Published online May 28, 2013. doi: 10.3748/wjg.v19.i20.3134
Revised: March 6, 2013
Accepted: March 23, 2013
Published online: May 28, 2013
Processing time: 180 Days and 22.6 Hours
AIM: To evaluate the correlation between nonalcoholic fatty liver disease (NAFLD) and microvascular complications in type 2 diabetes mellitus (T2DM).
METHODS: Data were obtained from 1217 inpatients with T2DM (757 females, 460 males; aged 63.39 ± 12.28 years). NAFLD was diagnosed by hepatic ultrasonography. Diabetic nephropathy (DN), diabetic peripheral neuropathy (DPN), and diabetic retinopathy (DR) were diagnosed according to their respective criteria. The prevalence of NAFLD and the independent correlations of clinical characteristics with NAFLD were determined by cross-tabulation and logistic regression, respectively.
RESULTS: Approximately 61% of inpatients with T2DM in Qingdao, China had NAFLD, which decreased significantly with increase in age and prolonged course of diabetes. The prevalence of NAFLD in patients presenting with DN, DPN and DR was 49.4%, 57.2% and 54.9%, respectively. These rates were significantly lower than those of patients without DN, DPN and DR (65.9%, 65.6% and 66.1%, respectively, P < 0.05). Participants with NAFLD had greater body weight, waist circumference (WC), body mass index (BMI), fasting blood glucose (FBG), hemoglobin A1c, alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase, blood pressure, as well as triglyceride (TG) levels and lower high-density lipoprotein (HDL) concentration than those without NAFLD (P < 0.05). NAFLD was positively correlated with BMI, WC, TG, FBG, diastolic blood pressure, and systolic blood pressure but negatively correlated with the duration of diabetes, DR, DPN, DN, and HDL.
CONCLUSION: Despite the benign nature of NAFLD, efforts should be directed toward early diagnosis, intensive blood glucose and blood pressure control, and effective dyslipidemia correction.
Core tip: Nonalcoholic fatty liver disease (NAFLD) and diabetic microangiopathy complications represent important burdens for patients with type 2 diabetes mellitus (T2DM). However based on the finding that the prevalence of NAFLD was negatively correlated with age and duration of T2DM, we suggest that NAFLD is benign process and efforts should be directed at strengthening early diagnosis, intensive blood glucose and pressure control, and effective dyslipidemia correction to prevent and minimize occurrence of NAFLD.
- Citation: Lv WS, Sun RX, Gao YY, Wen JP, Pan RF, Li L, Wang J, Xian YX, Cao CX, Zheng M. Nonalcoholic fatty liver disease and microvascular complications in type 2 diabetes. World J Gastroenterol 2013; 19(20): 3134-3142
- URL: https://www.wjgnet.com/1007-9327/full/v19/i20/3134.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i20.3134
Diabetes is an independent risk factor for the development of nonalcoholic fatty liver disease (NAFLD) and progression to advanced liver disease, including fibrosis, cirrhosis, and hepatocellular carcinoma[1]. Cross-sectional studies have reported that the prevalence of NAFLD in patients with type 2 diabetes mellitus (T2DM) ranges from 42.1% to 75.2% in China[2,3]. With the rising incidence and prevalence of T2DM in China[4], a close estimate of the prevalence of NAFLD, as well as its clinical risk factors, is important for predicting the number of patients that require monitoring for more advanced liver diseases, or those who may benefit from future disease-modifying agents.
Targher et al[5] have reported that most individuals with NAFLD are older, more likely to be male, and have a longer duration of diabetes than those without NAFLD. By contrast, Williamson et al[6] have reported that participants with definite steatosis (grade 3) are significantly younger and have shorter duration of diabetes than the combined normal/probably normal groups (grades 0-2). Zhou et al[3] have indicated that the prevalence of NAFLD in men aged < 50 years is higher than that in women. However, this finding is contrary for patients > 50 years; that is, the prevalence of NAFLD is higher in women[3]. Thus, further studies must be conducted to ascertain the differences in the prevalence of NAFLD between the sexes and the relationship between age, duration of diabetes, and prevalence of NAFLD.
The present cross-sectional study determined the prevalence and some risk factors for NAFLD and evaluated its correlations with microvascular complications in a large cohort of inpatients with T2DM in Qingdao, China. Differences in the prevalence of NAFLD between men and women, as well as the clinical and biochemical characteristics of the condition, are discussed.
A total of 1217 T2DM patients (757 women and 460 men) participated in this study. All patients were hospitalized between January 2008 and January 2012 at the Department of Internal Medicine, Affiliated Hospital of Medical College, Qingdao University, China. Most participants abstained from alcohol consumption (n = 1107; 91%) or drank minimally (alcohol consumption < 20 g/d; n = 110; 9%). Patient information such as sex, birth date, duration of diabetes, daily alcohol consumption, smoking status, and medications (including hepatotoxic drugs such as glucocorticoids, amiodarone, methotrexate, or antineoplastic drugs) were obtained by the questionnaire method. The heights and body weights (BWs) of the patients were measured (i.e., without wearing a heavy coat and shoes). Body mass index (BMI) was calculated by dividing the BW (kg) by the square of the height (m). Waist circumference (WC) was measured in a standing position at the level of the umbilicus. Fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), fasting serum triglyceride (TG), high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol were determined using standardized methods in our laboratory. Serology for viral hepatitis B and C was also assessed in all participants. Hepatic ultrasonography scanning was performed on patients after an overnight fast by assigned and experienced radiologists, who were blinded to the health conditions of the patients. The liver was graded for markers of hepatic steatosis by using established criteria: a bright hepatic echo pattern (compared with the echo response of the right kidney); increased attenuation of the echo beam; and presence of focal fatty sparing[6]. Participants manifesting symptoms of hepatic steatosis or showing abnormal blood tests of liver function tests were further investigated for other parameters such as antinuclear antibody, antismooth muscle antibody, antimitochondrial antibody, and ferritin.
This study was approved by the Ethics Committee of the Affiliated Hospital Medical College, Qingdao University, and all participants provided written informed consent.
NAFLD was defined as the presence of definite hepatic steatosis on ultrasound scan (i.e., grade 3) in the absence of a secondary cause for hepatic steatosis. Secondary causes were defined as: alcohol consumption ≥ 14 U/wk or participant report of alcohol excess; use of hepatotoxic medication (glucocorticoids, isoniazid, methotrexate, amiodarone, and tamoxifen) within 6 mo prior to the study; positive hepatitis B or C serology; ferritin concentration ≥ 1000 mg/L (milder hyperferritinemia can be associated with obesity, insulin resistance, and NAFLD); clinically significant positive immunology titers (antismooth muscle antibody titer ≥ 1:160 or antimitochondrial antibody titer ≥ 1:40); or previous diagnosis of a persistent secondary cause for chronic liver disease according to their medical records. Patients were excluded from calculations on the prevalence of NAFLD if their data on the above-mentioned measures were missing, such that a secondary cause could not be excluded.
Diabetic microvascular complications included diabetic nephropathy (DN), diabetic peripheral neuropathy (DPN), and diabetic retinopathy (DR). DN was diagnosed by a positive persistent proteinuria for at least three consecutive readings per year, serum creatinine > 130 µmol/L, and/or glomerular filtration rate < 60 mL/min. DPN was diagnosed in the presence of persistent numbness, paresthesia, loss of hearing of the tuning fork and sense of vibration, and failure to elicit a knee and/or ankle jerk. DR was diagnosed in the presence of retinal hemorrhage, exudates, and macular edema[7].
Analyses were conducted using SPSS version 17.0. Data are presented as means ± SD or proportions, where appropriate. Skewed variables (e.g., TG and HDL) were logarithmically transformed, and genomic means are presented. Independent t and χ2 tests were used to compare the differences in means or proportions between different subgroups. The independent associations of variables with NAFLD were determined by a binary logistic regression. A value of P < 0.05 was considered statistically significant.
The mean demographic and clinical characteristics of the patients were as follows: age, 63.39 ± 12.28 years; duration of diabetes, 9.58 ± 7.09 years; BW, 70.9 ± 11.75 kg; WC, 93.63 ± 10.48 cm; BMI, 26.26 ± 3.54 kg/m2; FBG, 8.48 ± 3.25 mmol/L; HbA1c, 8.73% ± 2.47%; TG, 1.85 ± 1.33 mmol/L; total cholesterol (TC), 5.0 ± 1.29 mmol/L; HDL, 1.20 ± 0.29 mmol/L; and LDL, 2.95 ± 0.86 mmol/L.
The baseline characteristics of the study participants according to their NAFLD status are presented in Table 1. The prevalence of NAFLD in patients with T2DM was 61%, and no statistical difference was indicated between men and women (59.1% vs 62.1%, P > 0.05). Participants with NAFLD had higher BW, WC, BMI, FBG, HbA1c, ALT, AST, GGT, blood pressure and TG levels, and lower HDL concentration than those without NAFLD (P < 0.05). Meanwhile, no statistical difference was observed in the smoking history, height, plasma TC, and LDL levels between the groups. However, participants diagnosed with NAFLD were younger and had shorter duration of diabetes than those without NAFLD (P < 0.05).
| Variables | Without NAFLD | With NAFLD | P value |
| n | 475 | 742 | |
| Sex (female/male) | 287/188 | 470/272 | > 0.05 |
| Age (yr) | 65.11 ± 11.70 | 62.29 ± 12.52 | < 0.05 |
| Diabetes duration (yr) | 11.31 ± 7.10 | 8.47 ± 6.87 | < 0.05 |
| Height (cm) | 163.99 ± 8.49 | 164.20 ± 8.24 | > 0.05 |
| BW (kg) | 65.54 ± 9.76 | 74.32 ± 11.64 | < 0.05 |
| WC (cm) | 88.75 ± 8.72 | 96.76 ± 10.32 | < 0.05 |
| BMI (kg/m2) | 24.33 ± 2.87 | 27.49 ± 3.37 | < 0.05 |
| Current smokers (%) | 17.7 | 14.3 | > 0.05 |
| SBP (mmHg) | 131.43 ± 17.93 | 141.56 ± 16.15 | < 0.05 |
| DBP (mmHg) | 76.94 ± 8.87 | 83.61 ± 9.57 | < 0.05 |
| FBG (mmol/L) | 7.72 ± 3.18 | 8.97 ± 3.20 | < 0.05 |
| HbA1c (%) | 8.45 ± 2.44 | 8.91 ± 2.47 | < 0.05 |
| ALT (mmol/L) | 17.16 ± 8.19 | 25.72 ± 13.01 | < 0.05 |
| AST (mmol/L) | 18.23 ± 6.74 | 22.34 ± 9.00 | < 0.05 |
| GGT (mmol/L) | 19.47 ± 16.32 | 25.05 ± 17.94 | < 0.05 |
| TG (mmol/L) | 1.45 ± 1.06 | 2.12 ± 1.43 | < 0.05 |
| TC (mmol/L) | 4.99 ± 1.41 | 5.04 ± 1.22 | > 0.05 |
| LDL (mmol/L) | 2.95 ± 0.92 | 2.95 ± 0.82 | > 0.05 |
| HDL (mmol/L) | 1.24 ± 0.29 | 1.17 ± 0.28 | < 0.05 |
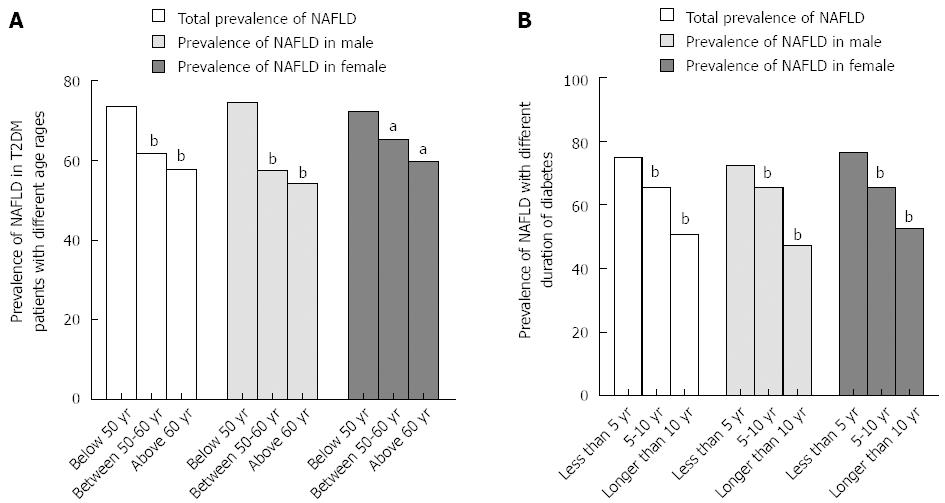
Patients were divided into three groups according to age: < 50, 50-60, and > 60 years. The prevalence rates of NAFLD for the three age groups were 73.4%, 61.6%, and 57.8%, respectively, indicating that the prevalence of NAFLD decreased significantly as age increased (Figure 1A, P < 0.05). This trend persisted even when patients were classified by sex (74.5%, 57.5%, and 54.2% in men and 72.3%, 65.1%, and 59.6% in women, P < 0.05). Although the prevalence of NAFLD in women was higher than that in men < 50 years and the reverse was evident at > 50 years, no significant difference was found in the prevalence of NAFLD between men and women at different ages (Figure 1A, P > 0.05).
When patients were divided into three groups according to duration of diabetes, that is, < 5 years, 5-10 years, and > 10 years, the prevalence rates of NAFLD were 74.9%, 65.5%, and 50.7%, respectively. This indicates that the prevalence of NAFLD decreased significantly as diabetes was prolonged (Figure 1B, P < 0.05). This trend persisted even when patients were classified by sex (72.3%, 65.6%, and 47.1% in men and 76.5%, 65.4%, and 52.6% in females, respectively, P < 0.05). No significant difference was indicated in the prevalence rate of NAFLD between men and women with different durations of diabetes (Figure 1B, P > 0.05).
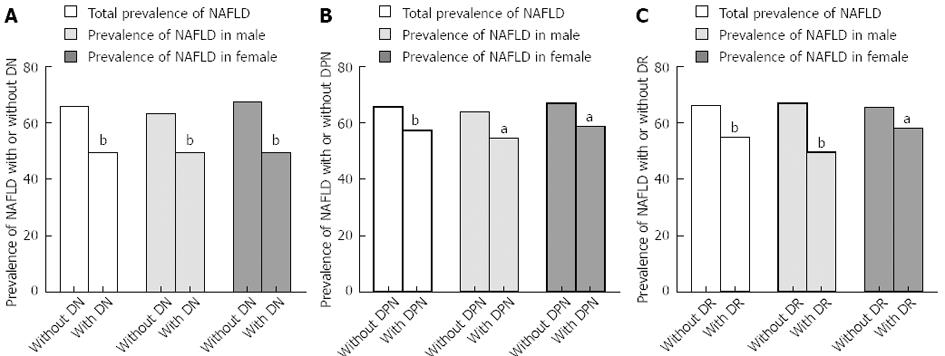
The incidence of NAFLD was found to correlate negatively with DN, as shown in Figure 2A (r = -0.154, P < 0.05). The general prevalence of NAFLD in T2DM with and without DN was 49.4% and 65.9%, respectively (P < 0.05). This trend persisted even when patients were stratified by sex (r = -0.13 in men, r = -0.17 in women, both P < 0.05). The prevalence of NAFLD in men without DN was significantly higher than that of patients with DN (63.3% and 49.3%, respectively, P < 0.05). Similarly, the prevalence of NAFLD in women without DN is significantly higher than that of patients with DN (67.5% and 49.5%, respectively, P < 0.05).
The incidence of NAFLD was found to correlate negatively with DPN, as shown in Figure 2B (r = -0.086, P < 0.05). The general prevalence of NAFLD in T2DM with and without DPN was 57.2% and 65.6%, respectively (P < 0.05). This trend also persisted when patients were stratified by sex (r = -0.095 in men, r = -0.084 in women, both P < 0.05). The prevalence of NAFLD in men without DPN was significantly higher than that of patients with DPN (63.9% and 54.5%, respectively, P < 0.05). Similar results were found in women (66.9% and 58.6%, respectively, P < 0.05).
The incidence of NAFLD was negatively correlated with DR, as shown in Figure 2C (r = -0.114, P < 0.05). The general prevalence of NAFLD in T2DM with and without DR was 54.9% and 66.1%, respectively (P < 0.05). This trend persisted even when patients were stratified by sex (r = -0.176 in men, r = -0.076 in women, both P < 0.05). The prevalence of NAFLD in men without DR was significantly higher than that of patients with DR (66.9% and 49.5%, respectively, P < 0.05). Similar results were determined in women (65.5% and 58.1%, respectively, P < 0.05).
With NAFLD as the dependent variable, the variables that were independently associated with NAFLD in T2DM patients were identified by binary stepwise logistic regression. Independent predictors of NAFLD were BMI (OR for log BMI: 1.238 × 107; 95%CI: 154915.06-9.897 × 107), WC (OR for log WC: 261.44; 95%CI: 1.691-4.422.03), TG (OR for log TG: 7.79; 95%CI: 3.99-15.19), FBG (OR = 1.08; 95%CI: 1.02-1.15), diastolic blood pressure (DBP) (OR = 1.03; 95%CI: 1.01-1.05), systolic blood pressure (SBP) (OR = 1.03; 95%CI: 1.021-1.04), duration of diabetes (OR = 0.96; 95%CI: 0.93-0.98), DR (OR = 0.71; 95%CI: 0.51-0.99), DPN (OR = 0.69; 95%CI: 0.50-0.95), DN (OR = 0.32; 95%CI: 0.22-0.46), and HDL (OR for log HDL: 0.17; 95%CI: 0.03-0.89) (Table 2).
| Variables | β | SE | P value | OR | 95%CI |
| Total patients with T2DM | |||||
| logBMI | 16.33 | 2.24 | 0.00 | 1.238 × 107 | 154915.06-9.897 × 108 |
| logWC | 5.57 | 2.57 | 0.03 | 261.45 | 1.69-40422.03 |
| logTG | 2.05 | 0.34 | 0.00 | 7.79 | 3.99-15.19 |
| FBG | 0.079 | 0.03 | 0.01 | 1.08 | 1.02-1.15 |
| DBP | 0.03 | 0.01 | 0.00 | 1.03 | 1.01-1.05 |
| SBP | 0.03 | 0.01 | 0.00 | 1.03 | 1.02-1.04 |
| Duration of diabetes | -0.05 | 0.01 | 0.00 | 0.96 | 0.93-0.98 |
| DR | -0.035 | 0.17 | 0.04 | 0.71 | 0.51-0.99 |
| DPN | -0.38 | 0.17 | 0.03 | 0.69 | 0.50-0.95 |
| DN | -1.15 | 0.19 | 0.00 | 0.32 | 0.22-0.46 |
| logHDL | -1.76 | 0.84 | 0.04 | 0.17 | 0.03-0.89 |
| Male patients with T2DM | |||||
| logBMI | 24.53 | 3.37 | 0.00 | 4.52 × 1010 | 6.128 × 107-3.334 × 1013 |
| logTG | 3.63 | 0.62 | 0.00 | 37.88 | 11.34-126.61 |
| DBP | 0.08 | 0.02 | 0.00 | 1.09 | 1.05-1.12 |
| Duration of diabetes | -0.07 | 0.02 | 0.00 | 0.94 | 0.90-0.98 |
| logHDL | -4.92 | 1.72 | 0.00 | 0.01 | 0.00-0.21 |
| Female patients with T2DM | |||||
| logBMI | 16.91 | 2.05 | 0.00 | 2.21 × 107 | 395927.04-1.23 × 109 |
| logTG | 1.25 | 0.42 | 0.00 | 3.48 | 1.54-7.90 |
| FBG | 0.2 | 0.04 | 0.00 | 1.22 | 1.13-1.31 |
| SBP | 0.04 | 0.01 | 0.00 | 1.04 | 1.03-1.05 |
| Duration of diabetes | -0.04 | 0.01 | 0.00 | 0.96 | 0.93-0.98 |
| DPN | -0.44 | 0.21 | 0.04 | 0.65 | 0.43-0.98 |
| DR | -0.49 | 0.21 | 0.02 | 0.61 | 0.41-0.93 |
| DN | -1.23 | 0.23 | 0.00 | 0.29 | 0.19-0.46 |
However, the risk factors for NAFLD in men and women were not identical. Independent predictors of NAFLD in men were BMI (OR for log BMI: 4.52 × 1010; 95%CI: 6.12 × 107-3.33 × 1013), TG (OR for log TG: 37.88; 95%CI: 11.34-126.61), DBP (OR = 1.09; 95%CI: 1.05-1.12), duration of diabetes (OR = 0.94; 95%CI: 0.90-0.98), and HDL (OR: for log HDL: 0.01; 95%CI: 0-0.21) (Table 2). Independent predictors of NAFLD in women were BMI (OR for log BMI: 2.21 × 107; 95%CI: 395 927.04-1.23 × 109), TG (OR for log TG: 3.48; 95%CI: 1.54-7.90), FBG (OR = 1.22; 95%CI: 1.13-1.31), SBP (OR = 1.04; 95%CI: 1.03-1.05), duration of diabetes (OR = 0.96; 95%CI: 0.93-0.98), DPN (OR = 0.65; 95%CI: 0.43-0.98), DR (OR = 0.61; 95%CI: 0.41-0.93), and DN (OR = 0.29; 95%CI: 0.19-0.46) (Table 2).
NAFLD represents an important burden of disease for patients with T2DM; however, the magnitude of the problem is currently unknown. In this study, the prevalence of NAFLD in hospitalized Chinese T2DM patients was calculated at 61%. This prevalence is lower than that reported by Lu et al[2] in 2009 (75.18%) but markedly higher compared with that reported by Zhou et al[3] conducted 5 years ago (42.1%).
A limitation of this study was that the diagnosis of NAFLD was based on ultrasound imaging. The patients did not undergo liver biopsy and histological examination, which is the gold standard technique for identifying steatosis. The sensitivity of ultrasonography in detecting steatosis varies between 60% and 94% and is dependent on the degree of steatosis. In particular, sensitivity is significantly low when the degree of steatosis is < 30%[5,8]. Furthermore, some participants with normal ultrasound scans may have undiagnosed hepatic fibrosis and thus be considered a severe case of NAFLD[6]. Therefore, the cases that were potentially misclassified by ultrasonography might have resulted in an underestimated prevalence of NAFLD. This limitation might have attenuated the magnitude of our effect measures to null. The findings of this study may then be considered conservative estimates of the prevalence of NAFLD in hospitalized Chinese T2DM patients.
This study revealed that the prevalence of NAFLD in male hospitalized T2DM patients was 59.1%, which was slightly lower than that in female patients (62.1%), but without statistical significance. When patients were divided into three groups according to their age (i.e., < 50, 50-60, and > 60 years), the prevalence of NAFLD in women was higher than in men aged < 50 years, whereas the reverse was evident when age was > 50 years. However, no significant difference was indicated in the prevalence of NAFLD between male and female patients at different ages. This finding differs from that of Zhou et al[3], who reported that the prevalence of fatty liver in patients < 50 years old was significantly higher in men than women; however, the reverse was evident when participants were older than 50 years. In the present study, several male patients with NFALD, especially those aged < 50 years, were excluded for overdrinking. With this factor considered, the prevalence of NAFLD in male hospitalized T2DM patients may be significantly higher than that of female patients.
In our study, patients were assessed according to three age groups. The prevalence rates of NAFLD significantly decreased as age increased. This trend persisted even when the patients were stratified by sex. These observations are inconsistent with those of Targher et al[5], who reported that the prevalence of NAFLD increased with age (i.e., 65.4% among participants aged 40-59 years and 74.6% among those aged ≥ 60 years; P < 0.001). By contrast, Lu et al[2] found that the prevalence of NAFLD in Chinese T2DM patients did not increase with age. In particular, T2DM patients in China aged 40-59 years are more prone to NAFLD. These findings are consistent with those published in a review by Duvnjak et al[9], which reports that the highest prevalence of NAFLD occurs in those aged 40-60 years. A potential explanation for these observations is that middle-aged persons are often too busy to participate in physical exercise and are more likely to dine outside of the home, whereas older and retired persons may have more time for physical exercise and devote more attention to their lifestyle. These behaviors among middle-aged persons may be one reason why Chinese patients with T2DM are more vulnerable to NAFLD, considering that the prevalence of NAFLD does not increase with age[2].
Patients were also assessed according to the duration of diabetes (i.e., < 5, 5-10, and > 10 years). The prevalence rates of NAFLD decreased significantly with the prolonged course of diabetes. This trend persisted even when patients were stratified by sex. No significant difference was observed in the prevalence of NAFLD between male and female patients with different durations of diabetes. These findings contradict those of Banerjee et al[10], who found that a longer duration of T2DM was significantly associated with NAFLD. The association of a shorter duration of diabetes with liver disease has been previously described. A possible explanation for this association may be that a greater degree of hyperinsulinemia in early T2DM increases the uptake of free fatty acids (FFAs) by hepatocytes[6]. Popović et al[11] presented a significant negative correlation between the duration of diabetes and fasting insulinemia as well as insulin resistance assessed by homeostatic model assessment-insulin resistance index. Increased fasting insulinemia and hepatic insulin resistance may trigger a reduced catabolism of lipoproteins rich in TGs and an increased hepatic very LDL production via changes in the rate of apolipoprotein B synthesis and degradation, as well as de novo lipogenesis or increased FFA flux from adipose tissue into the liver[12].
In this study, we demonstrated that the general prevalence of diabetic microangiopathy complications (i.e., DN, DPN and DR) increased significantly with age increased (data not shown). This trend persisted even when the patients were stratified by sex. When the patients were stratified by duration of T2DM, the prevalence rates of diabetic microangiopathy complications increased significantly with prolonged duration of T2DM (data not shown). In previous reports, the age and duration of diabetes were common risk factors for the three microvascular complications of T2DM[13-17]. Thus, chronic microvascular diabetic complications increase gradually with prolonged duration of T2DM, and the clinical symptoms tend to worsen progressively. Consequently, these patients need to pay more attention to their lifestyle and drug therapy. Therefore, the incidence of NAFLD does not increase with a prolonged duration of T2DM.
The development of NAFLD is a multifaceted cascade of physiological and biochemical events, including genetic, environmental, metabolic, and stress-related factors; the exact risk factors for NAFLD have not been clearly identified[18]. On the basis of the univariate analysis results, patients diagnosed with NAFLD were significantly younger and had a shorter duration of diabetes than those without NAFLD. The BW, WC, BMI, SBP, DBP, FBG, HbA1c, and TG were significantly higher, and HDL cholesterol was significantly lower in patients with NAFLD (Table 1, P < 0.05). Multivariate analysis identified the following as independent predictors of NAFLD: BMI, WC, TG, FBG, DBP, SBP, duration of diabetes, DR, DPN, DN, and HDL. This observation is consistent with the findings reported in literature that the prevalence of NAFLD is closely associated with obesity, dyslipidemia, hypertension, and glucose intolerance, a cluster of metabolic disorders that is now recognized as metabolic syndrome[10,18,19]. However, the negative correlations between the prevalence of NAFLD and the duration of diabetes, DR, DPN, and DN identified in this study are inconsistent with the data reported in previous studies[10]. Given the negative correlations between the prevalence of NAFLD as well as the duration of diabetes and diabetic chronic microvascular complications, more attention should be given to lifestyle and drug therapy, particularly in patients presenting an aggravation in any of the clinical symptoms mentioned above.
Despite the difference in the independent risk factors for NAFLD between men (Table 2) and women (Table 2), both positively correlated with BMI, TG, and hypertension and a negative correlation with the duration of diabetes. In men, the prevalence of NAFLD positively correlated with DBP but negatively with HDL (Table 2). In women, the prevalence of NAFLD positively correlated with FBG and SBP but negatively with DPN, DR and DN (Table 2). According to a previous study, SBP represents the most prevalent type in obese women, being a stronger predictor of cardiovascular disease than DBP[20]. Therefore, the regulation of SBP is more critical for women.
Although most patients with NAFLD presented with non-progressive bland steatosis, few others developed a histological subtype of nonalcoholic steatohepatitis (NASH), which can develop to cirrhosis, hepatocellular carcinoma, and liver-cancer-related death[9,21,22], especially for NASH patients with T2DM[21,23,24]. Thus, specialists have considered that NAFLD may not be a completely benign disorder[9,25]. On the contrary, we consider NAFLD a benign condition because of the negative relationship between the prevalence of NAFLD with age and duration of T2DM. No treatment to alleviate NAFLD or prevent its progression has been scientifically proven. However, various therapeutic alternatives aimed at interfering with the risk factors involved in the pathogenesis of this disorder have been applied to prevent the progression of NAFLD to end-stage liver disease. The most important therapeutic measure is to increase insulin sensitivity by motivating patients to change their lifestyle habits, particularly by diet modification and participation in physical activities to lose weight[26,27].
Tushuizen et al[28] reported that after 44 wk of exenatide therapy, patients experienced a 73% reduction in hepatic fat content (i.e., from a baseline of 15.8% to 4.3%), as evaluated by proton magnetic resonance spectroscopy. This finding is consistent with that reported by Ding et al[29] on the reduced hepatic lipid content in exenatide-treated ob/ob or obese mice compared with placebo-treated mice. Lazo et al[30] reported that a 12-mo intensive lifestyle intervention in patients with T2DM significantly reduced steatosis and NAFLD. In addition, Lo et al[24] have reported that diet-induced NASH fibrosis is exacerbated by diabetes and attenuated by insulin therapy. On the basis of these findings, we believe that NAFLD is reversible, and patients may benefit from its early diagnosis and treatment[31].
In conclusion, the results of this study suggest that NAFLD is extremely common in people with T2DM, positively correlates with BMI, WC, TG, FBG, DBP, and SBP, and negatively correlates with the duration of diabetes, DR, DPN, DN, and HDL. Given that hepatic fat content can be reversed with lifestyle changes and drugs, NAFLD should be included in future preventive public health initiatives, and the affected individuals should be motivated to adopt a healthier lifestyle[27]. Patients with T2DM should be always assessed for NAFLD to ensure early diagnosis and entry into proper and thorough medical care. Future efforts should be directed toward strengthening early diagnosis, intensive blood glucose and blood pressure control, and effective dyslipidemia correction to prevent and minimize the occurrence of NAFLD.
Diabetes is an independent risk factor for the development of nonalcoholic fatty liver disease (NAFLD) and its progression to more advanced liver diseases, including fibrosis, cirrhosis, and hepatocellular carcinoma. NAFLD and diabetic microangiopathy complications represent important burdens of disease for patients with type 2 diabetes mellitus (T2DM), Previous studies have shown that the prevalence of NAFLD and microangiopathy complications in patients with T2DM significantly correlate with age and duration of T2DM. However, few studies have focused on the relationship between NAFLD and diabetic microangiopathy complications.
Many studies have reported that individuals with NAFLD are older, more likely to be male, and have a longer duration of diabetes than those without NAFLD. However, recently, conflicting results have shown that participants with definite steatosis are significantly younger and have shorter durations of diabetes than the combined normal/probably normal groups. Further studies are required to ascertain the differences in the prevalence of NAFLD between the sexes, and the relationships between age, duration of diabetes, and prevalence of NAFLD.
The relatively larger cross-sectional study of inpatients with T2DM in Qingdao, China showed that the prevalence of NAFLD was 61%. However, based on the finding that the prevalence of NAFLD was negatively correlated with age and duration of T2DM, the authors believe that NAFLD is a benign process.
Our results suggest that patients with T2DM should be always assessed for NAFLD to ensure early diagnosis and entry into proper and thorough medical care. Future efforts should be directed at strengthening early diagnosis, intensive blood glucose and pressure control, and effective dyslipidemia correction to prevent and minimize the occurrence of NAFLD.
NAFLD is defined as the presence of definite hepatic steatosis on ultrasound scan in the absence of a secondary cause for hepatic steatosis. Diabetic microvascular complications included diabetic nephropathy (DN), diabetic peripheral neuropathy (DPN), and diabetic retinopathy (DR). DN is defined by increased urinary albumin excretion in the absence of urinary tract infection or other renal abnormalities. DPN is diagnosed in the presence of persistent numbness, paresthesia, loss of hearing of the tuning fork and sense of vibration, and failure to elicit a knee and/or ankle jerk. DR is diagnosed in the presence of retinal hemorrhages, exudates, and macular edema.
This was a good descriptive study in which the authors evaluated the correlation between NAFLD and microvascular complications in Chinese T2DM patients. The results are interesting and suggest that NAFLD is likely a benign process and efforts should be directed at early diagnosis, intensive blood glucose and blood pressure control, and effective dyslipidemia correction, which is of clinical interest.
| 1. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 899] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 2. | Lu H, Zeng L, Liang B, Shu X, Xie D. High prevalence of coronary heart disease in type 2 diabetic patients with non-alcoholic fatty liver disease. Arch Med Res. 2009;40:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Zhou J, Jia WP, Bao YQ, Ma XJ, Lu W, Yu M, Pan JM, Hu C, Xiang KS. [Study on prevalence and risk factors of fatty liver of patients with type 2 diabetes]. Zhonghua Yi Xue Zazhi. 2007;87:2249-2252. [PubMed] |
| 4. | Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2186] [Cited by in RCA: 2325] [Article Influence: 145.3] [Reference Citation Analysis (2)] |
| 5. | Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 708] [Article Influence: 37.3] [Reference Citation Analysis (1)] |
| 6. | Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, Frier BM, Van Look LA, Johnston GI, Reynolds RM. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139-1144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 301] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 7. | Alwakeel JS, Al-Suwaida A, Isnani AC, Al-Harbi A, Alam A. Concomitant macro and microvascular complications in diabetic nephropathy. Saudi J Kidney Dis Transpl. 2009;20:402-409. [PubMed] |
| 8. | Fierbinteanu-Braticevici C, Dina I, Petrisor A, Tribus L, Negreanu L, Carstoiu C. Noninvasive investigations for non alcoholic fatty liver disease and liver fibrosis. World J Gastroenterol. 2010;16:4784-4791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Duvnjak M, Lerotić I, Barsić N, Tomasić V, Virović Jukić L, Velagić V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13:4539-4550. [PubMed] |
| 10. | Banerjee S, Ghosh US, Dutta S. Clinicopathological profile of hepatic involvement in type-2 diabetes mellitus and its significance. J Assoc Physicians India. 2008;56:593-599. [PubMed] |
| 11. | Popović L, Zamaklar M, Lalić K, Vasović O. [Analysis of the effect of diabetes type 2 duration on beta cell secretory function and insulin resistance]. Srp Arh Celok Lek. 2006;134:219-223. [PubMed] |
| 12. | Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42:1331-1346. [PubMed] |
| 13. | Liu Z, Fu C, Wang W, Xu B. Prevalence of chronic complications of type 2 diabetes mellitus in outpatients - a cross-sectional hospital based survey in urban China. Health Qual Life Outcomes. 2010;8:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Chorny A, Lifshits T, Kratz A, Levy J, Golfarb D, Zlotnik A, Knyazer B. [Prevalence and risk factors for diabetic retinopathy in type 2 diabetes patients in Jewish and Bedouin populations in southern Israel]. Harefuah. 2011;150:906-910, 935. [PubMed] |
| 15. | Pradeepa R, Anjana RM, Unnikrishnan R, Ganesan A, Mohan V, Rema M. Risk factors for microvascular complications of diabetes among South Indian subjects with type 2 diabetes--the Chennai Urban Rural Epidemiology Study (CURES) Eye Study-5. Diabetes Technol Ther. 2010;12:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Pradeepa R, Rema M, Vignesh J, Deepa M, Deepa R, Mohan V. Prevalence and risk factors for diabetic neuropathy in an urban south Indian population: the Chennai Urban Rural Epidemiology Study (CURES-55). Diabet Med. 2008;25:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Yokoyama H, Yokota Y, Tada J, Kanno S. Diabetic neuropathy is closely associated with arterial stiffening and thickness in Type 2 diabetes. Diabet Med. 2007;24:1329-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Fang JG, Zhu J, Li XJ, Li R, Dai F, Song XM, Chen L, Li F, Chen SY. [Epidemiological survey of prevalence of fatty liver and its risk factors in a general adult population of Shanghai]. Zhonghua Gan Zang Bing Zazhi. 2005;13:83-88. [PubMed] |
| 19. | Tarantino G, Saldalamacchia G, Conca P, Arena A. Non-alcoholic fatty liver disease: further expression of the metabolic syndrome. J Gastroenterol Hepatol. 2007;22:293-303. [PubMed] |
| 20. | Tarantino G, Pizza G, Colao A, Pasanisi F, Conca P, Colicchio P, Finelli C, Contaldo F, Di Somma C, Savastano S. Hepatic steatosis in overweight/obese females: new screening method for those at risk. World J Gastroenterol. 2009;15:5693-5699. [PubMed] |
| 21. | Lam B, Younossi ZM. Treatment options for nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2010;3:121-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Bugianesi E, Vanni E, Marchesini G. NASH and the risk of cirrhosis and hepatocellular carcinoma in type 2 diabetes. Curr Diab Rep. 2007;7:175-180. [PubMed] |
| 23. | Adams LA, Harmsen S, St Sauver JL, Charatcharoenwitthaya P, Enders FB, Therneau T, Angulo P. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105:1567-1573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Lo L, McLennan SV, Williams PF, Bonner J, Chowdhury S, McCaughan GW, Gorrell MD, Yue DK, Twigg SM. Diabetes is a progression factor for hepatic fibrosis in a high fat fed mouse obesity model of non-alcoholic steatohepatitis. J Hepatol. 2011;55:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Tarantino G, Conca P, Riccio A, Tarantino M, Di Minno MN, Chianese D, Pasanisi F, Contaldo F, Scopacasa F, Capone D. Enhanced serum concentrations of transforming growth factor-beta1 in simple fatty liver: is it really benign? J Transl Med. 2008;6:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Raszeja-Wyszomirska J, Lawniczak M, Marlicz W, Miezyńska-Kurtycz J, Milkiewicz P. [Non-alcoholic fatty liver disease--new view]. Pol Merkur Lekarski. 2008;24:568-571. [PubMed] |
| 27. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1756] [Cited by in RCA: 1827] [Article Influence: 91.4] [Reference Citation Analysis (15)] |
| 28. | Tushuizen ME, Bunck MC, Pouwels PJ, van Waesberghe JH, Diamant M, Heine RJ. Incretin mimetics as a novel therapeutic option for hepatic steatosis. Liver Int. 2006;26:1015-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 457] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 30. | Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, Wagenknecht LE, Pi-Sunyer FX, Kahn SE, Clark JM. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 269] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 31. | Cusi K. Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2009;16:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
P- Reviewers Buechler C, Hsieh PS, Tarantino G S- Editor Wen LL L- Editor Kerr C E- Editor Zhang DN