Published online Jul 28, 2013. doi: 10.3748/wjg.v19.i28.4559
Revised: May 27, 2013
Accepted: June 1, 2013
Published online: July 28, 2013
Processing time: 103 Days and 18.2 Hours
AIM: To assess whole-body fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) in the management of small bowel obstructions (SBOs) secondary to gastric cancer and its role in treatment strategies.
METHODS: The medical records of all of the patients who were admitted for an intestinal obstruction after curative resection for gastric cancer were retrospectively reviewed. PET/CT was performed before a clinical treatment strategy was established for each patient. The patients were divided into 2 groups: patients with no evidence of a tumor recurrence and patients with evidence of a tumor recurrence. Tumor recurrences included a local recurrence, peritoneal carcinomatosis or distant metastases. The primary endpoint was the 1-year survival rate, and other variables included patient demographics, the length of hospital stay, complications, and mortality.
RESULTS: The median time between a diagnosis of gastric cancer and the detection of a SBO was 1.4 years. Overall, 31 of 65 patients (47.7%) had evidence of a tumor recurrence on the PET/CT scan, which was the only factor that was associated with poor survival. Open and close surgery was the main type of surgical procedure reported for the patients with tumor recurrences. R0 resections were performed in 2 patients, including 1 who underwent combined adjacent organ resection. In the group with no evidence of a tumor recurrence on PET/CT, bowel resections were performed in 7 patients, adhesiolysis was performed in 7 patients, and a bypass was performed in 1 patient. The 1-year survival curves according to PET/CT evidence of a tumor recurrence vs no PET/CT evidence of a tumor recurrence were significantly different, and the 1-year survival rates were 8.8% vs 93.5%, respectively. There were no significant differences (P = 0.71) in the 1-year survival rates based on surgical vs nonsurgical management (0% with nonoperative treatment vs 20% after exploratory laparotomy).
CONCLUSION: 18F-FDG PET/CT can be used to identify the causes of bowel obstructions in patients with a history of gastric cancer, and this method is useful for planning the surgical management of these patients.
Core tip: The management of patients who present with a small bowel obstruction (SBO) after treatment of primary carcinoma challenges the clinical judgement of even the most experienced surgeons when the feared cause is metastatic disease. It is difficult to predict whether the quality and/or the quantity of life in this group of patients will be improved by surgery. This study evaluated the clinical role of 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) in identifying SBOs and its role in subsequent clinical treatment strategies. We found that 18F-FDG PET/CT is an appropriate method to identity the causes of bowel obstructions secondary to gastric cancer, and this method is useful for the surgical management of these patients.
- Citation: Wu WG, Dong P, Wu XS, Li ML, Ding QC, Zhang L, Yang JH, Weng H, Ding Q, Tan ZJ, Lu JH, Gu J, Liu YB. Surgical management of patients with bowel obstructions secondary to gastric cancer. World J Gastroenterol 2013; 19(28): 4559-4567
- URL: https://www.wjgnet.com/1007-9327/full/v19/i28/4559.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i28.4559
An intestinal obstruction is a common problem in patients with an advanced malignancy. Approximately 3%-15% of all terminal cancer patients will develop an intestinal obstruction[1,2]. In advanced abdominal and pelvic malignancies, 5%-51% of patients with ovarian malignancies and 10%-28% of patients with gastrointestinal cancers will develop an intestinal obstruction[3-8]. An intestinal obstruction may be due to intra-abdominal adhesions, intra-abdominal hernias, local cancer recurrences, peritoneal carcinomatosis, or distant metastases from other tumors. Small bowel obstructions (SBOs) secondary to malignant disease are often a sign of end-stage disease and are associated with poor survival. The treatment of such patients presents a dilemma for the surgeon. Inappropriate surgery will not significantly improve morbidity and mortality outcomes and often has limited success in relieving symptoms. Nonoperative treatment is often ineffective at restoring bowel function, and when relief is obtained, early reobstruction frequently occurs[9,10]. The management of patients who present with a bowel obstruction after treatment of primary carcinoma challenges the clinical judgment of even the most experienced surgeons when the feared cause is metastatic or recurrent disease[11]. The management of these patients is difficult, and it is unclear which patients will benefit from surgery and which patients will have similar outcomes from medical management because many patients may have diffuse peritoneal metastatic disease and/or adhesions from previous surgery. It is difficult to predict whether the quality and/or the quantity of life in this group of patients will be improved by surgery because these patients have a poor prognosis at the time of presentation[12]. In addition, the management of these patients presents an additional difficulty because the intestinal obstruction may be due to more than one physiopathological process, such as an intraluminal obstruction from polypoid lesions that occlude the bowel lumen, an intramural obstruction from the infiltration of a tumor within the muscular coat of the bowel wall, and an extramural obstruction from mesenteric and omental masses and extrinsic compression from malignant adhesions.
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) detects the increased utilization of glucose by malignant cells to provide diagnostic information and is more accurate than conventional diagnostic methods in cases of primary and recurrent gastrointestinal tumors[12-15]. To date, the usefulness of integrated FDG PET/computed tomography (CT) in the treatment decisions for patients with bowel obstructions secondary to malignant disease has not been investigated. This study evaluated the clinical role of whole-body FDG PET/CT in the management of SBOs secondary to gastric cancer and its role in the formulation of subsequent clinical treatment strategies.
This retrospective chart review was approved by the institutional review board and was performed at the Department of General Surgery, Xinhua Hospital, School of Medicine, Shanghai Jiaotong University. A retrospective review of our electronic database was conducted to find all patients with a history of curative resection for gastric cancer who were admitted for an intestinal obstruction from August 1, 2008 to January 1, 2010. Adult patients with discharge diagnoses of bowel obstructions and gastric cancer were enrolled. Patients whose cancer was first diagnosed with the bowel obstruction and patients without radiographic confirmation of an obstruction were excluded. Patients with an early postoperative bowel obstruction, which is generally defined as a mechanical obstruction that occurs within 1 mo of abdominal surgery, were excluded. No patient with a bowel obstruction before a cancer diagnosis was included in this study. The diagnosis of a bowel obstruction was based on a combination of clinical signs and symptoms and radiologic findings. The enrolled patients had at least one of the following symptoms along with radiographic confirmation of an obstruction: nausea and vomiting, colicky pain, abdominal bloating, obstipation, or an inability to tolerate PO intake. Radiographic confirmation of the obstruction was usually by either plain abdominal films or a CT scan. In addition, at least one of the following findings was required: dilated loops of bowel with a paucity of air in the colon, air/fluid levels, or a transition from a dilated bowel to a decompressed bowel[16]. PET/CT was performed for each patient before the clinical treatment strategy was established. Based on the PET/CT results, the patients were divided into 2 groups: patients with no evidence of a tumor recurrence and patients with evidence of a tumor recurrence, which included a local recurrence, peritoneal carcinomatosis or distant metastases.
PET/CT imaging was performed using a GE Discovery ST 8-slice scanner. The patients were scanned after 6 h of fasting. Blood glucose levels were checked immediately before the scan. An average of 296-370 MBq (i.e., 8-10 mCi) FDG was injected intravenously, and whole-body images were obtained 1 h later. Low-dose CT images were used for attenuation correction. An oral contrast agent was administered to all of the patients for PET/CT imaging. A semiquantitative and visual analysis was made. The images were evaluated by 2 nuclear medicine specialists, and a consensus was required to prevent interobserver variability. The FDG uptake was defined as qualitatively positive when the focal FDG uptake was higher than the normal biodistribution of background FDG activity. In addition, to exclude the physiologic uptake, the FDG uptake in the bowel was considered positive only when wall thickening of the same bowel was simultaneously detected by CT. The PET/CT images were analyzed for the number and the sites with positive FDG uptake, and the standardized uptake value value of all of the positive FDG uptake values was measured.
The patients with an obstruction were divided into 2 treatment groups: patients who received conservative treatment and patients who underwent surgical management. The standard nonoperative management of small bowel obstructions consisted of fluid and electrolyte replacement, bowel rest, and tube decompression. A nonoperative course may be followed for 24-48 h. If the obstruction has not resolved within that time period, it is unlikely that the obstruction will ever resolve and laparotomy is usually advised. In the patients who underwent surgery for a bowel obstruction after curative resection of gastric cancer, the type of operation was determined by 3 expert gastrointestinal surgeons depending on the overall medical status of the patient, the wishes of the patient, and the abdominal examination. The primary endpoint of the analysis of surgical vs non-surgical treatment for the bowel obstructions in this study was the 1-year survival rate, and other recorded variables included patient demographics, the length of hospital stay, complications, and mortality. The modified Clavien system was used to grade any postoperative complications. In-hospital mortality was defined as the percentage of patients who died before hospital discharge. The length of hospital stay was defined as the number of days from the index procedure to hospital discharge. This study was approved by the Human Research Review Committee of our hospital.
Statistical analyses and graphics were generated using the SPSS 13.0 statistical package for Windows (SPSS, Inc., Chicago, IL, United States). If the P value was < 0.05, the results were considered statistically significant. Patency after palliation and the overall survival were estimated using the Kaplan-Meier actuarial method, and the curves were compared using the log-rank test. To identify the independent factors that influenced clinical success and the risk factors that were associated with the 1-year overall survival, univariate and multivariate analyses were performed. The results were expressed as the mean ± SD or as the percentages.
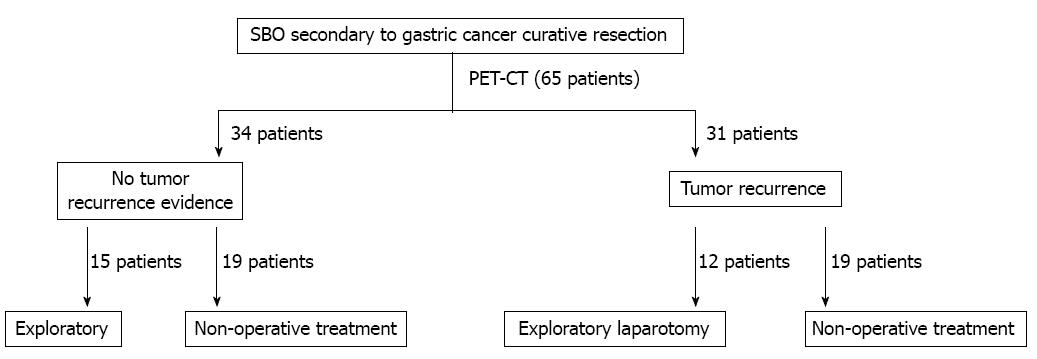
There were 72 cases of bowel obstructions in patients with a history of curative surgery for gastric cancer at our institution during the study period. Seven patients declined PET/CT imaging and were excluded from the analysis. The remaining 65 patients were all admitted for a SBO and were included in the analysis. The surgical decision-making process is shown in Figure 1. The average age at the time of the primary gastric cancer diagnosis was 62.5 ± 17.1 years. The mean age at admission for a SBO was 63.9 ± 15.6 years.
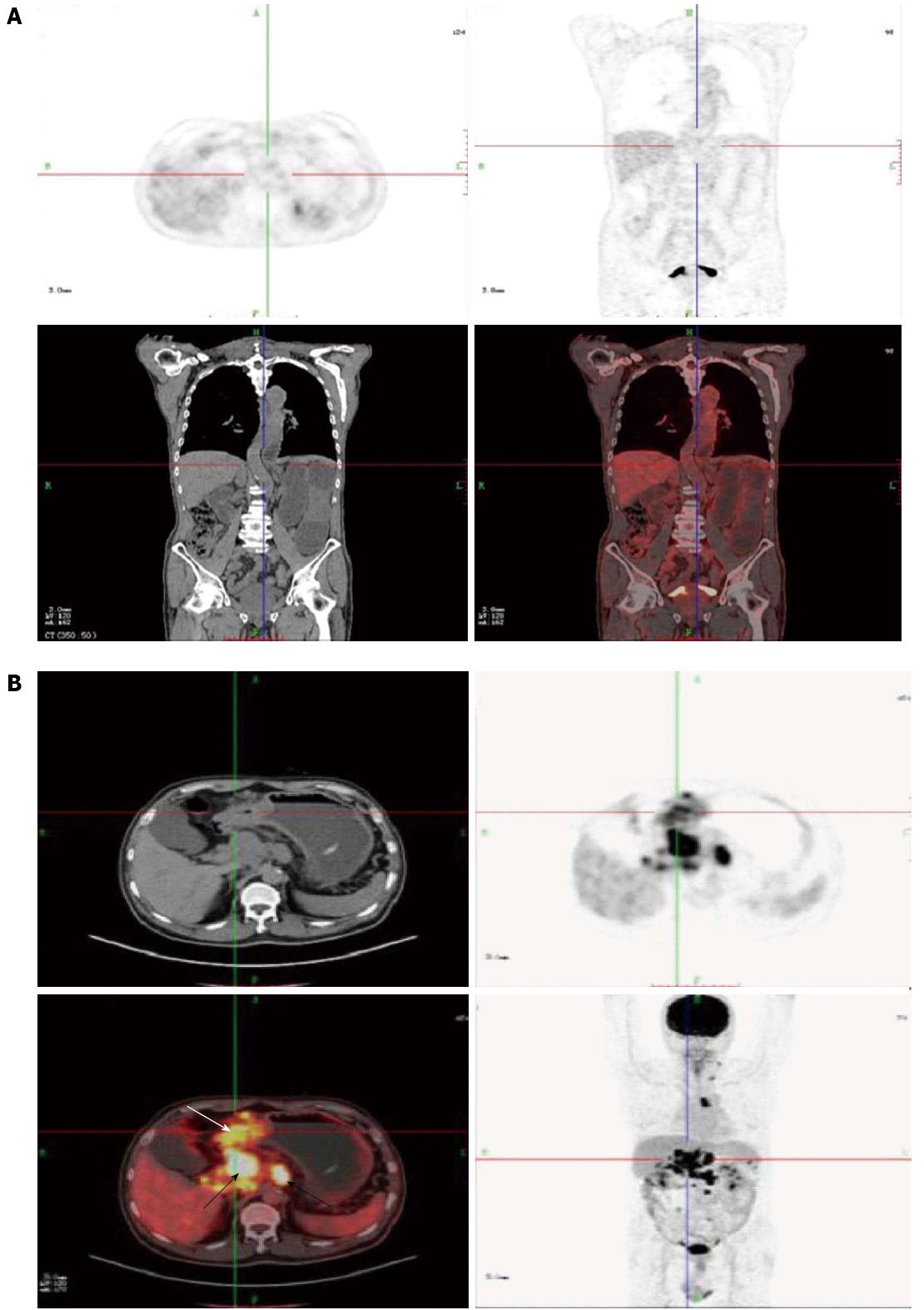
The median time between curative resection of gastric cancer and the detection of a SBO was 1.4 years. The clinicopathological data of the patients are listed in Table 1. Each patient underwent PET/CT before the final clinical treatment strategy was determined. PET/CT indicated that 31 patients (47.7%) had evidence of a tumor recurrence, including a local recurrence, peritoneal carcinomatosis, and distant metastases (Table 2; Figure 2A). The remaining 34 (52.3%) patients had no evidence of a tumor recurrence (Figure 2B). Both the univariate and multivariate analyses for factors that may have correlated with survival revealed that the only factor that was associated with poor survival was PET/CT evidence of a tumor recurrence (Table 3).
| Factors | Value |
| All | 65 (100.0) |
| Sex | |
| Female | 11 (16.39) |
| Male | 54 (83.1) |
| Age (yr) | |
| < 70 | 45 (69.2) |
| ≥ 70 | 20 (30.8) |
| Site | |
| Lower | 41 (63.1) |
| Middle | 8 (12.3) |
| Upper | 16 (24.6) |
| Diffuse | 0 (0.0) |
| Comorbidities | |
| Yes | 42 (64.6) |
| No | 23 (35.4) |
| Surgery | |
| Subtotal gastrectomy | 33 (50.8) |
| Total gastrectomy | 19 (29.2) |
| Extended total gastrectomy | 13 (20.0) |
| Types of digestive reconstruction | |
| Billroth I | 20 (30.8) |
| Billroth II | 15 (23.1) |
| Roux-en-Y | 30 (46.1) |
| Grading | |
| Well differentiated | 22 (33.8) |
| Moderately differentiated | 22 (33.8) |
| Poorly differentiated | 21 (32.4) |
| Undifferentiated | 0 (0.0) |
| T stage | |
| T1 | 0 (0.0) |
| T2 | 21 (32.3) |
| T3 | 38 (58.5) |
| T4 | 6 (9.2) |
| No. metastatic nodes | |
| N0 | 8 (12.3) |
| N1 | 22 (33.8) |
| N2 | 23 (35.4) |
| N3 | 12 (18.5) |
| M stage | |
| M0 | 65 (100.0) |
| M1 | 0 (0.0) |
| Intra-abdominal chemotherapy | |
| Yes | 35 (53.8) |
| No | 30 (46.2) |
| Postchemotherapy | |
| Yes | 55 (84.6) |
| No | 10 (15.4) |
| Recurrence in PET/CT | |
| Yes | 31 (47.7) |
| No | 34 (52.3) |
| Re-surgery | |
| Yes | 27 (41.5) |
| No | 38 (58.5) |
| Variables | n | SUV mean (range) | |
| Recurrence | Yes | 31 | 7.3 (2.6-28.3) |
| No | 34 | / | |
| Recurrence site | Locoregional recurrence | 2 | 6.8 (4.1-16.2) |
| (Remnant stomach or anastomosis site) | |||
| Distant metastasis | 29 | 7.9 (2.6-28.3) | |
| Lymph-node | 16 | 7.5 (2.6-28.3) | |
| Liver | 8 | 8.0 (2.8-15.5) | |
| lung | 6 | 7.1 (2.6-14.2) | |
| Other site (bone, skin, etc.) | 10 | 6.2 (3.2-12.5) | |
| Peritoneum | 12 | 5.4 (2.7-11.2) |
| Factors | Survive (n) | Death (n) | Univariate analysisPvalue | Multivariate analysisPvalue |
| Sex | 0.751 | |||
| Female | 6 | 5 | ||
| Male | 26 | 28 | ||
| Age (yr) | 0.180 | |||
| < 70 | 25 | 20 | ||
| ≥ 70 | 7 | 13 | ||
| Site | 0.336 | |||
| Lower | 20 | 21 | ||
| Middle | 1 | 7 | ||
| Upper | 11 | 5 | ||
| Diffuse | 0 | 0 | ||
| Comorbidities | 0.798 | |||
| Yes | 20 | 22 | ||
| No | 12 | 11 | ||
| Surgery | 0.683 | |||
| Subtotal gastrectomy | 17 | 16 | ||
| Total gastrectomy | 10 | 9 | ||
| Extended total gastrectomy | 5 | 8 | ||
| Types of digestive reconstruction | 0.446 | |||
| Billroth I | 12 | 8 | ||
| Billroth II | 6 | 9 | ||
| Roux-en-Y | 14 | 16 | ||
| Grading | 0.241 | |||
| Well differentiated | 11 | 11 | ||
| Moderately differentiated | 8 | 14 | ||
| Poorly differentiated | 13 | 8 | ||
| Undifferentiated | 0 | 0 | ||
| T stage | 0.447 | |||
| T1 | 0 | 0 | ||
| T2 | 8 | 13 | ||
| T3 | 21 | 17 | ||
| T4 | 3 | 3 | ||
| No. metastatic nodes | 0.105 | |||
| N0 | 1 | 7 | ||
| N1 | 11 | 11 | ||
| N2 | 14 | 19 | ||
| N3 | 6 | 6 | ||
| Intra-abdominal chemotherapy | 0.459 | |||
| Yes | 19 | 16 | ||
| No | 13 | 17 | ||
| Postchemotherapy | 0.511 | |||
| Yes | 26 | 29 | ||
| No | 6 | 4 | ||
| Recurrence in PET/CT | 0.000 | 0.000 | ||
| Yes | 2 | 29 | ||
| No | 31 | 3 | ||
| Re-surgery | 0.804 | |||
| Yes | 14 | 13 | ||
| No | 18 | 20 |
In patients who received surgical treatment, the type of operation was determined by 3 expert gastrointestinal surgeons based on abdominal examinations and the general condition of the patient. A total of 27 patients (12 in the group with PET/CT evidence of a tumor recurrence and 15 with no evidence of a tumor recurrence) underwent laparotomy. The types of surgical procedures that were performed are summarized in Table 4. Open and close surgery was the main type of surgical procedure reported for the patients with tumor recurrences. R0 resections were performed in 2 patients, including 1 who underwent combined adjacent organ resection. In the group with no evidence of tumor recurrences on PET/CT, bowel resections were performed in 7 patients, adhesiolysis was performed in 7 patients, and a bypass was performed in 1 patient. The overall incidence of postoperative complications was 44.4% (12 of 27 patients). There were 7 patients with Clavien grade I complications, including 3 with wound infections and 4 with pleural effusions. Another 3 patients were classified as having grade II complications, including 1 with an anastomotic leakage and 2 with pneumonia. One patient had the grade IIIb complication of an abdominal abscess, and 1 patient had the grade V complication of multiple organ failure and died in the hospital 1 wk after surgery.
| Procedure | n |
| Open and close | 10 |
| R0 resection | 2 |
| Bypass | 1 |
| Bowel resection | 7 |
| Adhesiolysis | 7 |
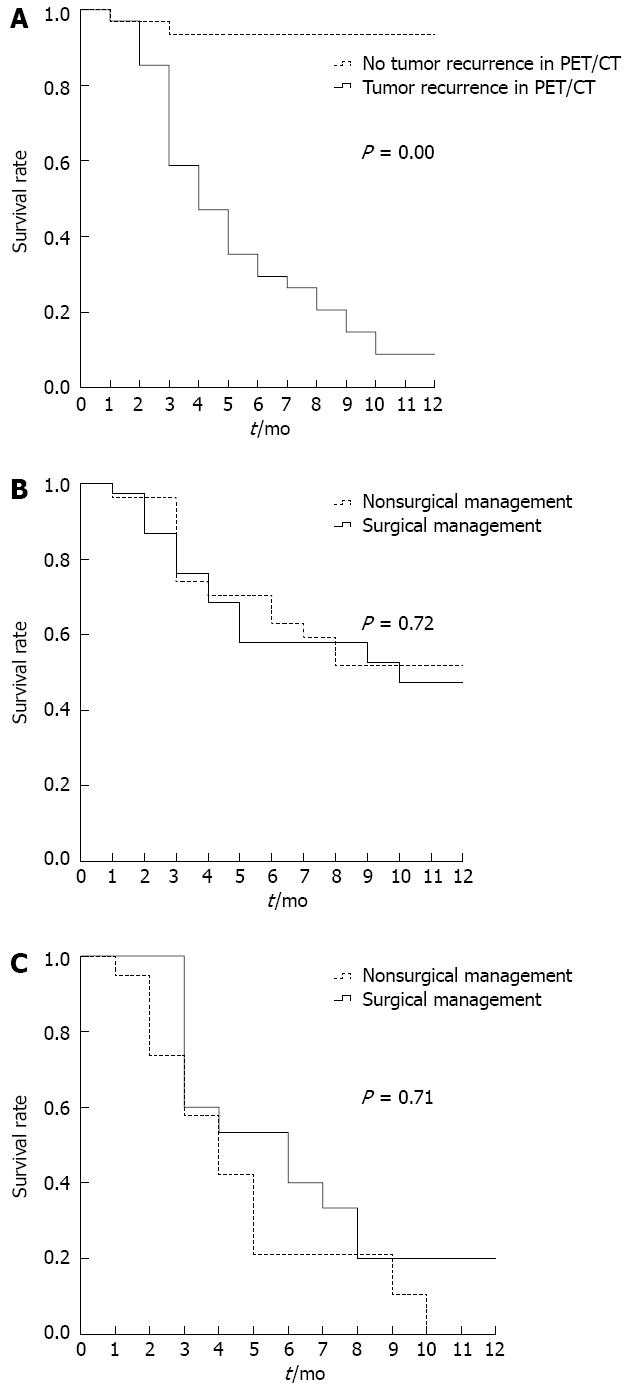
The 1-year survival curves according to the PET/CT findings are shown in Figure 3A. There was a significant difference in the survival between patients with and without evidence of recurrences on PET/CT, and the 1-year survival rates were 8.8%, and 93.5%, respectively (P = 0.00). The 1-year survival curves according to exploratory laparotomy and nonoperative treatment are shown in Figure 3B. There were no significant differences (P = 0.71) in the 1-year survival based on surgical vs nonsurgical management (0% with nonoperative treatment vs 20% after exploratory laparotomy). The 1-year survival curves according to evidence of a tumor recurrence on PET/CT are shown in Figure 3C.
Other variables in the analysis included 30-d readmission, the length of hospital stay, complications, and the mortality rates. These variables are listed in Table 5. In both the PET/CT-positive and -negative groups, exploratory laparotomy resulted in a shorter mean length of hospital stay than nonsurgical management (P < 0.05).
| Variables | No recurrence in PET/CT | Recurrence in PET/CT | ||
| Surgical management | Non-surgical management | Surgical management | Non-surgical management | |
| Mean length of stay (d) | 10.5 ± 2.3 | 18.2 ± 8.7 | 8.2 ± 3.1 | 19.1 ± 9.6 |
| 30-d re-admission | 0.00% | 21.10% | 25.00% | 26.10% |
| In-hospital mortality | 0.00% | 0.00% | 0.10% | 0.00% |
| Overall complications | 53.30% | 0.00% | 33.30% | 0.00% |
An intestinal obstruction is a common problem in patients with advanced cancers. Approximately 3%-15% of all terminally ill cancer patients will suffer from an intestinal obstruction and 10%-28% of patients with gastrointestinal cancers will develop an intestinal obstruction. Obstructions in patients with a history of gastric cancer may be secondary to a malignant process, either extrinsic or intrinsic to the bowel, or an underlying benign etiology, such as an intra-abdominal hernia or intraperitoneal adhesions. The current treatment options for patients with a bowel obstruction secondary to malignant disease include surgery to bypass/remove the obstruction, gastrointestinal decompression via a nasogastric tube, and medications (e.g., octreotide)[17]. In inoperable cases, decompression via a nasogastric tube may be the only treatment available. Nasogastric tube decompression can provide symptomatic relief but may cause mucosal erosion, esophagitis, or aspiration pneumonia, which further diminish quality of life. However, surgical treatment is often contraindicated because of the poor physical status of the patient, and many patients with gynecological malignancies, especially ovarian cancer, are not candidates for surgery because of the presence of diffuse intraperitoneal carcinomatosis, multiple partial obstruction points, ascites, and/or a history of previous radiotherapy. A critical step in the management of patients with a bowel obstruction and a history of curative resection of gastric cancer is to determine whether a malignant process is present. Identifying the underlying etiology of the bowel obstruction, malignant or benign, will significantly impact management decisions. Additionally, distinguishing between a malignant obstruction and a benign obstruction is a key measure in deciding which patients should undergo early operation.
It is well documented that the complete surgical removal of gastric tumors with lymph node dissection is the only curative treatment that is currently available; however, disease recurrence after radical surgery still occurs in approximately 22%-48% of patients, and its prognosis is poor[18-21]. Tumor marker evaluation, endoscopy, and imaging studies have previously been used to monitor patients for gastric cancer recurrences; however, there are several limitations to tumor markers and endoscopy. Tumor markers cannot be used to determine the site of recurrence, and endoscopy cannot detect extraluminal recurrences[22]. The most important limitation of CT in the detection of locally recurrent gastric cancer is the lack of specificity because the diagnostic ability of CT depends on the morphological changes of the involved organs and distorted anatomical features. In addition, CT uses size criteria. These factors result in difficulties in image interpretation, and CT cannot precisely identify the presence and the quality of tumors.
Whole-body 18F-FDG PET detects increased glucose metabolism in malignant cells to produce diagnostic evidence and can be widely applied for staging, re-staging, and monitoring therapy-induced tumor changes and response to therapy in patients with various cancers. The usefulness of integrated 18F-FDG PET/CT for the diagnosis of recurrences in patients with gastric cancer has been investigated in previous studies, which have indicated that 18F-FDG PET/CT is an effective and helpful diagnostic method in the evaluation of recurrences. Other trials have studied the impact of 18F-FDG PET/CT on the clinical decision-making process[23-25], FDG-PET results led to a radical change in the clinical management of 20% of the patients who were analyzed for resection of colorectal liver metastases. FDG-PET was considered a decisive technique for determining whether to perform surgery, and management was changed in 29% of the patients. This study has confirmed the critical role of whole-body 18F-FDG PET/CT during the clinical course of patients with a bowel obstruction and a history of gastric cancer.
Several patients in our study with a SBO and a history of gastric cancer did not have end-stage disease that was associated with poor survival. Distinguishing between a malignant obstruction and a benign obstruction is a key measure in deciding which patients should undergo early operation. Patients with metastatic cancer who develop a bowel obstruction have a short median survival time (approximately 3 mo)[16], and decisions regarding the treatment of bowel obstructions must be carefully weighed in these patients. Surgery can offer good palliative benefits for these patients; however, surgery may result in complications that reduce quality of life and cause patients to spend an excessive amount of time in the hospital, which could have been avoided. Therefore, optimal palliation may result from the nonoperative and medical management of symptoms and lead to a potential decrease in the length of hospital stay. Attempting surgery in these patients may not be the best decision, and the finding in our study that there were no differences in the survival of patients with recurrent disease based on the type of management shifts the focus of care for these patients from a selection process to surgical vs nonsurgical management.
Multiple specialists are usually involved in the treatment of these patients, including gastroenterologists, interventional and diagnostic radiologists, radiation oncologists, and medical and surgical oncologists. These specialists may have divergent opinions regarding definitive individualized treatment. Our results suggest that patients who had evidence of a tumor recurrence on a PET/CT scan face an end-of-life scenario and optimal symptom control is the goal for these patients. In addition, FDG PET/CT is a superior post-therapy surveillance modality for the diagnosis of recurrent gastric cancer compared with other imaging methods after initial surgery. In addition, FDG PET/CT has been specifically helpful in optimizing treatment plans and may play an important role in treatment stratification in the future[26]. Miller et al[11] compared operative therapy with nonoperative therapy in patients with small bowel obstructions secondary to malignant disease and found a rate of reobstruction that was 15% higher in the nonoperative group. Additionally, they reported shorter times to a reobstruction in patients who had received conservative nonoperative therapy, and they observed that a palpable abdominal mass was an important predictor of poor outcomes in their series. In this study, we concluded that patients with a history of gastric cancer who present with a SBO and who have no evidence of a tumor recurrence on PET/CT will receive benefits in both survival and quality of life after surgery to relieve the obstruction.
In conclusion, 18F-FDG PET/CT can be used to identify the causes of bowel obstructions in patients with a history of curative resection of gastric cancer, and this method is useful for planning the surgical management of these patients. Surgical intervention in a patient who has an obstruction after curative resection of gastric cancer and who has no evidence of a tumor recurrence on a PET/CT examination benefits the quality of life of the patient. Patients with poor survival, including patients with PET/CT evidence of a local recurrence, peritoneal carcinomatosis or distant metastases, would not benefit from surgery.
The management of patients who present with a small bowel obstruction (SBO) after treatment of primary carcinoma challenges the clinical judgement of even the most experienced surgeons when the feared cause is metastatic disease. It is difficult to predict whether the quality and/or the quantity of life in this group of patients will be improved by surgery because these patients have a poor prognosis at the time of presentation. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) detects the increased utilization of glucose by malignant cells and is more accurate than conventional diagnostic methods for the diagnosis of primary and recurrent gastrointestinal tumors.
The management of SBOs after treatment of primary carcinoma is difficult, and it is unclear which patients will benefit from surgery and which patients will have similar outcomes from medical management because many patients may have diffuse peritoneal metastatic disease and/or adhesions from previous surgery. It is difficult to predict whether the quality and/or the quantity of life in this group of patients will be improved by surgery because these patients have a poor prognosis at the time of presentation. In addition, the management of these patients presents an additional difficulty because the intestinal obstruction may be due to more than one physiopathological process, such as an intraluminal obstruction from polypoid lesions that occlude the bowel lumen, an intramural obstruction from the infiltration of a tumor within the muscular coat of the bowel wall, and an extramural obstruction from mesenteric and omental masses and extrinsic compression from malignant adhesions.
18F-FDG PET/computed tomography (CT) can be used to identify the causes of bowel obstructions in patients with a history of curative resection of gastric cancer, and this method is useful for planning the surgical management of these patients. Surgical intervention in a patient who has an obstruction after curative resection of gastric cancer and who has no evidence of a tumor recurrence on a PET/CT examination benefits the quality of life of the patient. Patients with poor survival, including patients with PET/CT evidence of a local recurrence, peritoneal carcinomatosis or distant metastases, may not benefit from surgery.
18F-FDG PET/CT can be used to identify the causes of bowel obstructions in patients with a history of gastric cancer, and this method is useful for planning the surgical management of these patients.
PET with 18F-FDG detects the increased utilization of glucose by malignant cells to provide diagnostic information and is more accurate than conventional diagnostic methods in cases of primary and recurrent gastrointestinal tumors.
This article is interesting. The authors present their experience of using whole-body PET/CT in the surgical management of patients with bowel obstructions secondary to gastric cancer. PET/CT significantly improves survival because of its ability to identify the causes of bowel obstructions. Overall, the paper is well written and acceptable for publication in its current form.
| 1. | Baines M, Oliver DJ, Carter RL. Medical management of intestinal obstruction in patients with advanced malignant disease. A clinical and pathological study. Lancet. 1985;2:990-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 158] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Fainsinger RL, Spachynski K, Hanson J, Bruera E. Symptom control in terminally ill patients with malignant bowel obstruction (MBO). J Pain Symptom Manage. 1994;9:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 290] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 4. | Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev. 2000;CD002764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3573] [Cited by in RCA: 2844] [Article Influence: 948.0] [Reference Citation Analysis (0)] |
| 5. | Yates MR, Morgan DE, Baron TH. Palliation of malignant gastric and small intestinal strictures with self-expandable metal stents. Endoscopy. 1998;30:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Rose PG, Piver MS, Tsukada Y, Lau TS. Metastatic patterns in histologic variants of ovarian cancer. An autopsy study. Cancer. 1989;64:1508-1513. [PubMed] |
| 7. | Dvoretsky PM, Richards KA, Angel C, Rabinowitz L, Beecham JB, Bonfiglio TA. Survival time, causes of death, and tumor/treatment-related morbidity in 100 women with ovarian cancer. Hum Pathol. 1988;19:1273-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Dvoretsky PM, Richards KA, Angel C, Rabinowitz L, Stoler MH, Beecham JB, Bonfiglio TA. Distribution of disease at autopsy in 100 women with ovarian cancer. Hum Pathol. 1988;19:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Tang E, Davis J, Silberman H. Bowel obstruction in cancer patients. Arch Surg. 1995;130:832-836; discussion 836-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Parker MC, Baines MJ. Intestinal obstruction in patients with advanced malignant disease. Br J Surg. 1996;83:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Miller G, Boman J, Shrier I, Gordon PH. Small-bowel obstruction secondary to malignant disease: an 11-year audit. Can J Surg. 2000;43:353-358. [PubMed] |
| 12. | Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 368] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 13. | Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, Lee JH, Ryu KW, Kim YW, Bae JM. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006;33:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 173] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Yoon YC, Lee KS, Shim YM, Kim BT, Kim K, Kim TS. Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection prospective study. Radiology. 2003;227:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Pameijer CR, Mahvi DM, Stewart JA, Weber SM. Bowel obstruction in patients with metastatic cancer: does intervention influence outcome? Int J Gastrointest Cancer. 2005;35:127-133. [PubMed] |
| 17. | Watari H, Hosaka M, Wakui Y, Nomura E, Hareyama H, Tanuma F, Hattori R, Azuma M, Kato H, Takeda N. A prospective study on the efficacy of octreotide in the management of malignant bowel obstruction in gynecologic cancer. Int J Gynecol Cancer. 2012;22:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Starzyńska T. Molecular epidemiology of gastric cancer. Dig Dis. 2007;25:222-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Kunisaki C, Makino H, Akiyama H, Otsuka Y, Ono HA, Kosaka T, Takagawa R, Nagahori Y, Takahashi M, Kito F. Clinical significance of the metastatic lymph-node ratio in early gastric cancer. J Gastrointest Surg. 2008;12:542-549. [PubMed] |
| 20. | Schwarz RE, Zagala-Nevarez K. Recurrence patterns after radical gastrectomy for gastric cancer: prognostic factors and implications for postoperative adjuvant therapy. Ann Surg Oncol. 2002;9:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 560] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 22. | Whiting J, Sano T, Saka M, Fukagawa T, Katai H, Sasako M. Follow-up of gastric cancer: a review. Gastric Cancer. 2006;9:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Sun L, Su XH, Guan YS, Pan WM, Luo ZM, Wei JH, Wu H. Clinical role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in post-operative follow up of gastric cancer: initial results. World J Gastroenterol. 2008;14:4627-4632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (12)] |
| 24. | Ruers TJ, Langenhoff BS, Neeleman N, Jager GJ, Strijk S, Wobbes T, Corstens FH, Oyen WJ. Value of positron emission tomography with [F-18]fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol. 2002;20:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Sim SH, Kim YJ, Oh DY, Lee SH, Kim DW, Kang WJ, Im SA, Kim TY, Kim WH, Heo DS. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer. 2009;9:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Bilici A, Ustaalioglu BB, Seker M, Kefeli U, Canpolat N, Tekinsoy B, Ozugur S, Gumus M. The role of 18F-FDG PET/CT in the assessment of suspected recurrent gastric cancer after initial surgical resection: can the results of FDG PET/CT influence patients’ treatment decision making? Eur J Nucl Med Mol Imaging. 2011;38:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
P- Reviewers Lin WY, Liu YY, Sun ZH S- Editor Gou SX L- Editor A E- Editor Zhang DN