Published online Jun 28, 2013. doi: 10.3748/wjg.v19.i24.3747
Revised: December 3, 2012
Accepted: December 15, 2012
Published online: June 28, 2013
Processing time: 315 Days and 3.8 Hours
AIM: To investigate cellular 5-HT4(-h/+h) receptor distribution, particularly in the epithelial layer, by laser microdissection and polymerase chain reaction (PCR) in porcine gastrointestinal (GI) tissues.
METHODS: A stepwise approach was used to evaluate RNA quality and to study cell-specific 5-HT4 receptor mRNA expression in the porcine gastric fundus and colon descendens. After freezing, staining and laser microdissection and pressure catapulting (LMPC), RNA quality was evaluated by the Experion automated electrophoresis system. 5-HT4 receptor and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expressions were examined by endpoint reverse transcription (RT)-PCR in mucosal and muscle-myenteric plexus (MMP) tissue fractions, in mucosal and MMP parts of hematoxylin and eosin (HE) stained tissue sections and in microdissected patches of the epithelial and circular smooth muscle cell layer in these sections. Pig gastric fundus tissue sections were also stained immunohistochemically (IHC) for enterochromaffin cells (EC cells; MAB352); these cells were isolated by LMPC and examined by endpoint RT-PCR.
RESULTS: After HE staining, the epithelial and circular smooth muscle cell layer of pig colon descendens and the epithelial cell layer of gastric fundus were identified morphologically and isolated by LMPC. EC cells of pig gastric fundus were successfully stained by IHC and isolated by LMPC. Freezing, HE and IHC staining, and LMPC had no influence on RNA quality. 5-HT4 receptor and GAPDH mRNA expressions were detected in mucosa and MMP tissue fractions, and in mucosal and MMP parts of HE stained tissue sections of pig colon descendens and gastric fundus. In the mucosa tissue fractions of both GI regions, the expression of h-exon containing receptor [5-HT4(+h) receptor] mRNA was significantly higher (P < 0.01) compared to 5-HT4(-h) receptor expression, and a similar trend was obtained in the mucosal part of HE stained tissue sections. Large microdissected patches of the epithelial and circular smooth muscle cell layer of pig colon descendens and of the epithelial cell layer of pig gastric fundus, also showed 5-HT4 receptor and GAPDH mRNA expression. No 5-HT4 receptor mRNA expression was detected in gastric LMPC-isolated EC cells from IHC stained tissues, which cells were positive for GAPDH.
CONCLUSION: Porcine GI mucosa predominantly expresses 5-HT4(+h) receptor splice variants, suggesting their contribution to the 5-HT4 receptor-mediated mucosal effects of 5-HT.
- Citation: Priem EK, Maeyer JHD, Vandewoestyne M, Deforce D, Lefebvre RA. Predominant mucosal expression of 5-HT4(+h) receptor splice variants in pig stomach and colon. World J Gastroenterol 2013; 19(24): 3747-3760
- URL: https://www.wjgnet.com/1007-9327/full/v19/i24/3747.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i24.3747
The 5-HT4 receptor is a G-protein coupled receptor (GPCR) that activates the adenylyl cyclase/cyclic adenosine monophosphate/protein kinase A pathway in response to serotonin (5-HT). The 5-HT4 receptor is expressed on excitatory motor neurons in the gut, facilitating acetylcholine release, which stimulates gastrointestinal (GI) motility[1-3]. This presynaptic facilitation is thought to be the principal mechanism for the prokinetic action of 5-HT4 receptor agonists, explaining their therapeutic use in GI dysmotility-related disorders, such as chronic constipation, gastroparesis and gastroesophageal reflux disease[4]. The selective 5-HT4 receptor agonist prucalopride is now used in patients with chronic laxative-resistant constipation; indeed, it facilitates acetylcholine release from cholinergic neurons towards human colonic circular[5], as well as longitudinal[6], smooth muscle. The non-selective 5-HT4 receptor agonist cisapride was, until it was withdrawn because of non-specific cardiac side effects, used for increasing gastric emptying in patients with gastroparesis[7]. In addition, prucalopride accelerates gastric emptying in humans[8], corresponding with its facilitating effect on acetylcholine release from cholinergic nerves towards human gastric circular muscle[9]. Our group has previously shown that the pig is a good model for human 5-HT4 receptors on GI cholinergic neurons; the presence of facilitatory 5-HT4 receptors on cholinergic neurons innervating pig gastric circular[10] and longitudinal[11] muscle and colonic circular muscle[12] was illustrated in functional assays.
However, apart from cholinergic neurons, other locations for the 5-HT4 receptor in the colon and stomach have been proposed. In the human colon, 5-HT4 receptors were reported to be present on circular smooth muscle cells, inducing relaxation[13]. A functional study by Borman et al[14] in 1996 reported that 5-HT-induced secretion in the human sigmoid colon is mediated via 5-HT2A receptors; however, in the ascending colon, a combination of 5-HT2A and 5-HT4 receptors appears to be involved. Nevertheless, a recent study showed the presence of mRNA of several 5-HT4 receptor splice variants in the mucosal layer of the human sigmoid colon[15]; 5-HT4 receptor mRNA was also reported in the pig colonic mucosa[16]. In the rat colon, it has been suggested that 5-HT-induced mucosal ion transport and Cl- secretion is mediated by 5-HT4 receptors[17-20]. Immunohistochemical and functional assays showed the presence of 5-HT4 receptors in mouse colonic epithelial cells, including enterochromaffin (EC) cells and goblet cells, inducing mucosal 5-HT release and Cl- secretion[20]. The presence of 5-HT4 receptor transcripts, detected by reverse transcription polymerase chain reaction (RT-PCR), has also been reported in the gastric mucosa of humans[21,22] and pigs[16], but cellular distribution within the epithelial layer has not yet been investigated.
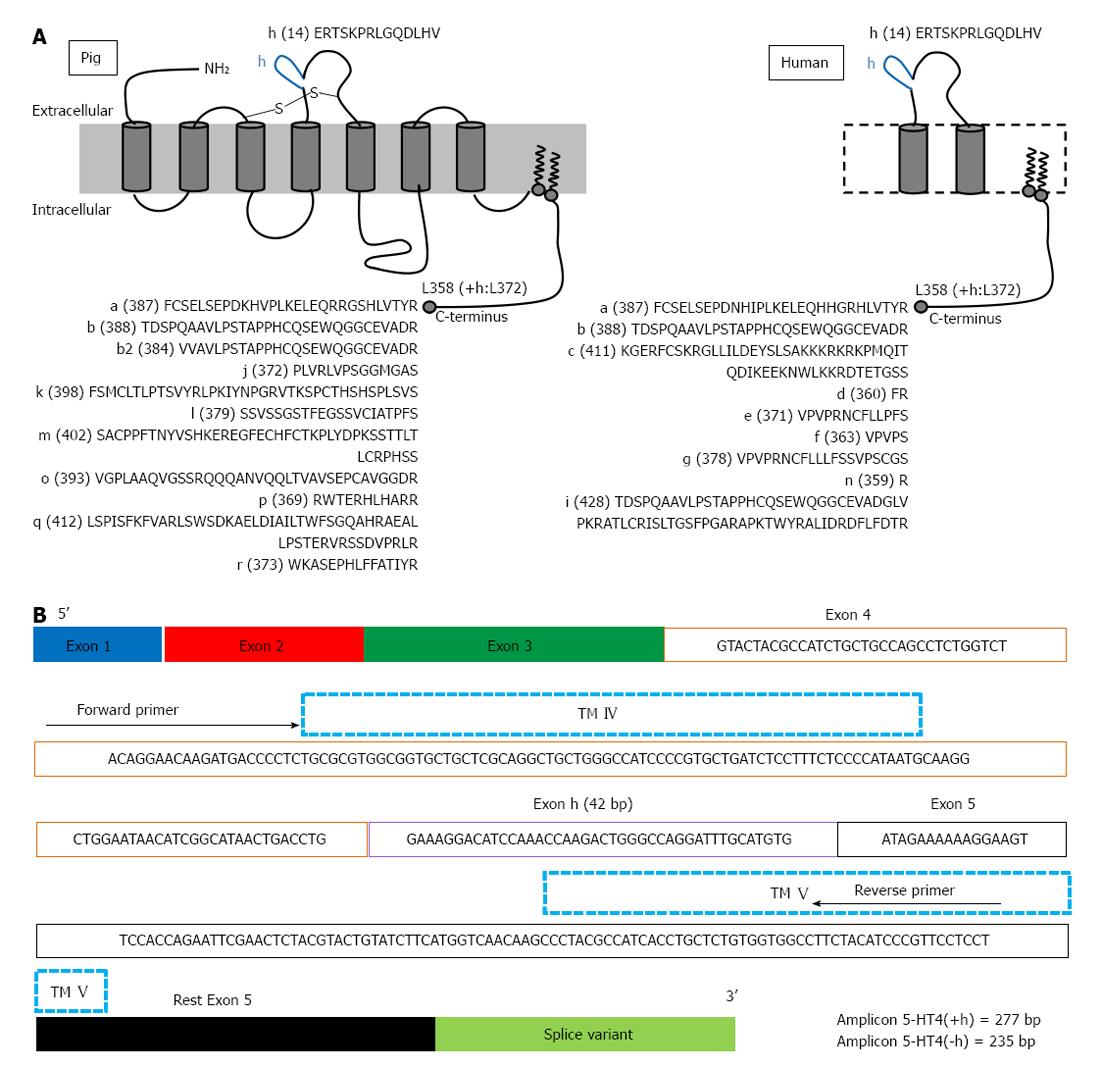
More detailed information on the expression and localization of GPCRs, with special attention to the 5-HT4 receptor, is needed in human enteric neuronal subpopulations, mast cells and epithelial cells, to provide a better understanding of function and activity of 5-HT4 receptors in the GI wall, which may offer new therapeutic perspectives[23]. To date, the majority of information on 5-HT4 receptor distribution is based on functional studies[12] or on 5-HT4 receptor expression studies using homogenates of tissues[15,16,21,22]. However, homogenates of tissues limit the potential of expression studies: important cell-specific transcript information is lost because of the heterogeneity of tissues, such as GI tissues. Techniques have been developed to enable collection of particular cells from mixed populations, which generally involve either fluorescence activated cell sorting (FACS) purification of dissociated cells or laser-assisted microdissection. In contrast to FACS, microdissection can be applied to most tissues[24] and laser microdissection has already been used in previously reported gene expression studies to investigate site-specific gene expression. In the laser microdissected enteric ganglia of the human intestine, 5-HT3A receptor mRNA expression was described[25] and in microdissected human colonic mucosal epithelium, transcripts encoding 5-HT3A, 5-HT3C, 5-HT3D and 5-HT3E subunits were detected[26]. In different species, 5-HT4 receptors show splice variation in the intracellular C-terminus starting after the common amino acid structure L358. In humans, nine splice variants have been described (Figure 1A). In pig, at least another nine different splice variants, not described in humans, have been reported (Figure 1A), as well as unique splice variation, with variants composed of duplicated exons[16]. Splice variants in the extracellular loops of GPCRs are rare[27]; however, the 5-HT4 receptor can have an extra insertion of 14 amino acids in the second extracellular loop, encoded by the h-exon (Figure 1A). In humans, this H variant has been described in combination with the b-terminal exon [5-HT4(hb)][28]. When comparing the pharmacology of the 5-HT4(hb) splice variant, when transiently expressed in cells being CV-1 (simian) in origin, and carrying the SV40 genetic material (COS)-7 cells, with that of the 5-HT4(b) and 5-HT4(a) splice variant, it showed a smaller fraction of receptors coupled to G-proteins and the 5-HT4 receptor antagonist GR113808 behaved as a partial agonist[28]. In the human GI tract, the h exon could be amplified in combination with the b exon only from the lower esophageal sphincter; however, h exon-carrying 5-HT4 transcripts were also obtained from other parts of the GI tract, suggesting that the h-exon might be expressed in combination with other C-terminal exons[28]. In pigs, De Maeyer et al[16] showed that the 5-HT4(h) splice variant also exists in combination with C-terminal exons other than 5-HT4(b), namely 5-HT4(ha), 5-HT4(hm) and 5-HT4(hr). H-exon containing 5-HT4 transcripts were also found along the porcine GI tract, with predominant expression in the mucosal layer. Therefore, the aim of the present study was to develop and validate an experimental protocol for the assessment of 5-HT4 receptor distribution (with and without the h exon) at the cellular level in laser microdissected porcine GI tissues, paying special attention to the mucosal layer of pig colon descendens and gastric fundus.
Young male pigs (10-12 wk, 15-25 kg-breed Line 36) were obtained from Rattlerow Seghers, Lokeren Belgium. The Ethical Committee for Animal Experiments from the Faculty of Medicine and Health Sciences at Ghent University approved all the experimental procedures.
The pigs were anaesthetized with an intramuscular injection of 5 mL Zoletil 100 (containing 50 mg/mL tiletamine and 50 mg/mL zolazepam; Virbac Belgium SA, Heverlee, Belgium). After exsanguination, the stomach and the colon descendens, prelevated 10 cm above the anus to the transverse colon, were removed and thoroughly washed in ice cold aerated (5% CO2/95% O2) phosphate buffered saline (PBS) at pH 7.4 (Life Technologies Europe, Ghent, Belgium). The gastric fundus was cut open along the lesser curvature and small pieces of tissue were cut in the direction of the circular muscle layer from the ventral side. The colon descendens was opened along the mesenteric border, fat tissue was removed and tissues were cut in the direction of the circular muscle layer.
Freezing tissue fractions for direct RNA processing: The GI tissues were divided by blunt dissection into a mucosal-submucosal (mucosa) fraction and a muscular-myenteric plexus (MMP) fraction. The fractions were cut into small pieces, put in an RNase-free vial (Life Technologies Europe), rapidly frozen in liquid N2 and stored at -80 °C. After frozen tissue homogenization and before RNA extraction, MMP samples were treated with proteinase K (Qiagen, Antwerp, Belgium) to increase the total RNA output. Proteinase K removes proteins such as the contractile proteins, connective tissue and collagen, which define a fibrous tissue such as the smooth muscle layer (Rneasy fibrous tissue handbook, Qiagen). RNA from mucosa and MMP fractions was extracted using the RNeasy Mini Kit (Qiagen) according to manufacturer’s guidelines and RNA samples were stored at -80 °C.
Freezing tissues for section preparation and laser microdissection: Whole tissues, containing the mucosal and the smooth muscle layers were cut into full-thickness small pieces with a sterile scalpel, placed in tissue embedding medium PELCO CryO-Z-T (Pelco International, CA, United States), rapidly frozen in liquid N2 containing cold isopentane and stored at -80 °C. The frozen tissue samples were cut into 8 μm-thick sections using a cryostat (Leica CM 1950; Leica Microsystems, Diegem, Belgium) with disposable RNase-free knives. Sections of 8 μm thickness are considered to represent a monolayer of cells[29,30]. The sections were placed on chilled (-20 °C) nuclease free polyethylene naphthalate-covered membrane slides (Carl Zeiss, Oberkochen, Germany) and immediately stored at -80 °C until the staining procedure. The membrane slides used for immunohistochemistry were extra coated with poly-L-Lysine (Sigma, Bornem, Belgium), which was diluted with 0.1% diethylpyrocarbonate (DEPC)-treated water. All materials (pincers, brushes) were treated with RNase ZAP (Sigma) and glassware and pincers were heated for 6 h at 200 °C, to remove all exogenous RNases.
To distinguish morphologically the different layers of the tissue sections for laser microdissection, the frozen tissue sections were stained with hematoxylin and eosin (Sigma) in RNase-free conditions. Hematoxylin and eosin (HE) staining started with fixing the slides in 70% ethanol for 1 min, followed by dipping the slides for 15 s in DEPC-treated water to remove PELCO CryO-Z-T embedding medium. Hematoxylin staining was carried out by placing the slides for 1 min in the hematoxylin solution (0.1%), followed by dipping the slides for 15 s in DEPC-treated water and 15 s in 70% ethanol. Slides were then placed for 1 min in eosin solution (0.25%), followed by dehydrating the slides for 15 s in the following order: DEPC-treated water, 70% ethanol, 100% ethanol. The staining procedure was finished with a 3 min xylene treatment and the slides were air dried for 10 min at room temperature, before scraping off the whole tissue section, or the mucosal and MMP part of the tissue section separately, or applying laser microdissection. Staining solutions based on ethanol and xylene were pre-cooled at -20 °C; aqueous solutions were pre-cooled at 4 °C. All solutions were diluted with 0.1% DEPC-treated water, kept in 50 mL RNase-free conical tubes (Life Technologies Europe) and kept on ice during the staining procedure.
Immunohistochemistry: To distinguish and isolate EC cells using the laser microdissection and pressure catapulting (LMPC) technique, visualization with cell-specific antibodies of these cells is required. To extract intact RNA of the cell samples, an immunohistochemically (IHC) protocol under RNase-free conditions was developed according to the staining procedure reported by Brown et al[24]. Cryosections were rinsed for 15 s with cold (4 °C) PBS (pH 7.4; Life Technologies Europe) and then fixed for 5 min in ice-cold (-20 °C) acetone. Acetone was removed by a cold PBS rinse (15 s) and slides were incubated for 30 min at 4 °C with blocking buffer (0.25% Triton X-100, 1% bovine serum albumin, 10% goat serum) supplemented with 1 mol/L NaCl. Then, sections were briefly rinsed with cold PBS and incubated overnight at 4 °C with the rat anti-serotonin primary antibody MAB352 (Milipore, Overijse, Belgium), used as a marker for EC cells. MAB352 was diluted 1:200 in PBS supplemented with 1 mol/L NaCl. Unbound primary antibody was removed by rinsing three times with cold PBS supplemented with 1 mol/L NaCl. Sections were then incubated with chicken anti-rat secondary antibody Alexa Fluor 488 (Life Technologies Europe) diluted 1:100 in PBS with 1 mol/L NaCl for 2 h at 4 °C. Unbound secondary antibody was removed by rinsing three times with cold PBS with 1 mol/L NaCl and excess NaCl was removed by a PBS rinse (5 s). Sections were dehydrated in 70% and then 100% ethanol (3 min each) and air dried for 10 min at room temperature before laser microdissection.
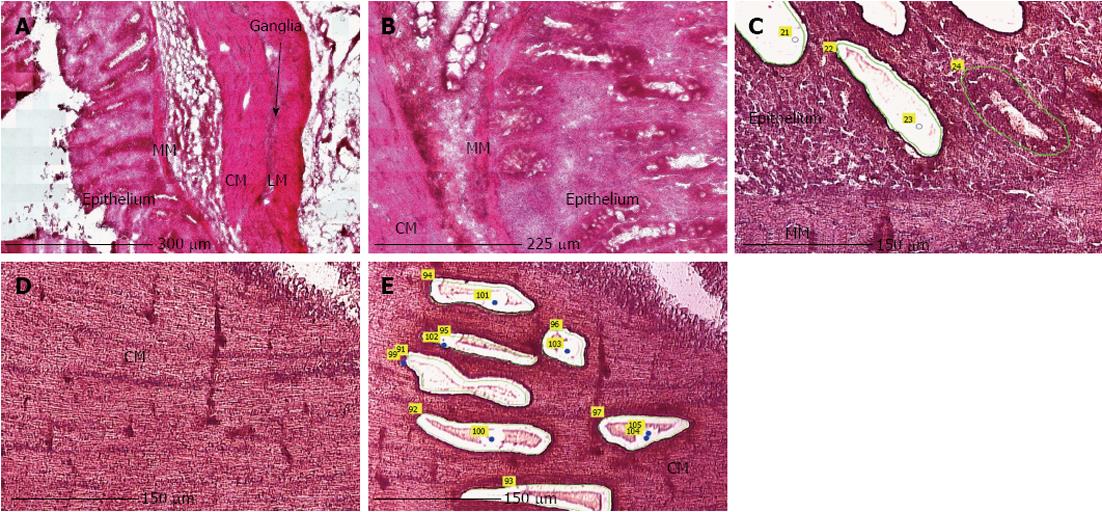
LMPC was performed using the laser microdissection system from PALM Technologies (Carl Zeiss) containing a PALM Microbeam, RoboStage and a PALM RoboMover (PALM RoboSoftware version 4). Under direct microscopic visualization, LMPC permits the procurement of histologically or immunohistologically defined tissue and cell samples (Figure 2). Approximately 15 large patches of cells from the epithelium or circular smooth muscle layer in HE stained sections, or 70 EC cells in IHC stained sections were laser-dissected and pressure-catapulted in 50 μL RLT lysis buffer (RNeasy kit, Qiagen). The cell collecting time was limited to 2 h per slide and after 2 h of cell sampling, the remaining tissue on the membrane slide was scraped off and RNA was extracted to determine if RNA integrity was preserved after 2 h. The samples were homogenized by vortexing, centrifuged and then placed at -80 °C for later use. Seven EC cell collections were pooled into one sample with a final volume of 350 μL, resulting in a collection of approximately 500 cells per sample. Total RNA from the cell samples was extracted using the RNeasy Micro kit (Qiagen), according to the manufacturer’s instructions.
Quantification of RNA was determined using a Nanodrop ND-1000 spectrophotometer (Isogen Life Science, Temse, Belgium) and the quality of RNA extracted from tissue fractions and tissue sections was assessed using the Experion automated electrophoresis system (BioRad, Nazareth Eke, Belgium).
cDNA of tissue fractions was prepared from 1 μg total RNA, whereas cDNA of whole tissue sections, parts of tissue sections and LMPC samples was prepared from the maximal input of total RNA as possible, as the amount of total RNA was less than 1 μg. The production of cDNA from sample RNA by RT was carried out according to manufacturer’s instructions, using SuperScript III Reverse Transcriptase SuperMix (Life Technologies Europe) containing random hexamers and oligo (dT)20. The obtained cDNA was stored at -20 °C before PCR. cDNA amplification reactions were carried out using the AccuPrime Pfx SuperMix (Life Technologies Europe). The template cDNA of mucosa and MMP tissue fractions for amplification was diluted 1:10. Expression of the 5-HT4 receptor within the samples was analysed using 5-HT4 receptor-specific primers spanning exon-intron-exon junctions: an exon 4-specific forward primer and an exon 5-specific reverse primer (Figure 1B). These primers will detect alternative splicing of the h-exon because the h-exon is located between exon 4 and exon 5. The quality of cDNA produced was assessed by amplifying cDNA for the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). To amplify cDNA of tissue fractions and tissue sections, PCR reactions were performed using the following protocol: 5 min at 95 °C, followed by 36 cycles with annealing temperature of 54 °C. The LMPC samples had a low RNA output; therefore, PCR reactions to amplify the cDNA for both 5-HT4 receptor and GAPDH, were performed using two rounds of PCR[15], according to the following protocol: a first reaction of 5 min at 95 °C followed by 36 cycles at 54 °C annealing temperature, followed by a second reaction using 1.5 μL of the product of the first reaction as a template for a second round of PCR (5 min at 95 °C followed by 36 cycles) with the same primers, but with a higher annealing temperature of 56 °C to increase specificity. RT and endpoint PCR reactions were processed on a C1000 Thermal Cycler (BioRad). PCR products were separated by 2% agarose gel electrophoresis and visualised by ethidium bromide staining. The primers (Eurogentec, Seraing, Belgium) used were published previously by De Maeyer et al[16]: 5-HT4R forward primer (5’-ACAGGAACAAGATGACCCCT-3’); 5-HT4R reverse primer (5’-AGGAGGAACGGGATGTAGAA-3’); GAPDH forward primer (5’-ACCACAGTCCATGCCATCAC-3’); GAPDH reverse primer (5’-TCCACCCTGTTGCTGTA-3’).
Semi-quantification of PCR products was determined by the intensity of PCR bands on the agarose gels using Image J 1.45 software. Band intensity was expressed as relative absorbance units, and the background of the image was determined and subtracted from the gel image. The ratio between the 5-HT4 receptor and GAPDH RNA was calculated to normalize for initial variations in sample concentration and as a control for reaction efficiency. Data presented are mean ± SE of the mean for animals (n). Statistical analyses were performed using Graphpad Prism software v.5.01 (United States). Differences in intensity were determined by an unpaired t test; P < 0.05 was considered statistically significant.
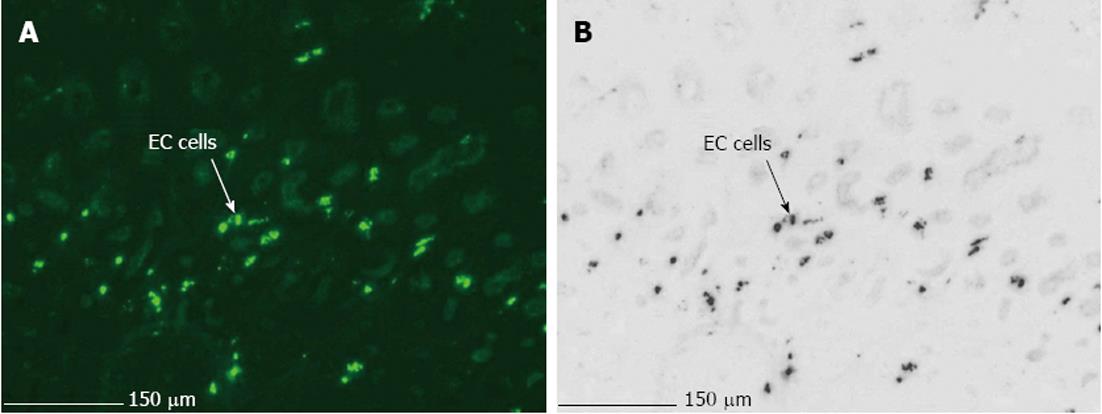
The main difficulties, when using LMPC to analyse gene expression of a specific cell type, are efficient and selective isolation of the desired cells, and obtaining RNA of good quality. Therefore, optimization of the LMPC experimental design was needed for the pig GI tissues, which are highly heterogeneous and rich in endogenous RNase and other enzymes. First, to select the desired cells from the heterogeneous GI tissues, a good visualization of the tissue layers and cells under direct microscopy was necessary. This requires that the morphology of the tissue be preserved, because fractures and air bubbles within the specimen will hamper the view. Tissues for section preparation and LMPC were therefore frozen in liquid N2 containing isopentane. After HE staining, the different layers of colon descendens (Figure 2) and gastric fundus (not shown) could be identified based on their morphological characteristics. After cell-specific IHC staining, MAB352-immunoreactive EC cells (Figure 3) were present in the crypts, villi and epithelial lining of the mucous membrane of the gastric fundus and were isolated by LMPC.
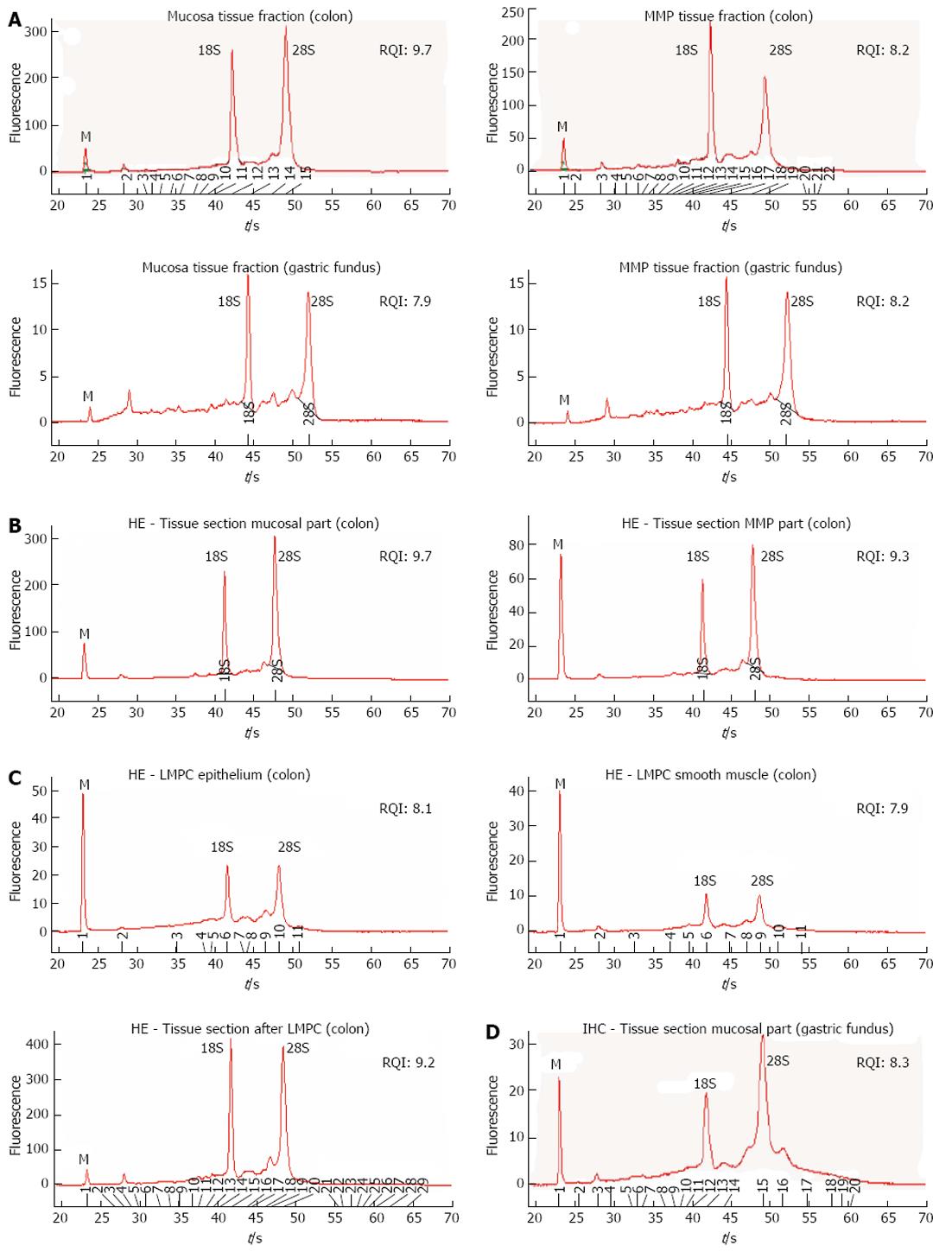
To evaluate the impact of the different protocol steps on RNA integrity, a systematic approach was followed by evaluating RNA yield and quality after each protocol step. RNA quality was assessed by comparing 28S and 18S and pre-18S ribosomal peaks to a set of degradation standards using the Experion automated electrophoresis system, where the RNA quality indicator (RQI) returns a number between 10 (intact RNA) and 1 (highly degraded RNA)[31]. The analysis showed that RNA quality was not affected after tissue fraction collection (Figure 4A), HE staining (Figure 4B), LMPC (Figure 4C) or after IHC staining (Figure 4D). However, electropherograms of RNA collected by LMPC could not be analysed systematically because the amount of RNA collected was too low. In Figure 4C, RNA quality from large microdissected patches of either epithelial or smooth muscle cells is shown, indicating that RNA was remained mostly intact, but the small ribosomal RNA peaks (18S/28S) indicate a low amount of RNA.
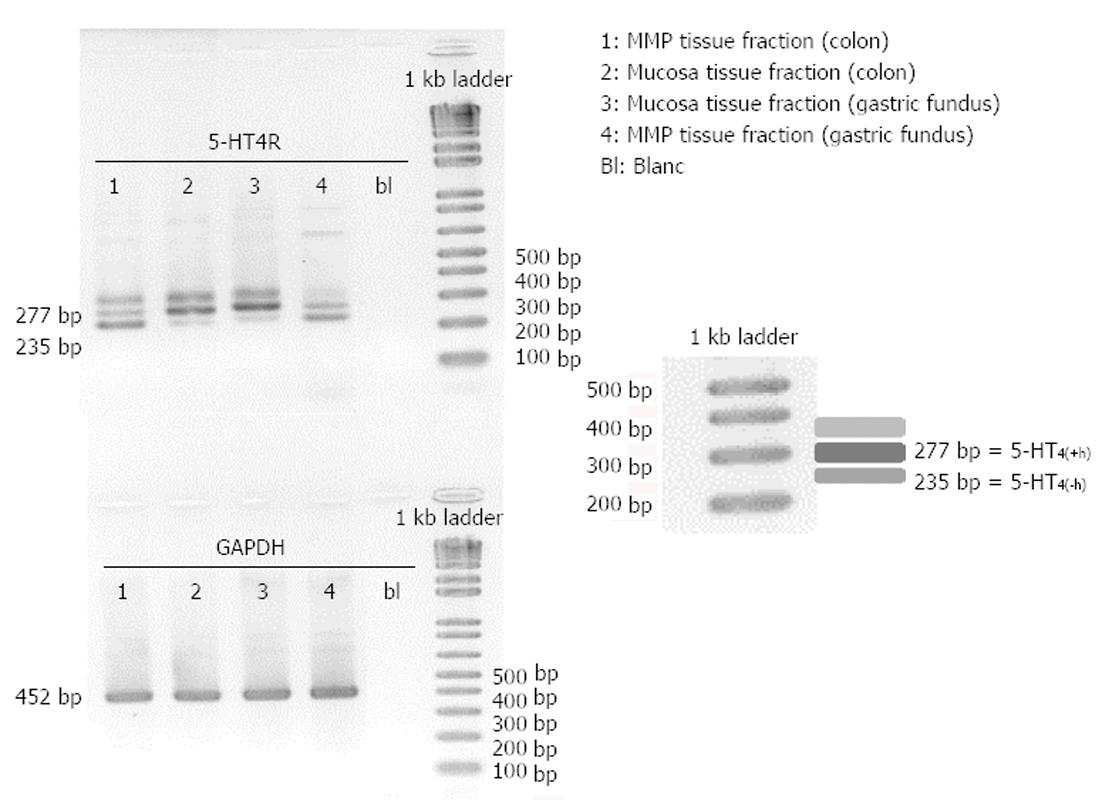
Tissue samples from pig colon descendens and gastric fundus were dissected into the mucosa fraction and the MMP fraction before freezing in liquid N2. After RNA extraction and endpoint PCR analysis of these fractions, 5-HT4 receptor expression was detected in mucosa as well as MMP fractions of the colon descendens and gastric fundus (Figure 5). All tissue samples were positive for GAPDH, confirming the integrity of the samples and completed PCR reactions. In all samples, 5-HT4 receptors containing the h-exon [277 bp; 5-HT4(+h) receptor] as well as 5-HT4 receptors without the h-exon [235 bp; 5-HT4(-h) receptor] were present (Figure 5). However, a third band, corresponding to a fragment of more than 300 bp was also observed. Therefore, we isolated this unknown PCR band using a QIA quick Gel extraction kit (Qiagen) and determined its DNA sequence with an ABI3130XL sequencer (Life Technologies Europe). After sequence analysis, we aligned the unknown sequence with the 5-HT4(+h) and 5-HT4(-h) receptor sequences and observed that the unknown band contained the same sequence and length as the 5-HT4(+h) receptor, but chromatogram details suggested the presence of additional nucleotides in the tail of the sequence, possibly caused by the formation of a heteroduplex with a 5-HT4(+h) strand and a 5-HT4(-h) strand, or formation of a triplex with other PCR fragments, resulting in a different electrophoretic separation.
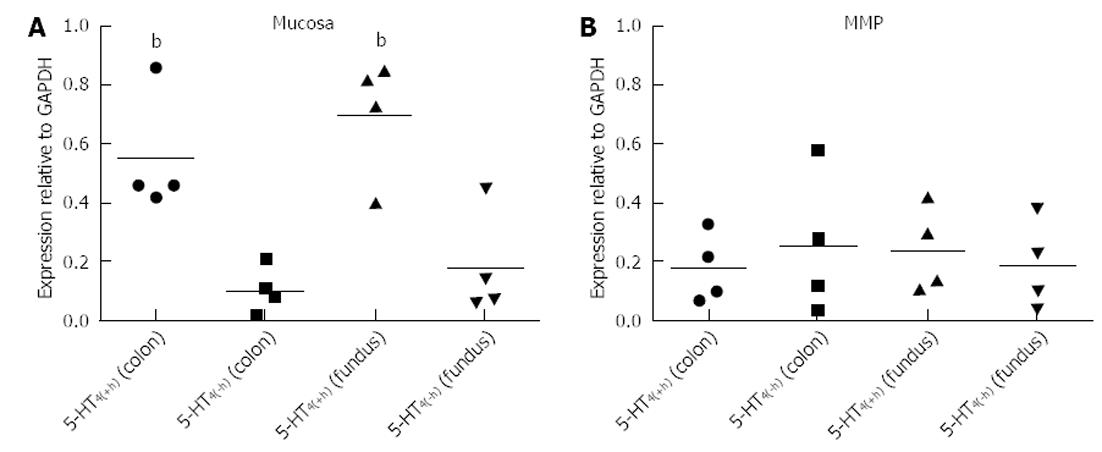
Mucosa fractions of the colon descendens and gastric fundus contained relatively more h-exon containing 5-HT4 receptors. Semi-quantification by expressing the intensity of the bands compared with the intensity of GAPDH and statistical analysis (Figure 6), confirmed the significantly (P < 0.01) more pronounced expression of 5-HT4(+h) receptor within the mucosa fractions (the ratio vs GAPDH was 0.55 ± 0.10 in the colon descendens and 0.69 ± 0.13 in the gastric fundus; n = 4) compared to the expression of the 5-HT4(-h) receptor (colon descendens: 0.12 ± 0.03; gastric fundus: 0.21 ± 0.10; n = 4). Within the MMP fraction of the colon descendens and gastric fundus there was no difference in expression of the 5-HT4(+h) receptor (colon descendens: 0.18 ± 0.06; and gastric fundus: 0.18 ± 0.05; n = 4) and 5-HT4(-h) receptor (colon descendens: 0.26 ± 0.12; gastric fundus: 0.17 ± 0.09; n = 4).
Whole tissue sections, and the mucosal or MMP part of tissue sections of the colon descendens and gastric fundus were scraped off a membrane slide and 5-HT4 receptor expression was analyzed.
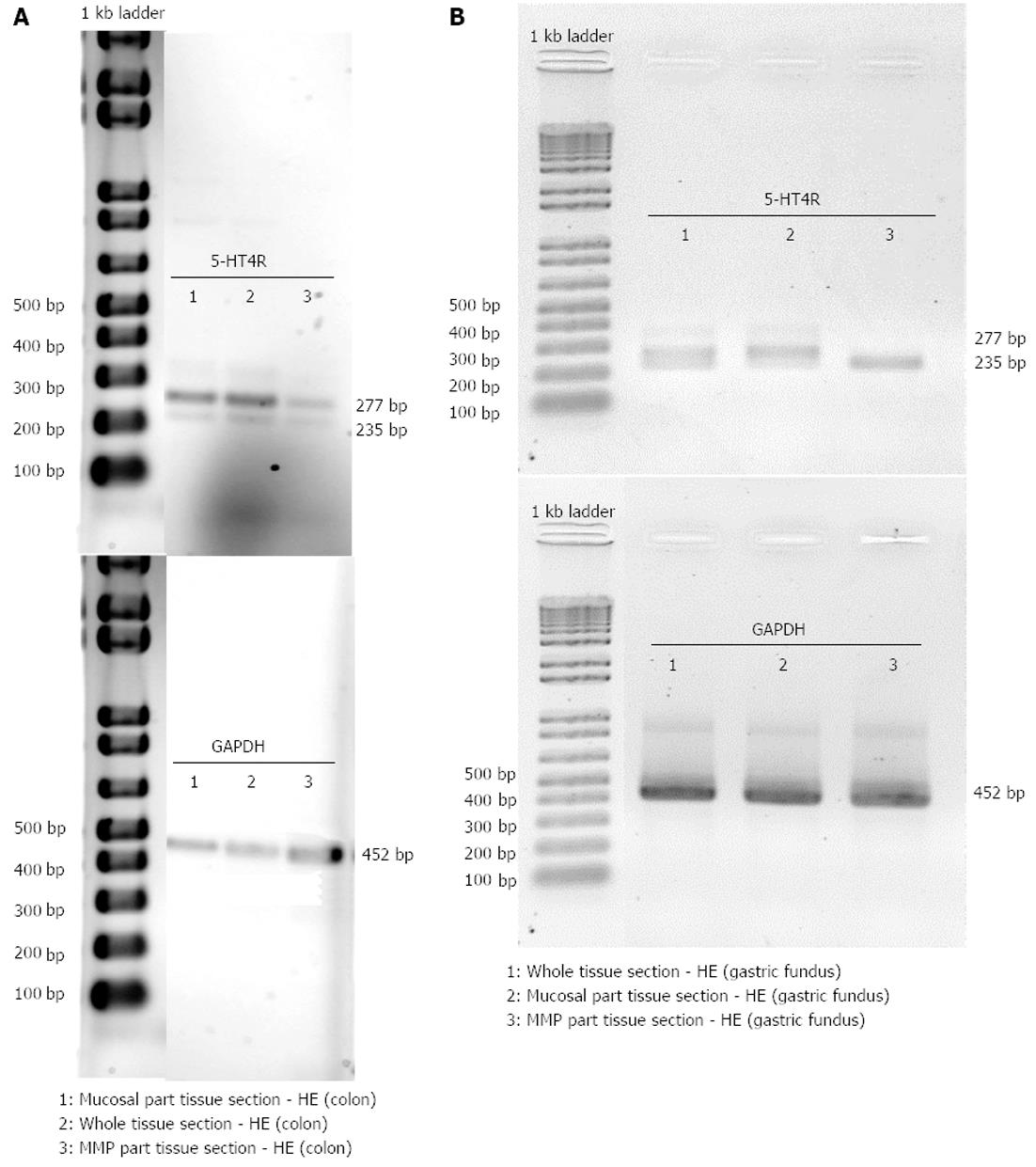
Colon descendens: 5-HT4 receptor and GAPDH expressions were detected in the whole tissue sections, and in the mucosal and MMP parts of tissue sections of the colon descendens (Figure 7A). After semi-quantification, the values for 5-HT4 receptor expression were 5-HT4(+h)R 1.21 ± 0.66 and 5-HT4(-h)R 0.63 ± 0.45 within whole tissue sections (n = 3); 5-HT4(+h)R 0.86 ± 0.39, 5-HT4(-h)R 0.28 ± 0.14 within the mucosal part of tissue sections (n = 3); and 5-HT4(+h)R 0.47 ± 0.05, 5-HT4(-h)R 0.24 ± 0.11 within the MMP part of tissue sections (n = 3). The tendency for more pronounced expression of the h-exon containing splice variant did not reach significance.
Gastric fundus: 5-HT4 receptor and GAPDH expression was also detected in the whole tissue sections, and mucosal and MMP parts of tissue sections of the gastric fundus (Figure 7B). After semi-quantification, expression values were 0.51 and 0.19 for 5-HT4(+h)R, 0.23 and 0.11 for 5-HT4(-h)R in whole tissue sections, 1.13 and 0.24 for 5-HT4(+h)R and 0.31 and 0.05 for 5-HT4(-h)R in the mucosal part of tissue sections. In the two samples with the MMP part of tissue sections, the 5-HT4(-h) receptor was found in both (0.24 and 0.45), while the 5-HT4(+h) receptor was only found in one of the samples (0.53) (Figure 7B lane 3, showing the result with only the 5-HT4(-h) receptor detected).
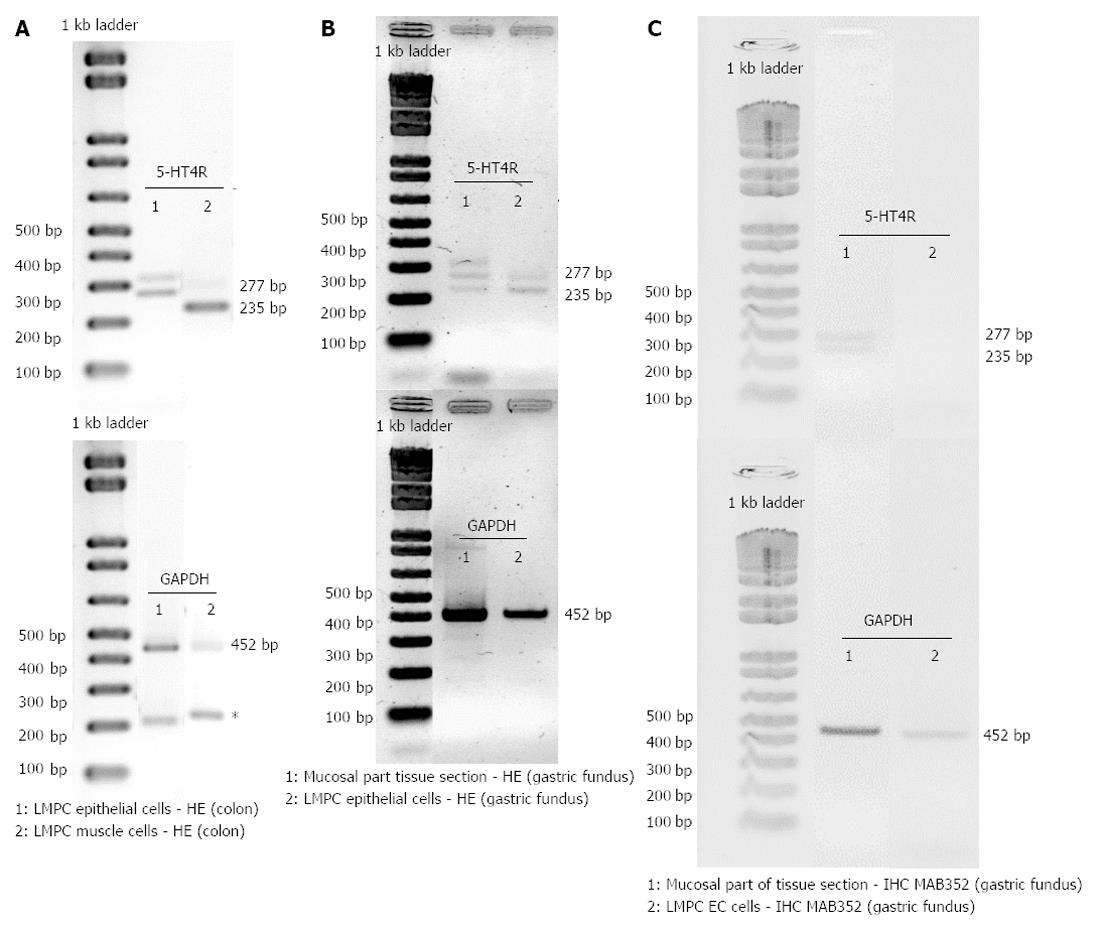
After HE staining, large microdissected patches of cells were taken from the epithelium and the circular smooth muscle layer of the colon descendens and from the epithelium of the gastric fundus. Low RNA yields meant that only after a second round of PCR, with the same 5-HT4 receptor specific-primers, was 5-HT4 receptor expression detected in large microdissected patches of epithelial cells (Figure 8A and B) or smooth muscle cells (Figure 8A). Non-specific amplification was occasionally observed because of the high number of cycles (Figure 8A). In the colon descendens (Figure 8A), higher expression of the 5-HT4(+h) receptor was observed compared with the 5-HT4(-h) receptor within the LMPC-isolated epithelial cells [5-HT4(+h)R 1.03 and 0.85, 5-HT4(-h)R 0.24 and 0.11], while the opposite occurred for the smooth muscle cells [5-HT4(+h)R 1.14 and 0.91, 5-HT4(-h)R 5.07 and 4.03]. In the gastric fundus epithelial cells (Figure 8B), the expression appeared similar for the 5-HT4(+h)R (0.45 and 0.20) and 5-HT4(-h)R (0.67 and 0.24).
After IHC staining, EC cells (MAB352; Figure 8C), were isolated by LMPC from gastric fundus tissue sections. After a second round of PCR with the same 5-HT4 receptor specific-primers, no 5-HT4 receptor expression was detected in LMPC-isolated EC cells, although the cells were positive for the GAPDH gene, confirming the integrity of the samples and completed PCR reactions at least for GAPDH. Additionally, in the mucosal part (Figure 8C) of IHC stained tissue sections, scraped off after LMPC of EC cells, 5-HT4 receptor and GAPDH expressions were detected.
The aim of this study was to investigate the 5-HT4 receptor distribution in the pig GI tract by confining gene expression analysis to site-specific regions of interest, with special attention being paid to the mucosal layer of the pig colon descendens and gastric fundus by isolating epithelial cells using the LMPC technique. A stepwise approach was used by first studying mucosal and MMP tissue fractions, then tissue sections where different cell layers were discerned morphologically by HE staining, and finally tissue sections stained for a particular cell type by IHC. The impact of the freezing method and staining method on the RNA quality was evaluated in mucosa and MMP tissue fractions (Figure 4A), and mucosal and MMP parts of HE stained tissue sections (Figure 4B) of the pig colon descendens and gastric fundus. The major advantages of LMPC are the isolation of biological material without direct user contact, thereby avoiding contamination, and the preservation of cellular integrity[32]. The main obstacles, when using LMPC to analyse 5-HT4 receptor mRNA expression in different cell types, are firstly to recognize the cells of interest and secondly to obtain RNA of good quality. To recognize the cells of interest while preserving RNA integrity, an HE protocol and a cell-specific IHC protocol were developed in RNase-free conditions. Developing a suitable IHC staining procedure was more complex compared with the HE staining, because a standardized IHC procedure requires a long overnight antibody incubation to obtain good antibody labeling; however, long incubation in aqueous buffers activates endogenous RNases, resulting in RNA degradation[33]. Our attempts to develop a fast IHC protocol resulted in diminished visualization because of the short antibody labeling time. Therefore, our IHC staining protocol was based on the report published by Brown et al[24], where overnight antibody incubation and RNA integrity could be maintained with the addition of 1 mol/L NaCl to all aqueous solutions, resulting in superior protection of RNA. It is not yet clear why a saline solution preserves RNA, although we can speculate that normal saline protects the integrity of cell membranes, which may prevent the release of intracellular RNases[34]. Our results confirm the preservation of RNA after HE staining (Figure 4B) as well as after overnight IHC staining (Figure 4D). EC cells in the mucosal layer of the pig gastric fundus were visualized by IHC staining with the MAB352 antibody against 5-HT (Figure 3). Indeed, Penkova et al[35] showed that the MAB352 antibody is selective for EC cells in the human gastric mucosa, as verified by electron microscopy.
In this study, 5-HT4 receptor expression was detected in both mucosa and MMP tissue fractions (Figures 5 and 6), and in mucosal and MMP parts of HE stained tissue sections (Figure 7) of the colon descendens, as well as of the gastric fundus, confirming previously reported data by De Maeyer et al[16]. Expressions of both a variant with the h-exon [5HT4(+h) receptor, 277 bp] and one without the h-exon [5HT4(-h) receptor, 235 bp] were observed. This was made possible by analysis of the 5-HT4 receptor mRNA expression profile using forward and reverse primers based on exon 4 and 5, which were designed to amplify part of the common receptor region encoded by exon 4 and 5, which flank exon h (Figure 1B). The study was not designed to discriminate the C-terminal tail associated with the h-exon; the 5HT4(+h) receptor detected might indeed be associated with several C-terminal splice variants, because the h-exon has already been associated with the a, m and r C-terminal in pigs[16]. Semi-quantitative analysis revealed that in the mucosa tissue fractions of colon descendens and gastric fundus, the expression level of the 5-HT4(+h) receptor was significantly higher compared with the 5-HT4(-h) receptor, and a similar trend was obtained in the mucosal part of HE stained tissue sections. While evaluation of 5-HT4 receptor RNA expression in human GI full-thickness tissue samples showed similar levels in the stomach compared with more distal levels[36], expression in human gastric mucosal specimens was much less pronounced than in mucosal specimens of more distal regions of the GI tract[21,22]. In the pig gastric fundus mucosa, however, the expression of the 5-HT4 receptor was similar to that in the mucosa of the colon descendens, with a clear-cut preponderance of the h-exon-containing receptor. The predominant mucosal location of h-exon containing 5-HT4 receptor splice variants might correspond to the preferential involvement of this type of 5-HT4 receptor splice variant in the mucosal effects of 5-HT4 receptor activation, such as goblet cell degranulation, chloride secretion and control of 5-HT release[21].
Both the mucosal and the MMP part of the GI tract contain several cell types on which the presence of 5-HT4 receptors has been suggested, at least in some regions in some species, such as EC cells, smooth muscle cells of the muscularis mucosae and submucosal intrinsic neurons in the mucosal part; and myenteric cholinergic neurons, smooth muscle cells and interstitial cells of Cajal in the MMP part[37-41]. To obtain more information on the cell-specific distribution of the 5-HT4 receptors, cell layers or particular cell types were isolated by LMPC. In the colon descendens, patches of the epithelial cell layer obtained by LMPC showed expression of 5-HT4 receptors, predominantly the 5HT4(+h) receptor. Possible cell types involved might be EC cells and goblet cells, which were recently shown to express 5-HT4 receptors in the mouse intestine[21]. In mouse, application of 5-HT4 receptor agonists led to mucosal 5-HT release and mucus secretion in a tetrodotoxin-insensitive manner, indicating direct activation of stimulatory 5-HT4 receptors on the EC cells and goblet cells[21]. In the porcine and human small intestine however, analysis of 5-HT release suggested the presence of inhibitory 5-HT4 receptors on the EC cells[42]. Relaxant 5-HT4 receptors have been proposed on circular smooth muscle in the human colon on the basis of functional data[13]; therefore, patches of cells were also obtained from the circular muscle layer of the pig colon descendens, which revealed 5-HT4 receptor expression. However, some authors were not able to confirm the presence of relaxant 5-HT4 receptors in human colonic circular muscle strips[43]; we were also unable to obtain evidence for muscular 5-HT4 receptors in pig colonic circular muscle strips[12]. Thus, we can not exclude the possibility that the 5-HT4 receptor expression observed in LMPC-isolated cell patches from the pig colonic circular muscle layer represents the 5-HT4 receptors on intercalated interstitial cells of Cajal. In addition, in the pig gastric fundus, LMPC-isolated cell patches of the epithelial cell layer showed 5-HT4 receptor expression. The human gastric mucosa was shown to contain a considerable number of EC cells scattered within the lining epithelium[35]; therefore, we stained EC cells in porcine gastric mucosa immunohistochemically and isolated them by LMPC. Although the 5-HT4 receptor was still detected in the full mucosal part of these IHC stained sections, 5-HT4 receptor expression was not detected in the isolated EC cells. The EC cells showed expression of GAPDH; therefore, this might be related to the small amount of 5-HT4 receptor RNA obtained, even when pooling 500 LMPC-isolated cells. In the gastric fundus, LMPC was not used to obtain cell patches from the muscle layer, as there are no functional data suggesting 5-HT4 receptors to be present on muscle cells in the stomach.
In conclusion, this study, using endpoint RT-PCR, confirmed the presence of 5-HT4 receptors in the mucosa and in the MMP part of porcine gastric fundus and colon descendens, and showed that the mucosa predominantly expresses h-exon-containing 5-HT4 receptors. The mucosal h-exon-containing 5-HT4 receptors might form additional sites of action for 5-HT4 receptor agonists. 5-HT4 receptors were detected in LMPC-isolated epithelial cell patches in the gastric fundus and colon descendens, and in circular muscle cell patches in the colon descendens. No 5-HT4 receptor expression was detected in gastric LMPC-isolated EC cells stained by immunohistochemistry; the expression of 5-HT4 receptors in individual cell types might be too low to detect by LMPC and endpoint PCR.
The authors thank Mrs. An Neesen, Sandra Soetaert and Trees Lepez for their technical help.
5-HT4 receptors are distributed throughout the gastrointestinal (GI) tract. They are expressed on excitatory motor neurons, promoting the stimulatory effect of these neurons on GI motility. This explains the therapeutic use of 5-HT4 receptor agonists in conditions with impaired GI motility, such as constipation. Several reports indicate that 5-HT4 receptors are also expressed in the GI mucosa. 5-HT4 receptors are G-protein coupled receptors containing seven transmembrane domains. The intracellular tail shows splice variation, with different lengths of the intracellular amino acid sequence. Exceptionally among G-protein coupled receptors, 5-HT4 receptors can also have an extra insertion of 14 amino acids in the second extracellular loop, encoded by the h-exon (42 base pairs). Previous distribution studies of 5-HT4 receptors in the GI tract did not consider the all or none presence of the h-sequence.
Using homogenates of tissues limits the potential to study cell-specific expression: important cell-specific transcript information is lost because of cell heterogeneity of tissues such as GI tissues. Techniques have been developed to enable collection of particular cells from mixed populations, which generally involve either fluorescence activated cell sorting (FACS) purification of dissociated cells or laser-assisted microdissection. In contrast to FACS, microdissection can be applied to most tissues.
The major advantages of laser microdissection and pressure catapulting (LMPC) are the isolation of biological material without direct user contact, thereby avoiding contamination, and the preservation of cellular integrity. The main concerns when using LMPC to analyse 5-HT4 receptor mRNA expression of a specific cell type is the efficient and selective isolation of the right cells, and obtaining RNA of good quality. To address these issues, and to optimize a LMPC experimental design for GI tissue that is highly heterogeneous, rich in endogenous RNase and enzymes, a systematic approach was required to evaluate the impact on RNA integrity of different critical steps. Therefore, RNA yield and quality were determined using the Experion automated electrophoresis system after tissue collection, hematoxylin and eosin (HE) staining or immunohistochemistry (IHC) staining, and LMPC by analysing RNA extracted from mucosal and muscular myenteric plexus (MMP) tissue fractions, and from scraped off tissue sections after staining and after LMPC. Developing a suitable IHC staining procedure was more complex compared with the HE staining, because a standard IHC procedure requires a long overnight antibody incubation to obtain good antibody labeling, but long incubation in aqueous buffers activates endogenous RNases, resulting in RNA degradation. The authors attempted to develop a fast IHC protocol, which resulted in diminished visualization because the short antibody labeling time. Therefore, the authors used an overnight antibody incubation for the IHC staining protocol whereby RNA integrity was maintained by the addition of 1 mol/L NaCl to all aqueous solutions, resulting in superior protection of RNA. It is not yet clear why a saline solution preserves RNA, although the authors speculate that normal saline protects the integrity of cell membranes, preventing the release of intracellular RNases.
This study, using endpoint reverse transcription-polymerase chain reaction (PCR) and LMPC, confirms the presence of 5-HT4 receptors in the mucosal and MMP parts of the porcine gastric fundus and colon descendens, and shows that the mucosa predominantly expresses h-exon containing 5-HT4 receptors. The mucosal h-exon-containing 5-HT4 receptors might be preferentially involved in the mucosal response to 5-HT4 receptor activation and might form potential drug targets for 5-HT4(+h) receptor-selective agonists.
5-HT4 receptors: The 5-HT4 receptor is a G-protein coupled receptor that activates the adenylyl cyclase/cyclic adenosine monophosphate/protein kinase A pathway in response to serotonin (5-hydroxytryptamine; 5-HT). The 5-HT4 receptor is expressed on excitatory motor neurons in the gut, facilitating acetylcholine release, which stimulates GI motility; LMPC: Under direct microscopic visualization, LMPC permits sampling of histologically or immunohistologically defined tissue and cell samples. The LMPC system uses a focused pulsed nitrogen UV-A laser beam (wavelength 355 nm) whose source is positioned below the material and the high energy generated beam is focused through a microscope ocular lens onto the biological material on the slide.The RoboMover stage is used to move the sample through the laser beam path to allow the user to control the size and shape of the area to be cut. The beam is then defocused and this energy is used to catapult the membrane and corresponding biological material from the slide. When the laser beam strikes the material, it is blasted off the glass surface and catapulted into the cap of the vial.
This manuscript describes the expression of the +h 5-HT4 receptor variant in porcine stomach and colon. The significance of this work is the use of LMPC and end-point PCR to allow the positive identification of the specific HTR4 variant (+ exon h) in discernible functional cell types and tissue regions relevant to serotoninergic responses. This study also discusses several technique modifications and improvements and artifacts in detail, which might be helpful for wider application of similar interests.
| 1. | Ren J, Zhou X, Galligan JJ. 5-HT4 receptor activation facilitates recovery from synaptic rundown and increases transmitter release from single varicosities of myenteric neurons. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1376-G1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 1162] [Article Influence: 61.2] [Reference Citation Analysis (2)] |
| 3. | Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1148-G1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Manabe N, Wong BS, Camilleri M. New-generation 5-HT4 receptor agonists: potential for treatment of gastrointestinal motility disorders. Expert Opin Investig Drugs. 2010;19:765-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Leclere PG, Prins NH, Schuurkes JA, Lefebvre RA. 5-HT4 receptors located on cholinergic nerves in human colon circular muscle. Neurogastroenterol Motil. 2005;17:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Prins NH, Akkermans LM, Lefebvre RA, Schuurkes JA. 5-HT(4) receptors on cholinergic nerves involved in contractility of canine and human large intestine longitudinal muscle. Br J Pharmacol. 2000;131:927-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Dutta U, Padhy AK, Ahuja V, Sharma MP. Double blind controlled trial of effect of cisapride on gastric emptying in diabetics. Trop Gastroenterol. 1999;20:116-119. [PubMed] |
| 8. | Bouras EP, Camilleri M, Burton DD, Thomforde G, McKinzie S, Zinsmeister AR. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 220] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Leclere PG, Lefebvre RA. Presynaptic modulation of cholinergic neurotransmission in the human proximal stomach. Br J Pharmacol. 2002;135:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Priem E, Van Colen I, De Maeyer JH, Lefebvre RA. The facilitating effect of prucalopride on cholinergic neurotransmission in pig gastric circular muscle is regulated by phosphodiesterase 4. Neuropharmacology. 2012;62:2126-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | De Maeyer JH, Prins NH, Schuurkes JA, Lefebvre RA. Differential effects of 5-hydroxytryptamine4 receptor agonists at gastric versus cardiac receptors: an operational framework to explain and quantify organ-specific behavior. J Pharmacol Exp Ther. 2006;317:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Priem EK, Lefebvre RA. Investigation of neurogenic excitatory and inhibitory motor responses and their control by 5-HT(4) receptors in circular smooth muscle of pig descending colon. Eur J Pharmacol. 2011;667:365-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | McLean PG, Coupar IM, Molenaar P. A comparative study of functional 5-HT4 receptors in human colon, rat oesophagus and rat ileum. Br J Pharmacol. 1995;115:47-56. [PubMed] |
| 14. | Borman RA, Burleigh DE. Human colonic mucosa possesses a mixed population of 5-HT receptors. Eur J Pharmacol. 1996;309:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Chetty N, Coupar IM, Tan YY, Desmond PV, Irving HR. Distribution of serotonin receptors and interacting proteins in the human sigmoid colon. Neurogastroenterol Motil. 2009;21:551-558, e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | De Maeyer JH, Aerssens J, Verhasselt P, Lefebvre RA. Alternative splicing and exon duplication generates 10 unique porcine 5-HT 4 receptor splice variants including a functional homofusion variant. Physiol Genomics. 2008;34:22-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Budhoo MR, Kellum JM. Evidence for a 5-HT4 receptor pathway mediating chloride secretion in the rat distal colon. J Surg Res. 1994;57:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Budhoo MR, Harris RP, Kellum JM. 5-Hydroxytryptamine-induced Cl- transport is mediated by 5-HT3 and 5-HT4 receptors in the rat distal colon. Eur J Pharmacol. 1996;298:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Albuquerque FC, Smith EH, Kellum JM. 5-HT induces cAMP production in crypt colonocytes at a 5-HT4 receptor. J Surg Res. 1998;77:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Ning Y, Zhu JX, Chan HC. Regulation of ion transport by 5-hydroxytryptamine in rat colon. Clin Exp Pharmacol Physiol. 2004;31:424-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844-854.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 22. | van Lelyveld N, Ter Linde J, Schipper ME, Samsom M. Regional differences in expression of TPH-1, SERT, 5-HT(3) and 5-HT(4) receptors in the human stomach and duodenum. Neurogastroenterol Motil. 2007;19:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | van Nassauw L, Timmermans JP. Detailed knowledge of cellular expression of G protein-coupled receptors in the human enteric nervous system is essential for understanding their diverse actions. Neurogastroenterol Motil. 2010;22:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Brown AL, Smith DW. Improved RNA preservation for immunolabeling and laser microdissection. RNA. 2009;15:2364-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Böttner M, Bär F, Von Koschitzky H, Tafazzoli K, Roblick UJ, Bruch HP, Wedel T. Laser microdissection as a new tool to investigate site-specific gene expression in enteric ganglia of the human intestine. Neurogastroenterol Motil. 2010;22:168-172, e52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Kapeller J, Möller D, Lasitschka F, Autschbach F, Hovius R, Rappold G, Brüss M, Gershon MD, Niesler B. Serotonin receptor diversity in the human colon: Expression of serotonin type 3 receptor subunits 5-HT3C, 5-HT3D, and 5-HT3E. J Comp Neurol. 2011;519:420-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Kilpatrick GJ, Dautzenberg FM, Martin GR, Eglen RM. 7TM receptors: the splicing on the cake. Trends Pharmacol Sci. 1999;20:294-301. [PubMed] |
| 28. | Bender E, Pindon A, van Oers I, Zhang YB, Gommeren W, Verhasselt P, Jurzak M, Leysen J, Luyten W. Structure of the human serotonin 5-HT4 receptor gene and cloning of a novel 5-HT4 splice variant. J Neurochem. 2000;74:478-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Bevilacqua C, Makhzami S, Helbling JC, Defrenaix P, Martin P. Maintaining RNA integrity in a homogeneous population of mammary epithelial cells isolated by Laser Capture Microdissection. BMC Cell Biol. 2010;11:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Pinzani P, Orlando C, Pazzagli M. Laser-assisted microdissection for real-time PCR sample preparation. Mol Aspects Med. 2006;27:140-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Denisov V, Strong W, Walder M, Gingrich J, Wintz H. Development and Validation of RQI: An RNA Quality Indicator for the Experion Automated Electrophoresis System. Bio-rad Technical note. 2008;5761. |
| 32. | Kuhn DE, Roy S, Radtke J, Khanna S, Sen CK. Laser microdissection and capture of pure cardiomyocytes and fibroblasts from infarcted heart regions: perceived hyperoxia induces p21 in peri-infarct myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H1245-H1253. [PubMed] |
| 33. | Fend F, Emmert-Buck MR, Chuaqui R, Cole K, Lee J, Liotta LA, Raffeld M. Immuno-LCM: laser capture microdissection of immunostained frozen sections for mRNA analysis. Am J Pathol. 1999;154:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 267] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 34. | Vincek V, Nassiri M, Knowles J, Nadji M, Morales AR. Preservation of tissue RNA in normal saline. Lab Invest. 2003;83:137-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Penkova NI, Baltadjiev GA, Koeva YA, Atanassova PK, Andonov VN, Trichkova VA. Serotonin-producing cells in human gastric mucosa--immunohistochemical and electron microscopic study. Folia Med (Plovdiv). 2010;52:31-37. [PubMed] |
| 36. | Mader R, Kocher T, Haier J, Wieczorek G, Pfannkuche HJ, Ito M. Investigation of serotonin type 4 receptor expression in human and non-human primate gastrointestinal samples. Eur J Gastroenterol Hepatol. 2006;18:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Poole DP, Xu B, Koh SL, Hunne B, Coupar IM, Irving HR, Shinjo K, Furness JB. Identification of neurons that express 5-hydroxytryptamine4 receptors in intestine. Cell Tissue Res. 2006;325:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Sakurai-Yamashita Y, Yamashita K, Kanematsu T, Taniyama K. Localization of the 5-HT(4) receptor in the human and the guinea pig colon. Eur J Pharmacol. 1999;383:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Sakurai-Yamashita Y, Yamashita K, Yoshimura M, Taniyama K. Differential localization of 5-hydroxytryptamine3 and 5-hydroxytryptamine4 receptors in the human rectum. Life Sci. 2000;66:31-34. [PubMed] |
| 40. | Wouters MM, Farrugia G, Schemann M. 5-HT receptors on interstitial cells of Cajal, smooth muscle and enteric nerves. Neurogastroenterol Motil. 2007;19 Suppl 2:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115:370-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 269] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 42. | Schwörer H, Ramadori G. Autoreceptors can modulate 5-hydroxytryptamine release from porcine and human small intestine in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:548-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Cellek S, John AK, Thangiah R, Dass NB, Bassil AK, Jarvie EM, Lalude O, Vivekanandan S, Sanger GJ. 5-HT4 receptor agonists enhance both cholinergic and nitrergic activities in human isolated colon circular muscle. Neurogastroenterol Motil. 2006;18:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
P- Reviewers Hori M, TacheY S- Editor Huang XZ L- Editor Stewart GJ E- Editor Li JY