Published online May 14, 2013. doi: 10.3748/wjg.v19.i18.2731
Revised: March 19, 2013
Accepted: April 3, 2013
Published online: May 14, 2013
Processing time: 81 Days and 6.1 Hours
Cathelicidins, are host defense peptides synthesized and stored in circulating leukocytes and numerous types of epithelial tissues in particular the gastrointestinal (GI) tract and skin. They have been known for their antimicrobial activities against a variety of microbes. Recently it was discovered that they have other significant biological functions and produce appealing pharmacological actions against inflammation and cancer in the GI tract through defined mechanisms. Experimental evidence shows that these actions could be tissue and disease specific and concentration dependent. This article reviews some of the physiological functions of cathelicidins and also their therapeutic potential in the treatment of inflammation and cancer and also the delivery system for this peptide as targeted therapy for various disorders in the GI tract both in animals and humans.
Core tip: Cathelicidin is one of the most important host defense peptides known today. It carries multiple and yet unique biological functions against pathogens which contribute to the induction and also progression of infection, inflammation and cancer, the three major types of diseases in mankind. Deficiency of such peptide would cause multiple dysfunctions in the body. In this review we highlight the physiological role and therapeutic potential of cathelicidin in inflammation and cancer and also mucosal repair in the gut. All these information would shed new lights on the development of cathelicidin as therapeutic agent for different disorders in the gastrointestinal tract.
- Citation: Chow JYC, Li ZJ, Kei WK, Cho CH. Cathelicidin a potential therapeutic peptide for gastrointestinal inflammation and cancer. World J Gastroenterol 2013; 19(18): 2731-2735
- URL: https://www.wjgnet.com/1007-9327/full/v19/i18/2731.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i18.2731
Cathelicidins are innate immunity peptides. They are antimicrobial peptides (AMPs) that are produced by organisms as part of the defensive mechanism against various pathogenic microbes in humans and animals[1,2]. This class of pleiotropic peptides provides the first-line defense against infection by eliminating pathogens. Each AMP is encoded by a distinct gene. They show a great diversity in structures but have some common features, including: (1) relatively small molecular sizes (usually less than 50 amino acid residues); (2) cationic nature; (3) amphipathic helix structure; and (4) a substantial portion of hydrophobic amino acids[2,3]. Human cathelicidin (LL-37) consists of a long amphipathic helix spanning residues 2-31 with the C-terminal residues 32-37 unstructured. Another feature is that the structure is curved with a train of hydrophobic side chains. Such a cationic structure is perfect to associate with anionic micelles[4]. Indeed the cationic cathelicidin reacts electrostatically with anionic membrane components in particular cancer cells and microbes to disrupt cell membranes and induce cell death, while normal cells are neutral[5,6]. This specific property would enable cathelicidins directly and selectively attack membranes of microbes and cancer cells but spare the normal cells[7]. This uniqueness would make these peptides naturally exist and relatively non-toxic to normal mammalian system and have significant clinical implications as therapeutic agents for various diseases in particular those bacterial-related inflammation and cancer in the gastrointestinal (GI) tract[1,4,8].
Cathelicidins, a family of host defense peptides naturally expressed by cells of the GI tract. LL-37 is the mature form of human cathelicidin. It is produced constitutively by differentiated surface and upper crypt epithelial cells in the colon and by the Brunner glands in the duodenum[9]. In normal stomach, the expression of the peptide is restricted to differentiated surface of various types of cells including epithelial cells, chief cells and parietal cells and is also present in the gastric secretion. They are upregulated during infection, inflammation and wound healing both in animals and humans[9-12]. These biological responses to external challenges could have significant implications as a host defense in protection against different disorders in the GI tract.
One good example is in the course of Helicobacter pylori (H. pylori) infection in which the expression of LL-37 is induced along the gastric glands. Induction of LL-37 may help to fight against bacterial infection at the early stage. However, the expression of LL-37 is dysregulated during H. pylori-associated gastric carcinogenesis. During the progression from atrophic gastritis to adenocarcinoma, the expression of LL-37 is reduced[12]. All these findings indicate that cathelicidin could play a significant role in preventing bacteria related inflammation and perhaps also carcinogenesis in the GI tract. It is envisaged that deficiency of this host defense peptide could facilitate the formation of inflammation and cancer.
Wound repair is a crucial adaptation to tissue damage. Based on the above information it comes to no surprise that soluble peptides like cathelicidins could evolve to orchestrate wound healing in response to mucosal damage in the GI tract. Along this line, LL-37 and mouse cathelicidin (mCRAMP) are strongly expressed in skin epithelium during wound healing in humans and mice[13]. In addition, the expression of LL-37 is low or absent in chronic ulcers, and antibodies to this peptide inhibit post-wounding re-epithelialization[14].
Induction of angiogenesis by cathelicidin further highlights its potential role in wound repair[15]. In this context, it has been proposed that the healing-promoting effect of the peptide may be mediated through modification of growth factor and receptor interactions[16,17]. However the exact mechanisms by which cathelicidins promote wound healing have not yet been fully clarified. A recent study conducted by Yang et al[11] in 2006 showed that rat cathelicidin can promote gastric ulcer healing in rats through induction of cell proliferation and angiogenesis. The same peptide stimulates cultured gastric epithelial cells through a transforming growth factor α-dependent transactivation of epidermal growth factor and its related pathway to induce proliferation of gastric cells[11].
Experimental evidence shows that cathelicidin can modulate inflammation by altering cytokine response and chemoattraction of inflammatory cells in diseased tissues[1,18,19]. A recent study demonstrates that bacterial DNA upregulates cathelicidin expression via a Toll-like receptor and mitogen-activated protein kinases/Erk pathway in colonic murine mucosa[20]. Clinical study also showed that cathelicidin expression was altered in inflammatory bowel diseases (IBD) patients. It was increased in both inflamed and non-inflamed mucosa in ulcerative colitis (UC) patients but not in Crohn’s disease patients. The distribution of cathelicidin was also changed. Cathelicidin mainly expressed in the upper crypt of colons in healthy people in contrast to the basal part in IBD patients[21]. In another study, deficiency of cathelicidin in mCRAMP-knockout mice present more severe symptoms and mucosal disruption than the wild-type mice in response to dextran sulfate sodium challenge to induce UC. The inflammatory cytokines and the number of apoptotic cells are increased together with mucus secretion and gene expression are impaired. All these abnormalities are reversed by intrarectal administration of mCRAMP or mCRAMP-encoding plasmid[22]. On the other hand the increase of endogenous cathelicidin by agents such as butyrate and vitamin D has been suggested to modulate inflammatory responses either induced by chemical or bacteria in colonic cells[21,23-26]. Indeed butyrate treatment has been demonstrated to improve rectal histopathology in humans and eradicate Shigella in vitro[23,24] and vitamin D can prevent mucosal injury in chemical-induced acute colitis in mice[25].
The peptide also significantly reduces the increased number of fecal microflora in UC animals[27]. Indeed exogenous cathelicidin modulates Clostridium difficile (C. difficile) colitis. In addition, C. difficile-induced colitic mice treated with cathelicidin inhibits toxin A-associated intestinal inflammation[28]. In view of the current UC therapies mainly focus on relieving the inflammatory responses or reducing the pathogenic microbes, cathelicidin would have both actions, and it further promotes the mucosal defensive mechanism through mucus secretion via a MAP kinase pathway[29]. All these actions would provide us a better therapeutic option in the treatment of inflammation in the colon. In this context, Cho and his group develop a new form of transporting system for this peptide by combining a probiotic Lactococcus lactis with cathelicidin gene into a single preparation. This preparation given orally instead of intrarectal administration[22] produces similar protection against UC in mice[30]. In a similar approach, we have applied the same mCRAMP-secreting strain of Lactococcus lactis to reduce H. pylori density in the stomach as well as the associated inflammatory cell infiltration and cytokine production[31]. These findings show the feasibility of using the transformed food-graded probiotic to deliver cathelicidin to the diseased organs and exert targeted therapy. This new biological preparation would have significant clinical applications in the future as potential therapeutic agent to alleviate inflammation induced by H. pylori infection in the stomach and bacteria overgrowth in the colon.
Although studies have demonstrated that LL-37 could promote tumorigenesis in some cancers including lung and breast cancers as well as epithelial ovarian cancer[32-34]. Other reports have shown that LL-37 may induce cell death in many tissues. In human airway epithelial cells, LL-37 has been shown to result in apoptotic TUNEL positive cells in a caspases-dependent manner[35]. Analogue of LL-37 could induce the caspase-independent apoptosis in an oral squamous cell line SAS-H1 but not normal cells[36]. The anti-tumorigenic effect of LL-37 is dependent on its ability to induce DNA break and mitochondrial damage in Jurkat T leukemia and A549 cells which are independent of caspase activation[37]. It is likely that low tissue expression of LL-37 could promote tumor formation. Indeed downregulation of LL-37 in cancer tissues has also been reported in the GI tract. In normal gastric mucosa, LL-37 is expressed in surface epithelial cells and chief cells as well as parietal cells in the fundic glands. Immunochemical staining of LL-37 has revealed that the expression of LL-37 is down-regulated in gastric hyperplastic polyps, tubular adenomas, and adenocarcinomas[12]. After H. pylori infection, LL-37 is markedly up-regulated in the epithelium and gastric secretions. Such upregulation could not be detected in patients with H. pylori-independent gastric inflammation. Moreover, a higher level of LL-37 expression has been demonstrated in wild-type H. pylori infection of cultured gastric epithelial cells and this higher production of LL-37 requires an intact type IV secretion system[4,12]. Therefore, it is indicated that expression of LL-37 may be in a tissue- and disease-specific manner.
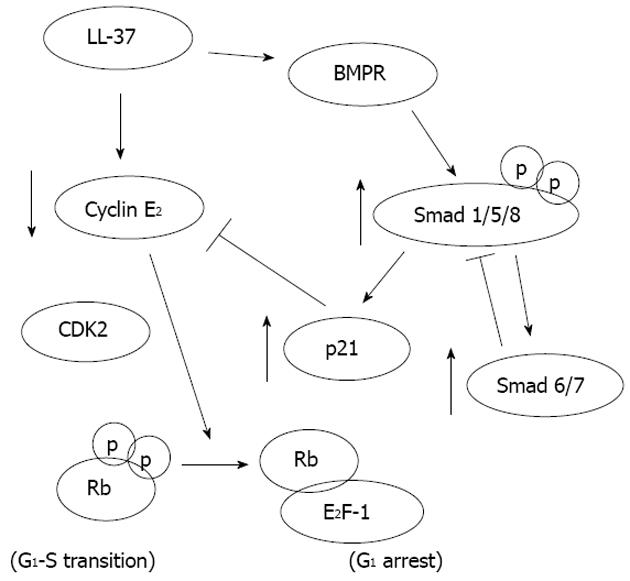
Our recent study shows that LL-37 may function as a putative tumor-suppressing gene in gastric carcinogenesis. We found that exogenous LL-37 inhibits proliferation and induces G0/Gi-phase cell cycle arrest through a defined signal pathway in gastric cancer cells (Figure 1). Furthermore depletion of endogenous LL-37 stimulates gastric cancer cell DNA synthesis suggesting that the antiproliferative effect of LL-37 occurs at physiological concentrations. The direct anti-cancer action has also been confirmed in a gastric xenograft cancer model in nude mice[38]. In the lower GI tract, it has been shown that LL-37 is strongly expressed in the human normal colon mucosa but downregulated in colon cancer tissues. In both settings it is correlated with the number of apoptotic cells in colonic mucosa. To this end, the pro-apoptotic activity of LL-37 is confirmed in colon cancer cells in which the peptide activates a GPCR-p53-Bax/Bak/Bcl-2 signaling cascade that triggers off the AIF/EndoG-mediated apoptosis in colon cancer cells[39]. All these findings suggest that cathelicidin could be a tumor suppressor gene in the stomach and colon. Supplementation of which would have a great potential as a therapeutic agent for gastric and colon cancers.
The host defense peptide cathelicidin is highly expressed in the GI mucosa. This peptide and its recombinant protein in a deliverable preparation represent an appealing option for the treatment of inflammation and cancer and also promotion of mucosal repair in the GI tract (Table 1). This is especially true for those diseases associated with bacteria including gastritis and UC. Depletion of cathelicidin by unknown epigenetic mechanisms in the gastric and colonic tissues could be one of the causative factors in the promotion of inflammation and carcinogenesis in both organs. Supplementation with this host defense peptide orally through an effective delivery system seems to be a promising approach to treat different disorders in the GI tract.
| Type of GI disorders | Functional effects | Mechanisms | Ref. |
| Ulcer | Increases of cell proliferation, re-epithelialization and angiogenesis | Activation of growth factors and their receptors | [11,13-17] |
| Inflammation | Decrease of pathogenic microbes, inflammatory cytokines and apoptosis; increase of mucus secretion | Activation of MAP kinase, formyl peptide receptor and mucin genes; electrostatic interaction on microbial membrane | [1,5,8,23] |
| Cancer | Induction of apoptosis and cell cycle arrest | Release of AIF/EndoG; activation of BMPR and Smads | [29,30] |
| 1. | Wu WK, Wong CC, Li ZJ, Zhang L, Ren SX, Cho CH. Cathelicidins in inflammation and tissue repair: Potential therapeutic applications for gastrointestinal disorders. Acta Pharmacol Sin. 2010;31:1118-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6194] [Cited by in RCA: 6390] [Article Influence: 266.3] [Reference Citation Analysis (0)] |
| 3. | Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2870] [Cited by in RCA: 3221] [Article Influence: 169.5] [Reference Citation Analysis (0)] |
| 4. | Wu WK, Wang G, Coffelt SB, Betancourt AM, Lee CW, Fan D, Wu K, Yu J, Sung JJ, Cho CH. Emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. Int J Cancer. 2010;127:1741-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Schweizer F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol. 2009;625:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 385] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 6. | Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 379] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 7. | Boman HG. Antibacterial peptides: basic facts and emerging concepts. J Intern Med. 2003;254:197-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 807] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 8. | Ahluwalia A, Tarnawski AS. Cathelicidin gene therapy: a new therapeutic option in ulcerative colitis and beyond? Gene Ther. 2013;20:119-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun. 2002;70:953-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 232] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Wong CC, Zhang L, Ren SX, Shen J, Chan RL, Cho CH. Antibacterial peptides and gastrointestinal diseases. Curr Pharm Des. 2011;17:1583-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Yang YH, Wu WK, Tai EK, Wong HP, Lam EK, So WH, Shin VY, Cho CH. The cationic host defense peptide rCRAMP promotes gastric ulcer healing in rats. J Pharmacol Exp Ther. 2006;318:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, Obonyo M, Gallo RL, Eckmann L, Kagnoff MF. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology. 2003;125:1613-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 169] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, Agerberth B, Gudmundsson GH, Gallo RL. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 427] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 14. | Heilborn JD, Nilsson MF, Kratz G, Weber G, Sørensen O, Borregaard N, Ståhle-Bäckdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 410] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Koczulla R, von Degenfeld G, Kupatt C, Krötz F, Zahler S, Gloe T, Issbrücker K, Unterberger P, Zaiou M, Lebherz C. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Gallo RL, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M, Bernfield M. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci USA. 1994;91:11035-11039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 263] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Li J, Post M, Volk R, Gao Y, Li M, Metais C, Sato K, Tsai J, Aird W, Rosenberg RD. PR39, a peptide regulator of angiogenesis. Nat Med. 2000;6:49-55. [PubMed] |
| 18. | Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 744] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 19. | Metz-Boutigue MH, Shooshtarizadeh P, Prevost G, Haikel Y, Chich JF. Antimicrobial peptides present in mammalian skin and gut are multifunctional defence molecules. Curr Pharm Des. 2010;16:1024-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Koon HW, Shih DQ, Chen J, Bakirtzi K, Hing TC, Law I, Ho S, Ichikawa R, Zhao D, Xu H. Cathelicidin signaling via the Toll-like receptor protects against colitis in mice. Gastroenterology. 2011;141:1852-1863.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 21. | Schauber J, Rieger D, Weiler F, Wehkamp J, Eck M, Fellermann K, Scheppach W, Gallo RL, Stange EF. Heterogeneous expression of human cathelicidin hCAP18/LL-37 in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2006;18:615-621. [PubMed] |
| 22. | Tai EK, Wu WK, Wang XJ, Wong HP, Yu L, Li ZJ, Lee CW, Wong CC, Yu J, Sung JJ. Intrarectal administration of mCRAMP-encoding plasmid reverses exacerbated colitis in Cnlp(-/-) mice. Gene Ther. 2013;20:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, Nasirul Islam KM, Gudmundsson GH, Andersson J, Agerberth B. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci USA. 2006;103:9178-9183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Raqib R, Sarker P, Mily A, Alam NH, Arifuzzaman AS, Rekha RS, Andersson J, Gudmundsson GH, Cravioto A, Agerberth B. Efficacy of sodium butyrate adjunct therapy in shigellosis: a randomized, double-blind, placebo-controlled clinical trial. BMC Infect Dis. 2012;12:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 26. | Lagishetty V, Chun RF, Liu NQ, Lisse TS, Adams JS, Hewison M. 1-α-hydroxylase and innate immune responses to 25-hydroxyvitamin D in colonic cell lines. J Steroid Biochem Mol Biol. 2010;121:228-233. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Tai EK, Wu WK, Wong HP, Lam EK, Yu L, Cho CH. A new role for cathelicidin in ulcerative colitis in mice. Exp Biol Med (Maywood). 2007;232:799-808. [PubMed] |
| 28. | Hing TC, Ho S, Shih DQ, Ichikawa R, Cheng M, Chen J, Chen X, Law I, Najarian R, Kelly CP. The antimicrobial peptide cathelicidin modulates Clostridium difficile-associated colitis and toxin A-mediated enteritis in mice. Gut. 2012;Jul 3; Epub ahead of print. [PubMed] |
| 29. | Tai EK, Wong HP, Lam EK, Wu WK, Yu L, Koo MW, Cho CH. Cathelicidin stimulates colonic mucus synthesis by up-regulating MUC1 and MUC2 expression through a mitogen-activated protein kinase pathway. J Cell Biochem. 2008;104:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Wong CC, Zhang L, Li ZJ, Wu WK, Ren SX, Chen YC, Ng TB, Cho CH. Protective effects of cathelicidin-encoding Lactococcus lactis in murine ulcerative colitis. J Gastroenterol Hepatol. 2012;27:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Zhang L, Yu J, Wong CC, Ling TK, Li ZJ, Chan KM, Ren SX, Shen J, Chan RL, Lee CC. Cathelicidin protects against Helicobacter pylori colonization and the associated gastritis in mice. Gene Ther. 2012;Dec 20; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Coffelt SB, Waterman RS, Florez L, Höner zu Bentrup K, Zwezdaryk KJ, Tomchuck SL, LaMarca HL, Danka ES, Morris CA, Scandurro AB. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int J Cancer. 2008;122:1030-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Heilborn JD, Nilsson MF, Jimenez CI, Sandstedt B, Borregaard N, Tham E, Sørensen OE, Weber G, Ståhle M. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer. 2005;114:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | von Haussen J, Koczulla R, Shaykhiev R, Herr C, Pinkenburg O, Reimer D, Wiewrodt R, Biesterfeld S, Aigner A, Czubayko F. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59:12-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Lau YE, Bowdish DM, Cosseau C, Hancock RE, Davidson DJ. Apoptosis of airway epithelial cells: human serum sensitive induction by the cathelicidin LL-37. Am J Respir Cell Mol Biol. 2006;34:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Okumura K, Itoh A, Isogai E, Hirose K, Hosokawa Y, Abiko Y, Shibata T, Hirata M, Isogai H. C-terminal domain of human CAP18 antimicrobial peptide induces apoptosis in oral squamous cell carcinoma SAS-H1 cells. Cancer Lett. 2004;212:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Aarbiou J, Tjabringa GS, Verhoosel RM, Ninaber DK, White SR, Peltenburg LT, Rabe KF, Hiemstra PS. Mechanisms of cell death induced by the neutrophil antimicrobial peptides alpha-defensins and LL-37. Inflamm Res. 2006;55:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Wu WK, Sung JJ, To KF, Yu L, Li HT, Li ZJ, Chu KM, Yu J, Cho CH. The host defense peptide LL-37 activates the tumor-suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells. J Cell Physiol. 2010;223:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Ren SX, Cheng AS, To KF, Tong JH, Li MS, Shen J, Wong CC, Zhang L, Chan RL, Wang XJ. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012;72:6512-6523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
P- Reviewer Koon HW S- Editor Wang JL L- Editor A E- Editor Li JY