[45] and from Gallotti et al[46]. The first one evaluates ARFI values calculated on HCCs, metastases, cholangiocarcinomas and hemangiomas, the second one evaluated in addition adenomas and focal nodular hyperplasia (FNHs), but did not consider cholangiocarcinomas.
In regard to hemangiomas, Gallotti et al[46] agree with Cho et al[45] about the great variability of this type of lesions (mean wave velocity value of the lesion 2.30 m/s; mean wave velocity value of the surrounding parenchyma 1.45 m/s), since its stiffness depends on the amount of fibrotic septa which divide the dilated vascular space.
For the first time in Gallotti et al[46] paper also FNHs and adenomas were studied. FNH resulted the stiffest lesions after metastases and cholangiocarcinomas, indipendently from their dimensions and from the presence or absence of central scar. In fact, even if present, the ROI has to be located out of the fibrotic central scar. The high stiffness (mean wave velocity value of the lesion 2.75 m/s; mean wave velocity value of the surrounding parenchyma 1.57 m/s[46]) is explained with the well known high fibrotic content of this type of lesion. Thus, if the result will be confirmed by further studies, the cut-off of 2 m/s, suggested by Cho et al[45] to distinguish benign from malignant lesions, can no longer be used.
On the other hand, adenomas showed wave velocity values similar to those observed in the surrounding liver[46]: this is a solid focal liver lesion, the softest analysed (mean wave velocity value of the lesion 1.25 m/s; mean wave velocity value of the surrounding parenchyma 1.40 m/s). The presence of cells similar to normal hepatocytes and few stroma explain the low mean wave velocity value calculated in adenomas compared to other focal liver lesions.
According to Fahey et al[44], but inconsistent with results of Cho et al[45] despite the similar diameter of the lesions, in Gallotti et al[46] almost all the HCCs evaluated resulted in softer lesions compared to the surrounding cirrhotic liver (mean wave velocity value of the lesion 2.17 m/s; mean wave velocity value of the surrounding parenchyma 2.99 m/s), and the main elastographic value was significantly lower than that of the surrounding parenchyma. This discrepancy might be explained by the difference in the severity of the cirrhosis of the background liver in each study population. In Cho et al[45] the degree of liver cirrhosis of patients with HCCs was likely to be less severe (15 out of 20 patients with HCCs had chronic liver disease of Child-Pugh classification A) compared with that seen in the other studies, assuming that the liver is stiffer with more severe liver cirrhosis.
There is concordance[44-46] about the fact that all metastatic lesions (mean wave velocity value of the lesion 2.87 m/s; mean wave velocity value of the surrounding parenchyma 1.63 m/s[46]) and, when considered, cholangiocarcinomas, are stiffer than the surrounding liver. This is probably due to the presence of fibrous content potentially found in many of these lesions. The presence of necrotic degeneration, mainly in the biggest masses, does not influence the results since the ROI for the stiffness calculation has to be accurately positioned out of the necrotic portion. Summarizing, based on the preliminary results of the study of solid focal liver lesions[44-46], it can be also concluded that ARFI seems to be an useful in the following scenarios: (1) for differential diagnosis between adenomas and FNHs; (2) for the study of metastases; and (3) for the study of HCCs in cirrhotic liver. Future perspective could be the application of ARFI in liver lesion detection by using volumetric automated acquisition.
Virtual Touch Tissue Quantification is a new non invasive imaging based technique able to estimate liver stiffness diagnosing cirrhosis with a good accuracy. The first assessment of patients with a suspicion of liver disease can be therefore easily performed with both conventional ultrasonography and Virtual Touch Tissue Quantification for liver stiffness assessment in a single step.
In conclusion, several studies about ARFI application in diffuse liver pathology have been made, and most of them state that ARFI itself can be used in the study of the liver with similar accuracy than transient elastography in diagnosing significant fibrosis[7,20,21] or cirrhosis[12,16,19]. However, ARFI has got some advantages in respect to TE since it does not require separate equipment and consequently it is not necessary an additional examination in addition to conventional US, saving time and costs. Moreover, during TE examination, only A-mode imaging is displayed on the screen in order to select the area of scanning and, consequently, ligaments, vascular structures or even lesions, may inadvertently be included in the ROI, possibly affecting the final results.
Another interesting finding is that Virtual Touch Tissue Quantification measurements can be successfully carried out almost in every patient while TE is unsuccessful in 7% of cases (e.g., in patients with narrow intercostal spaces and in those with morbid obesity), as reported also in literature[22-26]. On the contrary, there are just few indications about ARFI and focal liver lesions, so further studies are needed in order to find ARFI the correct place in the everyday clinical practice.
| 1. | Fahey BJ, Palmeri ML, Trahey GE. The impact of physiological motion on tissue tracking during radiation force imaging. Ultrasound Med Biol. 2007;33:1149-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Nightingale K, Bentley R, Trahey G. Observations of tissue response to acoustic radiation force: opportunities for imaging. Ultrason Imaging. 2002;24:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | McAleavey SA, Menon M, Orszulak J. Shear-modulus estimation by application of spatially-modulated impulsive acoustic radiation force. Ultrason Imaging. 2007;29:87-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Cross TJ, Mitchell JD, Cramp ME. Elastography for the non-invasive assessment of liver disease: limitations and future developments. Gut. 2009;58:1171-1172; author reply 1172. [PubMed] |
| 5. | Nightingale K, McAleavey S, Trahey G. Shear-wave generation using acoustic radiation force: in vivo and ex vivo results. Ultrasound Med Biol. 2003;29:1715-1723. [PubMed] |
| 6. | Nightingale KR, Palmeri ML, Nightingale RW, Trahey GE. On the feasibility of remote palpation using acoustic radiation force. J Acoust Soc Am. 2001;110:625-634. [PubMed] |
| 7. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 8. | Zhai L, Palmeri ML, Bouchard RR, Nightingale RW, Nightingale KR. An integrated indenter-ARFI imaging system for tissue stiffness quantification. Ultrason Imaging. 2008;30:95-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (4)] |
| 9. | Palmeri ML, Wang MH, Dahl JJ, Frinkley KD, Nightingale KR. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med Biol. 2008;34:546-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 444] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 10. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1408] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 11. | Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523-525. [PubMed] |
| 12. | Piscaglia F, Salvatore V, Di Donato R, D’Onofrio M, Gualandi S, Gallotti A, Peri E, Borghi A, Conti F, Fattovich G. Accuracy of VirtualTouch Acoustic Radiation Force Impulse (ARFI) imaging for the diagnosis of cirrhosis during liver ultrasonography. Ultraschall Med. 2011;32:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Sporea I, Sirli RL, Deleanu A, Popescu A, Focsa M, Danila M, Tudora A. Acoustic radiation force impulse elastography as compared to transient elastography and liver biopsy in patients with chronic hepatopathies. Ultraschall Med. 2011;32 Suppl 1:S46-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Takahashi H, Ono N, Eguchi Y, Eguchi T, Kitajima Y, Kawaguchi Y, Nakashita S, Ozaki I, Mizuta T, Toda S. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303-310. [PubMed] |
| 17. | Fierbinteanu-Braticevici C, Andronescu D, Usvat R, Cretoiu D, Baicus C, Marinoschi G. Acoustic radiation force imaging sonoelastography for noninvasive staging of liver fibrosis. World J Gastroenterol. 2009;15:5525-5532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (2)] |
| 18. | Colombo S, Buonocore M, Del Poggio A, Jamoletti C, Elia S, Mattiello M, Zabbialini D, Del Poggio P. Head-to-head comparison of transient elastography (TE), real-time tissue elastography (RTE), and acoustic radiation force impulse (ARFI) imaging in the diagnosis of liver fibrosis. J Gastroenterol. 2012;47:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 19. | Boursier J, Isselin G, Fouchard-Hubert I, Oberti F, Dib N, Lebigot J, Bertrais S, Gallois Y, Calès P, Aubé C. Acoustic radiation force impulse: a new ultrasonographic technology for the widespread noninvasive diagnosis of liver fibrosis. Eur J Gastroenterol Hepatol. 2010;22:1074-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 21. | Sporea I, Sirli R, Popescu A, Danilă M. Acoustic Radiation Force Impulse (ARFI)--a new modality for the evaluation of liver fibrosis. Med Ultrason. 2010;12:26-31. [PubMed] |
| 22. | Friedrich-Rust M, Schwarz A, Ong M, Dries V, Schirmacher P, Herrmann E, Samaras P, Bojunga J, Bohle RM, Zeuzem S. Real-time tissue elastography versus FibroScan for noninvasive assessment of liver fibrosis in chronic liver disease. Ultraschall Med. 2009;30:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D, Bertet J, Couzigou P, de Lédinghen V. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411-412. [PubMed] |
| 24. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 661] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 25. | Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, Milani S, Lorefice E, Petrarca A, Romanelli RG. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57:1288-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] |
| 27. | Ebinuma H, Saito H, Komuta M, Ojiro K, Wakabayashi K, Usui S, Chu PS, Umeda R, Ishibashi Y, Takayama T. Evaluation of liver fibrosis by transient elastography using acoustic radiation force impulse: comparison with Fibroscan(®). J Gastroenterol. 2011;46:1238-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 28. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 985] [Article Influence: 61.6] [Reference Citation Analysis (1)] |
| 29. | Chon YE, Choi EH, Song KJ, Park JY, Kim do Y, Han KH, Chon CY, Ahn SH, Kim SU. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One. 2012;7:e44930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 30. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 545] [Article Influence: 30.3] [Reference Citation Analysis (2)] |
| 31. | Poynard T, Munteanu M, Imbert-Bismut F, Charlotte F, Thabut D, Le Calvez S, Messous D, Thibault V, Benhamou Y, Moussalli J. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 32. | D’Onofrio M, Gallotti A, Mucelli RP. Tissue quantification with acoustic radiation force impulse imaging: Measurement repeatability and normal values in the healthy liver. AJR Am J Roentgenol. 2010;195:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (2)] |
| 33. | Eiler J, Kleinholdermann U, Albers D, Dahms J, Hermann F, Behrens C, Luedemann M, Klingmueller V, Alzen GF. Standard value of ultrasound elastography using acoustic radiation force impulse imaging (ARFI) in healthy liver tissue of children and adolescents. Ultraschall Med. 2012;33:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1581] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 35. | Goertz RS, Zopf Y, Jugl V, Heide R, Janson C, Strobel D, Bernatik T, Haendl T. Measurement of liver elasticity with acoustic radiation force impulse (ARFI) technology: an alternative noninvasive method for staging liver fibrosis in viral hepatitis. Ultraschall Med. 2010;31:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 36. | Han JY, Cho JH, Kwon HJ, Nam KJ. Predicting portal hypertension as assessed by acoustic radiation force impulse: correlations with the Doppler ultrasound. Br J Radiol. 2012;85:e404-e409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (3)] |
| 37. | Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology. 2002;36:S152-S160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Assy N, Nasser G, Djibre A, Beniashvili Z, Elias S, Zidan J. Characteristics of common solid liver lesions and recommendations for diagnostic workup. World J Gastroenterol. 2009;15:3217-3227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Trillaud H, Bruel JM, Valette PJ, Vilgrain V, Schmutz G, Oyen R, Jakubowski W, Danes J, Valek V, Greis C. Characterization of focal liver lesions with SonoVue-enhanced sonography: international multicenter-study in comparison to CT and MRI. World J Gastroenterol. 2009;15:3748-3756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 134] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (2)] |
| 40. | Vilgrain V. Advancement in HCC imaging: diagnosis, staging and treatment efficacy assessments: hepatocellular carcinoma: imaging in assessing treatment efficacy. J Hepatobiliary Pancreat Sci. 2010;17:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Hohmann J, Albrecht T, Hoffmann CW, Wolf KJ. Ultrasonographic detection of focal liver lesions: increased sensitivity and specificity with microbubble contrast agents. Eur J Radiol. 2003;46:147-159. [PubMed] |
| 42. | Numminen K, Isoniemi H, Halavaara J, Tervahartiala P, Makisalo H, Laasonen L, Hockerstedt K. Preoperative assessment of focal liver lesions: multidetector computed tomography challenges magnetic resonance imaging. Acta Radiol. 2005;46:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 337] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 44. | Fahey BJ, Nelson RC, Bradway DP, Hsu SJ, Dumont DM, Trahey GE. In vivo visualization of abdominal malignancies with acoustic radiation force elastography. Phys Med Biol. 2008;53:279-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (3)] |
| 45. | Cho SH, Lee JY, Han JK, Choi BI. Acoustic radiation force impulse elastography for the evaluation of focal solid hepatic lesions: preliminary findings. Ultrasound Med Biol. 2010;36:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Gallotti A, D’Onofrio M, Romanini L, Cantisani V, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) ultrasound imaging of solid focal liver lesions. Eur J Radiol. 2012;81:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 47. | Yuen MF, Lai CL. Screening for hepatocellular carcinoma: survival benefit and cost-effectiveness. Ann Oncol. 2003;14:1463-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 48. | Sporea I, Bota S, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, Badea R, Lupsor M, Fierbinteanu-Braticevici C, Petrisor A. Acoustic Radiation Force Impulse elastography for fibrosis evaluation in patients with chronic hepatitis C: an international multicenter study. Eur J Radiol. 2012;81:4112-4118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 49. | Karlas T, Hempel M, Tröltzsch M, Huster D, Günther P, Tenckhoff H, Mössner J, Berg T, Keim V, Wiegand J. Non-invasive evaluation of hepatic manifestation in Wilson disease with transient elastography, ARFI, and different fibrosis scores. Scand J Gastroenterol. 2012;47:1353-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Jaffer OS, Lung PF, Bosanac D, Patel VM, Ryan SM, Heneghan MA, Quaglia A, Sidhu PS. Acoustic radiation force impulse quantification: repeatability of measurements in selected liver segments and influence of age, body mass index and liver capsule-to-box distance. Br J Radiol. 2012;85:e858-e863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Marginean CO, Marginean C. Elastographic assessment of liver fibrosis in children: A prospective single center experience. Eur J Radiol. 2012;81:e870-e874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Crespo G, Fernández-Varo G, Mariño Z, Casals G, Miquel R, Martínez SM, Gilabert R, Forns X, Jiménez W, Navasa M. ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. J Hepatol. 2012;57:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 53. | Kircheis G, Sagir A, Vogt C, Vom Dahl S, Kubitz R, Häussinger D. Evaluation of acoustic radiation force impulse imaging for determination of liver stiffness using transient elastography as a reference. World J Gastroenterol. 2012;18:1077-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 54. | Yoon KT, Lim SM, Park JY, Kim do Y, Ahn SH, Han KH, Chon CY, Cho M, Lee JW, Kim SU. Liver stiffness measurement using acoustic radiation force impulse (ARFI) elastography and effect of necroinflammation. Dig Dis Sci. 2012;57:1682-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 55. | Sporea I, Badea R, Sirli R, Lupsor M, Popescu A, Danila M, Focsa M, Deleanu A. How efficient is acoustic radiation force impulse elastography for the evaluation of liver stiffness. Hepat Mon. 2011;11:532-538. [PubMed] |
| 56. | Noruegas MJ, Matos H, Gonçalves I, Cipriano MA, Sanches C. Acoustic radiation force impulse-imaging in the assessment of liver fibrosis in children. Pediatr Radiol. 2012;42:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 57. | Rizzo L, Calvaruso V, Cacopardo B, Alessi N, Attanasio M, Petta S, Fatuzzo F, Montineri A, Mazzola A, L’abbate L. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2011;106:2112-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 58. | Karlas T, Pfrepper C, Wiegand J, Wittekind C, Neuschulz M, Mössner J, Berg T, Tröltzsch M, Keim V. Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of liver fibrosis: examination standards and evaluation of interlobe differences in healthy subjects and chronic liver disease. Scand J Gastroenterol. 2011;46:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (3)] |
| 59. | Sporea I, Sirli R, Bota S, Fierbinţeanu-Braticevici C, Petrişor A, Badea R, Lupşor M, Popescu A, Dănilă M. Is ARFI elastography reliable for predicting fibrosis severity in chronic HCV hepatitis. World J Radiol. 2011;3:188-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Son CY, Kim SU, Han WK, Choi GH, Park H, Yang SC, Choi JS, Park JY, Kim do Y, Ahn SH. Normal liver elasticity values using acoustic radiation force impulse imaging: a prospective study in healthy living liver and kidney donors. J Gastroenterol Hepatol. 2012;27:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Rifai K, Cornberg J, Mederacke I, Bahr MJ, Wedemeyer H, Malinski P, Bantel H, Boozari B, Potthoff A, Manns MP. Clinical feasibility of liver elastography by acoustic radiation force impulse imaging (ARFI). Dig Liver Dis. 2011;43:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 62. | Kuroda H, Kakisaka K, Tatemichi Y, Sawara K, Miyamoto Y, Oikawa K, Miyasaka A, Takikawa Y, Masuda T, Suzuki K. Non-invasive evaluation of liver fibrosis using acoustic radiation force impulse imaging in chronic hepatitis patients with hepatitis C virus infection. Hepatogastroenterology. 2010;57:1203-1207. [PubMed] |
| 63. | Popescu A, Sporea I, Sirli R, Bota S, Focşa M, Dănilă M, Nicoliţă D, Martie A, Sendroiu M, Juchiş A. The mean values of liver stiffness assessed by Acoustic Radiation Force Impulse elastography in normal subjects. Med Ultrason. 2011;13:33-37. [PubMed] |
| 64. | Toshima T, Shirabe K, Takeishi K, Motomura T, Mano Y, Uchiyama H, Yoshizumi T, Soejima Y, Taketomi A, Maehara Y. New method for assessing liver fibrosis based on acoustic radiation force impulse: a special reference to the difference between right and left liver. J Gastroenterol. 2011;46:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 65. | Horster S, Mandel P, Zachoval R, Clevert DA. Comparing acoustic radiation force impulse imaging to transient elastography to assess liver stiffness in healthy volunteers with and without valsalva manoeuvre. Clin Hemorheol Microcirc. 2010;46:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 66. | Goertz RS, Amann K, Heide R, Bernatik T, Neurath MF, Strobel D. An abdominal and thyroid status with Acoustic Radiation Force Impulse Elastometry--a feasibility study: Acoustic Radiation Force Impulse Elastometry of human organs. Eur J Radiol. 2011;80:e226-e230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 67. | Ye XP, Ran HT, Cheng J, Zhu YF, Zhang DZ, Zhang P, Zheng YY. Liver and spleen stiffness measured by acoustic radiation force impulse elastography for noninvasive assessment of liver fibrosis and esophageal varices in patients with chronic hepatitis B. J Ultrasound Med. 2012;31:1245-1253. [PubMed] |
| 68. | Chen SH, Li YF, Lai HC, Kao JT, Peng CY, Chuang PH, Su WP, Chiang IP. Effects of patient factors on noninvasive liver stiffness measurement using acoustic radiation force impulse elastography in patients with chronic hepatitis C. BMC Gastroenterol. 2012;12:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Sporea I, Sirli R, Bota S, Popescu A, Sendroiu M, Jurchis A. Comparative study concerning the value of acoustic radiation force impulse elastography (ARFI) in comparison with transient elastography (TE) for the assessment of liver fibrosis in patients with chronic hepatitis B and C. Ultrasound Med Biol. 2012;38:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212-e219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 71. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 815] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 72. | Leroy V, Halfon P, Bacq Y, Boursier J, Rousselet MC, Bourlière M, de Muret A, Sturm N, Hunault G, Penaranda G. Diagnostic accuracy, reproducibility and robustness of fibrosis blood tests in chronic hepatitis C: a meta-analysis with individual data. Clin Biochem. 2008;41:1368-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 73. | Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, Fouchard-Hubert I, Gallois Y, Oberti F, Bertrais S. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 522] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 74. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 517] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
P- Reviewers Diamantis I, Faintuch J, Maruyama H S- Editor Huang XZ L- Editor A E- Editor Li JY