Published online Apr 28, 2013. doi: 10.3748/wjg.v19.i16.2492
Revised: November 9, 2012
Accepted: January 5, 2013
Published online: April 28, 2013
Processing time: 196 Days and 23.1 Hours
AIM: To examine fibrinogen-like protein 2 (fgl2) expression during taurocholate-induced acute pancreatitis progression in rats and its correlation with pancreatic injury severity.
METHODS: Forty-eight male Sprague-Dawley rats were randomly divided into the severe acute pancreatitis (SAP) group (n = 24) and the sham operation (SO) group (n = 24). Sodium taurocholate (4% at doses of 1 mL/kg body weight) was retrogradely injected into the biliopancreatic ducts of the rats to induce SAP. Pancreatic tissues were prepared immediately after sacrifice. At the time of sacrifice, blood was obtained for determination of serum amylase activity and isolation of peripheral blood mononuclear cells (PBMCs). Pancreatic tissue specimens were obtained for routine light microscopy including hematoxylin and eosin staining, and the severity of pancreatic injury was evaluated 1, 4 and 8 h after induction. Expression of fgl2 mRNA was measured in the pancreas and PBMCs using reverse transcription polymerase chain reaction. Expression of fgl2 protein was evaluated in pancreatic tissues using Western blotting and immunohistochemical staining. Masson staining was also performed to observe microthrombosis.
RESULTS: At each time point, levels of fgl2 mRNAs in pancreatic tissues and PBMCs were higher (P < 0.05) in the SAP group than in the SO group. For pancreatic tissue in SAP vs SO, the levels were: after 1 h, 3.911 ± 1.277 vs 1.000 ± 0.673; after 4 h, 9.850 ± 3.095 vs 1.136 ± 0.609; and after 8 h, 12.870 ± 3.046 vs 1.177 ± 0.458. For PBMCs in SAP vs SO, the levels were: after 1 h, 2.678 ± 1.509 vs 1.000 ± 0.965; after 4 h, 6.922 ± 1.984 vs 1.051 ± 0.781; and after 8 h, 13.533 ± 6.575 vs 1.306 ± 1.179. Levels of fgl2 protein expression as determined by Western blotting and immunohistochemical staining were markedly up-regulated (P < 0.001) in the SAP group compared with those in the SO group. For Western blotting in SAP vs SO, the results were: after 1 h, 2.183 ± 0.115 vs 1.110 ± 0.158; after 4 h, 2.697 ± 0.090 vs 0.947 ± 0.361; and after 8 h, 3.258 ± 0.094 vs 1.208 ± 0.082. For immunohistochemical staining in SAP vs SO, the results were: after 1 h, 1.793 ± 0.463 vs 0.808 ± 0.252; after 4 h, 4.535 ± 0.550 vs 0.871 ± 0.318; and after 8 h, 6.071 ± 0.941 vs 1.020 ± 0.406. Moreover, we observed a positive correlation in the pancreas (r = 0.852, P < 0.001) and PBMCs (r = 0.735, P < 0.001) between fgl2 expression and the severity of pancreatic injury. Masson staining showed that microthrombosis (%) in rats with SAP was increased (P < 0.001) compared with that in the SO group and it was closely correlated with fgl2 expression in the pancreas (r = 0.842, P < 0.001). For Masson staining in SAP vs SO, the results were: after 1 h, 26.880 ± 9.031 vs 8.630 ± 3.739; after 4 h, 53.750 ± 19.039 vs 8.500 ± 4.472; and after 8 h, 80.250 ± 12.915 vs 10.630 ± 7.003.
CONCLUSION: Microthrombosis due to fgl2 overexpression contributes to pancreatic impairment in rats with SAP, and fgl2 level may serve as a biomarker during early stages of disease.
- Citation: Ye XH, Chen TZ, Huai JP, Lu GR, Zhuge XJ, Chen RP, Chen WJ, Wang C, Huang ZM. Correlation of fibrinogen-like protein 2 with progression of acute pancreatitis in rats. World J Gastroenterol 2013; 19(16): 2492-2500
- URL: https://www.wjgnet.com/1007-9327/full/v19/i16/2492.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i16.2492
Severe acute pancreatitis (SAP) is a pathogenic condition that progresses rapidly and has a high mortality[1-3], but the underlying pathophysiological mechanisms remain incompletely defined. SAP is currently considered to be complicated by microcirculatory disturbances and coagulation abnormalities[4,5]. Inflammatory mediators such as interleukin (IL)-6, IL-1β, and tumor necrosis factor α (TNF-α) released during acute inflammatory reactions are not just involved in the inflammatory process but may also be responsible for the systemic activation of hemostasis in patients with SAP[6,7]. Intravascular coagulation and thromboembolism are believed to play an important role in the pathogenesis of SAP and are related to its severity[8,9]. Acute inflammatory events during disease progression can lead to dysregulation of the coagulation cascade[10]. In SAP patients, thrombin and platelets are deposited not only in the local pancreatic blood vessels but also in the connective tissue and intercellular spaces[4]. Studies suggest that biochemical variables such as prothrombin time, D-dimer, and clotting time may have prognostic value, and direct anticoagulant therapy has been shown to be helpful in the treatment of SAP[4,10-12]. These facts suggest that coagulation and inflammation in SAP are correlated, thus microthrombosis plays a crucial role in SAP[10]. However, the exact pathophysiological mechanism remains unknown.
Fibrinogen-like protein 2 (fgl2)/fibroleukin (also termed fgl2 prothrombinase) was determined to be a new member of the fibrinogen-related protein superfamily (fibrinogen-related domain), which includes fibrinogen, tenascin, ficolin, and angiopoietin[13-15]. fgl2 is a direct prothrombinase with serine protease activity. fgl2 can cleave prothrombin to thrombin via a noncanonical pathway, resulting in fibrin deposition[16,17]. fgl2 leads to histopathological lesions and ischemic injury by mediating “immune coagulation”, fibrin deposition, and microthrombosis[18-21]. Microvascular disturbances are caused by microthrombi that are activated and produced as a consequence of fgl2 action[18,21-23]. Nevertheless, whether fgl2 contributes to the pathogenesis of SAP is unclear.
In the present study, we used 4% sodium taurocholate to induce SAP in rats. We then investigated the expression and localization of fgl2 in pancreatic tissues. We also assessed fgl2 expression and its correlation with severity of pancreatic injury and microthrombi in rats with SAP to provide new insight into the pathogenesis of this disease.
Forty-eight male Sprague-Dawley rats, weighing 200-250 g, were obtained from the Experimental Animal Center of Wenzhou Medical College, Wenzhou, China. All animals were fed standard rat chow, had free access to water, and were housed at a constant room temperature of 25 °C and a 12-h day/night cycle. All animals were acclimated for at least one week before the experiments were initiated. All procedures were performed in accordance with the Guidelines for Animal Experiments of Wenzhou Medical College.
All rats received intraperitoneal injection of 10% chloraldurate (2 mL/kg body weight; Solarbio, Beijing, China) for anesthesia. The rats were divided into the SAP group (n = 24) and the sham operation (SO) group (n = 24). In the SAP group, a laparotomy was performed through a midline incision. Sodium taurocholate (4%; 1 mL/kg body weight; Sigma, St. Louis, MO, United States) was retrogradely injected into the biliopancreatic duct through the papilla using a segmental epidural catheter via a microinjection pump at a speed of 0.2 mL/min. A microclip was placed in the hepatic portion of the biliopancreatic duct to avoid reflux before the injection. SO rats underwent surgery but without infusion. After each operation, the abdomen was closed in two layers. All procedures were carried out using sterile techniques.
At defined time points (1, 4 and 8 h; n = 8 per time point) after SAP induction, rats were anesthetized with 10% chloraldurate (2 mL/kg body weight) and euthanized by exsanguination. Pancreatic tissues were harvested immediately and divided into two pieces. Portions of the tissues were fixed in 4% paraformaldehyde for immunohistochemical staining and microscopic observation, and other portions were removed and stored in liquid nitrogen until use. Blood samples (5 mL) were obtained via postcava puncture, and 4 mL of each sample was collected and stored in 5-mL tubes without anticoagulants (Generay, Shanghai, China). The blood samples were centrifuged at 1200 ×g for 20 min, and the serum was collected for determination of amylase activity (U/L) with a fully automatic biochemical analyzer (Hitachi, Tokyo, Japan). The remaining 1 mL blood was stored in ethylene diamine tetraacetic acid-containing tubes (Generay) and used to isolate peripheral blood mononuclear cells (PBMCs).
PBMC isolation was performed with density gradient centrifugation. The blood sample (1 mL) was diluted with 1 mL 0.9% saline. Subsequently, the diluted cell suspension was carefully laid over 2 mL Bandicoot percoll (Solarbio) and centrifuged at 2000 ×g for 20 min at 20 °C. The PBMC layer was carefully transferred into a new tube, and the volume was brought to 5 mL with 0.9% saline and centrifuged (2000 ×g, 5 min, 20 °C). This step was repeated. Finally, the supernatant was carefully removed, reserving the PBMCs at the bottom of the tube. PBMCs were immediately stored in liquid nitrogen until use.
Pancreatic tissue samples were fixed in 4% paraformaldehyde for histological analysis. The samples were dehydrated and embedded in paraffin. Pancreas sections were stained with hematoxylin and eosin (HE) for routine light microscopy. Slides were examined in a blinded fashion by two pathologists who were unaware of the treatment protocol according to the modified method of Schmidt et al[24] and Eşrefoğlu et al[25]. Variables of edema, hemorrhage, acinar cell degeneration, and interstitial inflammation were scored in 10 random fields of each slide to assess the severity of pancreatic injury under a light microscope (CX31, Tokyo, Japan; HE staining, × 200). Each variable was scored as follows: (1) edema: 0 = absent, 1 = focally in the interlobular space, 2 = increased in the intralobular space, 3 = isolated-island appearance of pancreatic acinus; (2) hemorrhage: 0 = absent, 1 = slight, 2 = moderate, 3 = severe; (3) acinar cell degeneration: 0 = absent, 1 = focal (< 5%); 2 = and/or sublobular (< 20%), 3 = and/or lobular (> 20%); and (4) inflammation: 0 = absent, 1 = slight, 2 = moderate, 3 = severe. The sum of these four variables for each pancreas section could have a maximum score of 12.
Pancreatic tissue sections were also stained with Masson stain to observe microthrombi in microvessels. One hundred microvessels on each slide were randomly selected, and the percent of microthrombus-positive vessels was calculated.
Total RNA was extracted from pancreatic tissues and PBMCs using Trizol reagent (Invitrogen, Carlsbad, CA, United States), and cDNA was synthesized with the First Strand cDNA synthesis kit (MBI Fermentas, Burlington, Canada) according to the manufacturer’s protocols. The samples were subsequently amplified using Moloney murine leukemia virus reverse transcriptase and Taq DNA polymerase (Invitrogen) using an ABI 7500 Sequence Detection System (Applied Biosystems Inc., Carlsbad, CA, United States). The sequences of the primers (Generay) were as follows: fgl2 (153 bp): 5’-CCTGGAGATTGTGGTTTCGT-3’ (forward) and 5’-TACCATGCCTTTCTCCAAGG-3’ (reverse), β-actin (153 bp): 5’-TGTCACCAACTGGGACGATA-3’ (forward) and 5’-GGGGTGTTGAAGGTCTCAAA-3’ (reverse). The cDNA was denatured at 95 °C for 5 min and amplified for 40 cycles of 95 °C (15 s), 60 °C (45 s), and 72 °C (60 s), followed by a final extension at 72 °C (5 min). The samples were tested in triplicate, and the results were calculated using the 2−ΔΔCT method. The expression of fgl2 mRNA was shown as relative to β-actin.
Pancreatic proteins were separated with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Millipore, Billerica, MA, United States). The membrane was blocked with 5% skim milk in Tris-buffered saline and then incubated with a polyclonal antibody against fgl2 (Biosynthesis Biotechnology, Beijing, China; 1:200) at 4 °C overnight. The membrane was washed three times with Tris-buffered saline and incubated with secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, United States) conjugated to horseradish peroxidase for 2 h at room temperature. The immunoreactive bands were visualized with an enhanced chemiluminescence reagent (Pierce, Rockford, IL, United States). Protein expression levels were normalized to β-actin.
Immunohistochemical staining was performed to assess fgl2 protein expression in the pancreas using EnVision reagents (Dako, Glostrup, Denmark). Four-μm-thick paraffin sections were routinely cut. Microwave antigen retrieval was conducted for 20 min in citrate buffer (pH 6.0) to activate antigens before quenching endogenous peroxidase activity in 0.3% H2O2 for 10 min. After three times of washing with phosphate-buffered saline (Generay) and then the primary reaction solution, the sections were incubated with rabbit polyclonal anti-rat fgl2 (Biosynthesis Biotechnology; 1:100) for 2 h at 37 °C. Following the same washing procedure, EnVision reagents were applied and incubated for 30 min at 37 °C. Finally, the reaction was developed with 0.05% diaminobenzidine and counterstained with hematoxylin for microscopy. Phosphate-buffered saline was used as a negative control instead of the primary antibody. To measure fgl2 protein expression, 10 randomly selected fields across each section were evaluated at × 200 magnification.
All data represent the mean ± SD. SPSS 15.0 software (SPSS, Chicago, IL, United States) was used for statistical analysis. Differences between the SAP and SO groups were analyzed with the Student’s t test. One-way analysis of variance was used to check for statistical significance among the three time points in the same group. Pearson’s correlation coefficient was calculated to determine the strength of the association between two continuous variables. P < 0.05 was considered statistically significant.
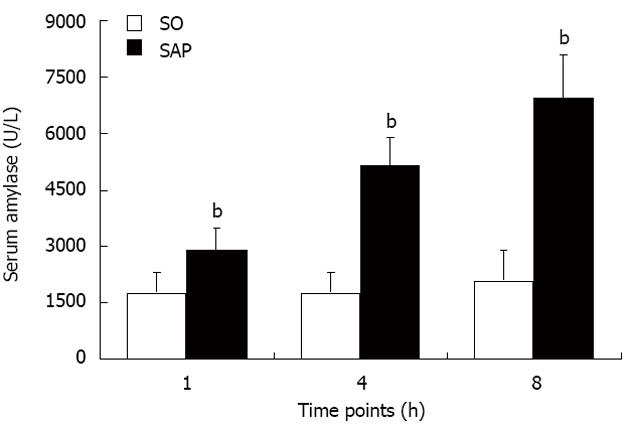
Serum amylase is the most commonly used biochemical indicator of acute pancreatitis. Levels of serum amylase were markedly elevated (P < 0.01) in the SAP group compared with the SO group at each time point. There was no significant difference in the levels of serum amylase among the three time points in the SO group (Figure 1).
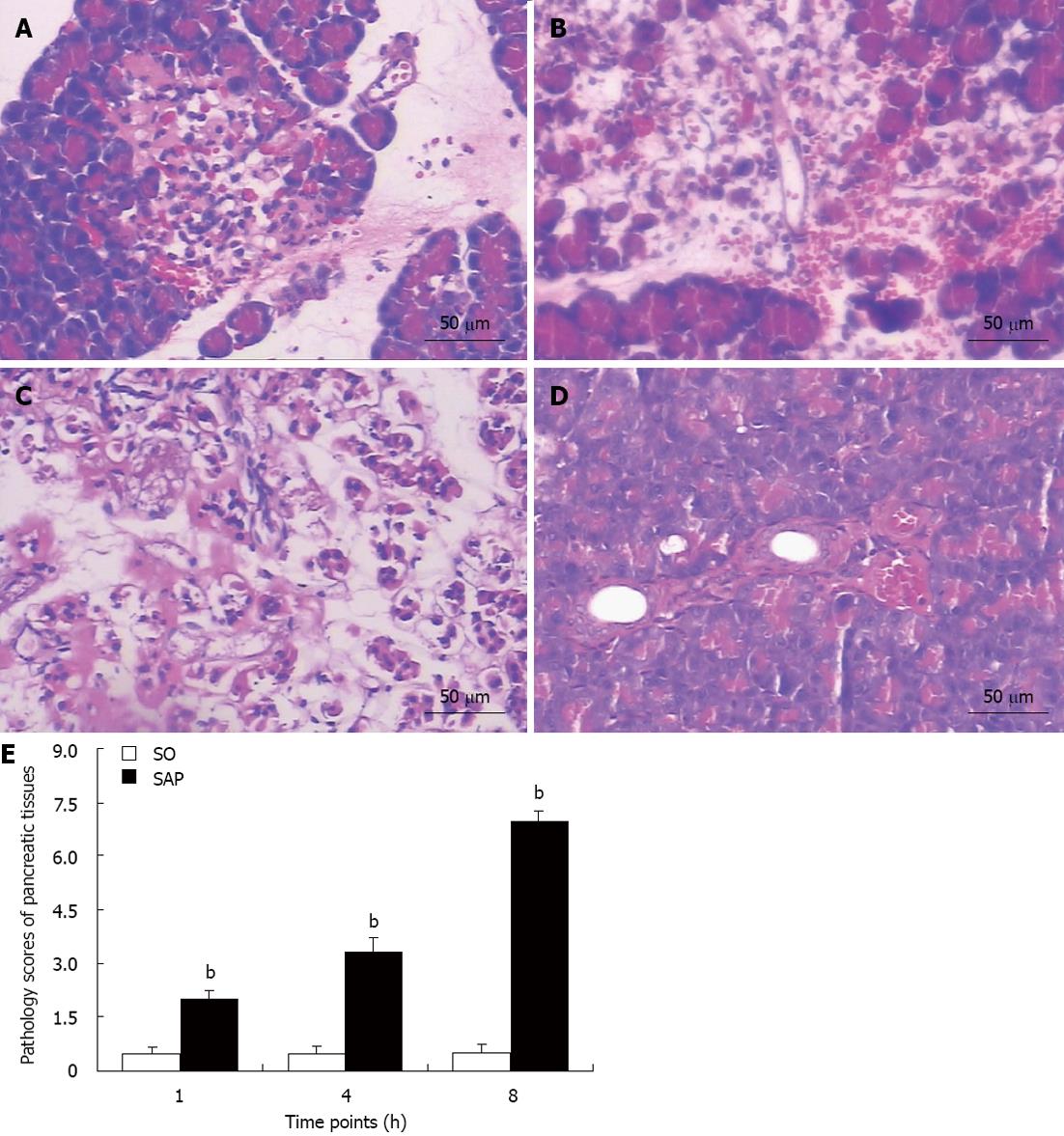
Compared with the SO group, the pancreatic tissues in the SAP group (Figure 2A-C) at 1 h (A), 4 h (B), and 8 h (C) appeared much more severely damaged. The pancreatic tissue of the SO group (Figure 2D) appeared morphologically normal at 8 h. Microscopic examination of the pancreas in the SAP group showed edema, hemorrhage complicated by microthrombosis, acinar cell degeneration, and inflammation (Figure 2A-C). The mean pathological score of each rat with SAP was higher (P < 0.01) than that of control rats at each time point. Pancreatitis worsened over time, as demonstrated by the increasing pathological score (P < 0.01, Figure 2E).
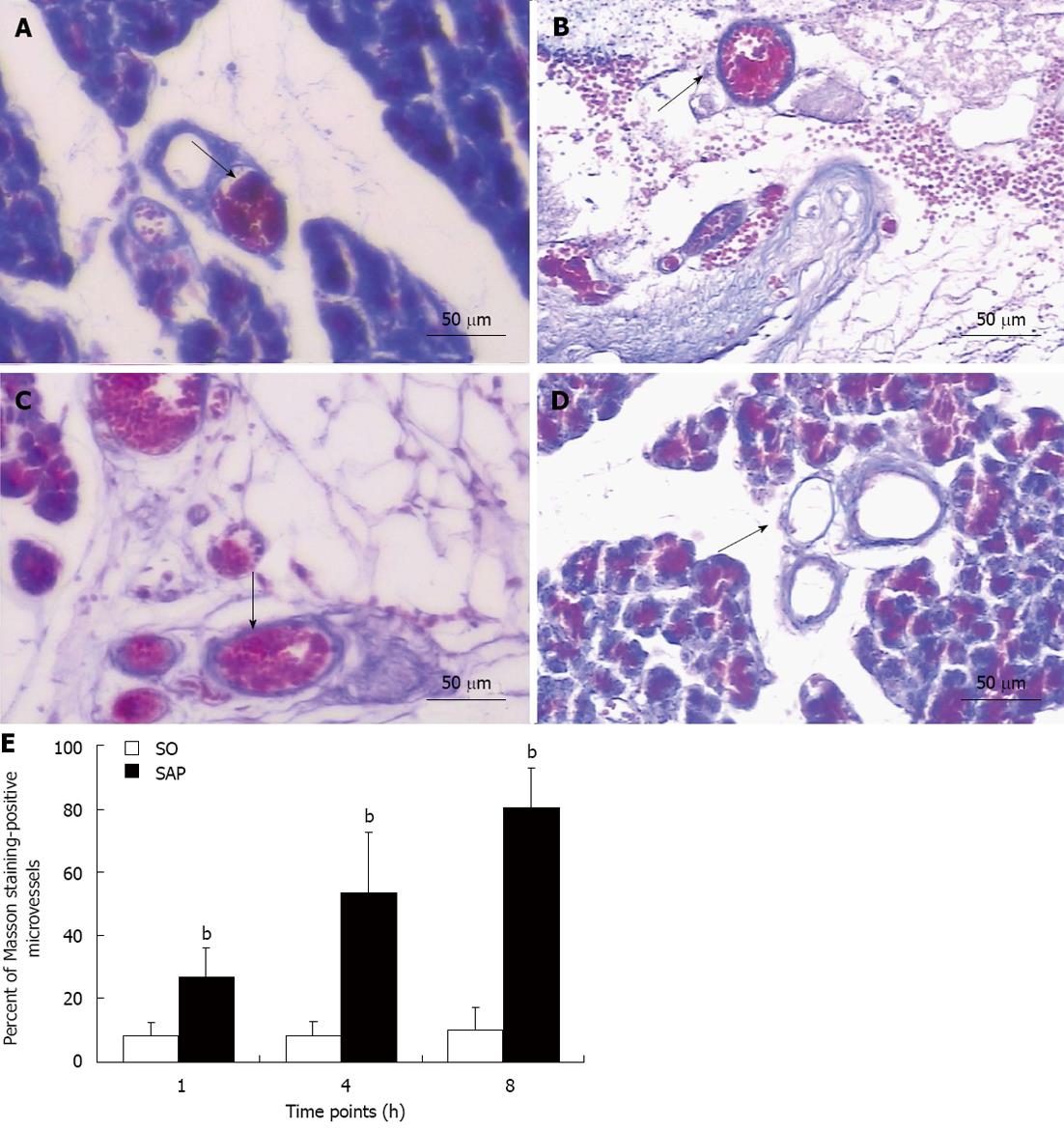
Masson staining was used to observe microthrombosis, which was seen as very bright red regions under light microscope. Microthrombi were localized and tightly combined with the microvascular endothelium of the pancreatic tissues, which suggested that the microthrombi formed in situ with the formation of fibrin. The percent of Masson staining-positive microvessels in the pancreas of the SAP group was higher (P < 0.01) than that in control rats at all time points and tended to increase (P < 0.01, Figure 3).
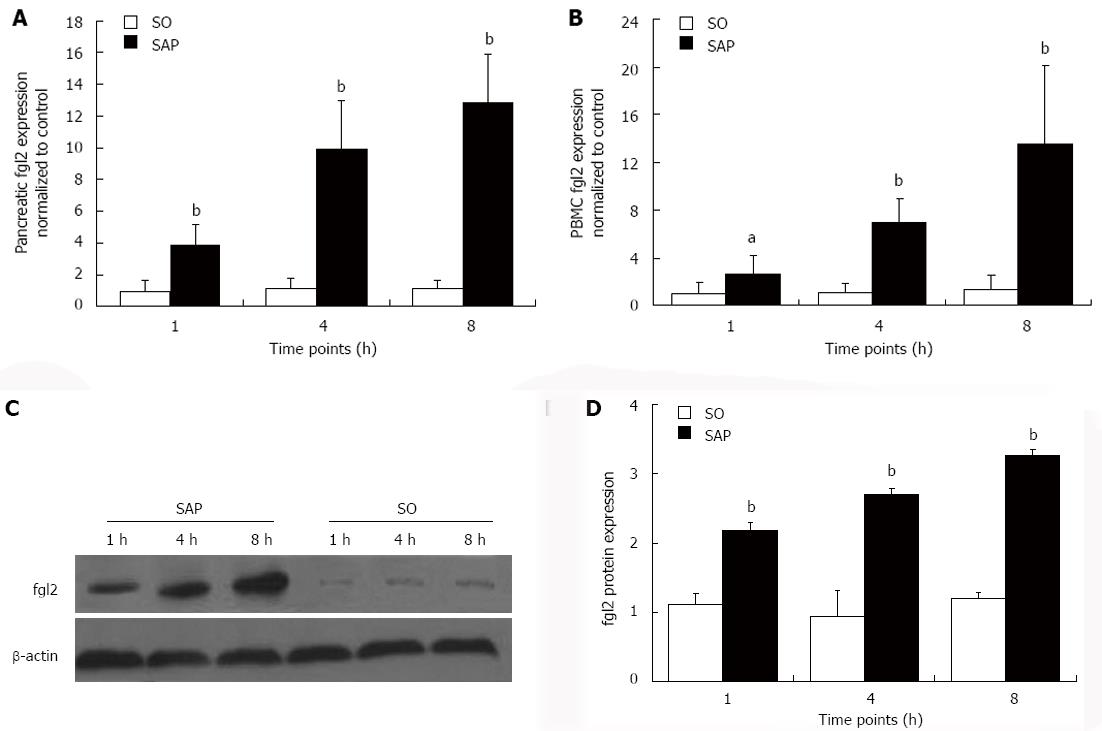
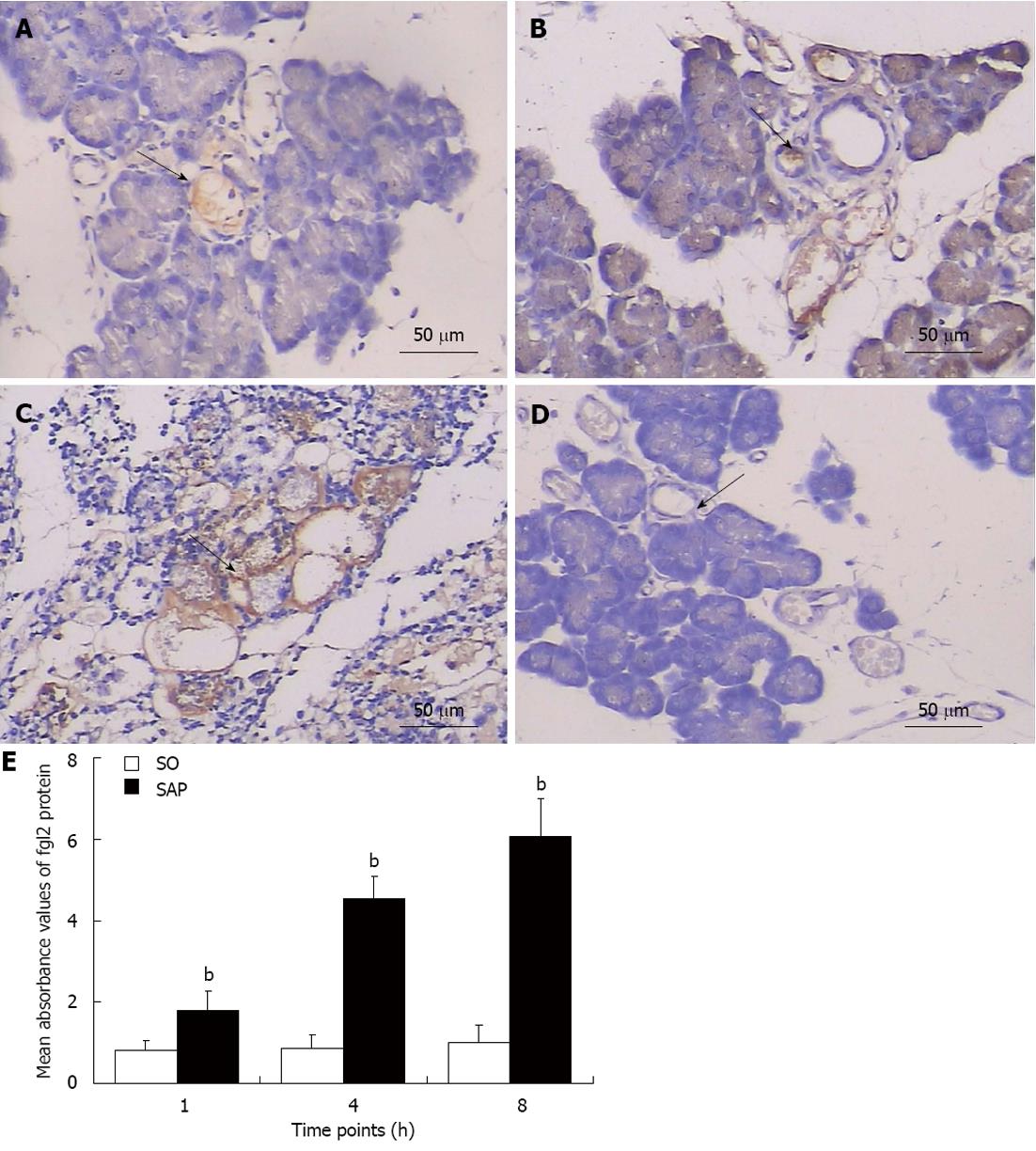
fgl2 expression was evaluated with real-time polymerase chain reaction, Western blotting, and immunohistochemical staining. The level of fgl2 mRNA was elevated in both pancreatic tissues and PBMCs (P < 0.05) beginning at 1 h after injection of 4% sodium taurocholate compared to the SO group. fgl2 mRNA increased over time in the SAP group (P < 0.01, Figure 4A and B). Western blot analysis revealed that fgl2 protein expression in the pancreas was higher (P < 0.01) in the SAP group than in the SO group and showed a tendency to increase over time (P < 0.01, Figure 4C and D). Immunohistochemical staining demonstrated that fgl2 was strongly expressed and localized in microvascular endothelial cells in pancreatic tissues in rats with SAP (Figure 5A-C). Only low levels of fgl2 expression were found in control rats (Figure 5D). In accordance with fgl2 mRNA level, fgl2 protein level was elevated as indicated by the mean absorbance value (P < 0.01) in the SAP group compared to the control rats and tended to increase (P < 0.01, Figure 5E). Pearson’s correlation coefficient analysis was used to compare fgl2 expression and the proportion of Masson staining-positive microvessels, and the results showed a correlation (r = 0.842, P < 0.01). This result suggested that elevated fgl2 expression may contribute to microthrombosis.
fgl2 expression and the severity of pancreatic injury of rats with SAP (as indicated by the pathological score) were higher compared with control rats, indicating a correlation between fgl2 expression and disease severity upon induction of SAP. Moreover, fgl2 expression in the pancreas (r = 0.852, P < 0.01) and PBMCs (r = 0.735, P < 0.01) correlated with the severity of pancreatic injury.
SAP is an inflammatory disorder mediated by up-regulated expression of proinflammatory cytokines such as TNF-α[4,10]. Inflammation and coagulation are interactive events during SAP. Microthrombosis is found in the early stages of the SAP rat model[1]. The “immune coagulation” hypothesized by Levy means that fgl2 could be transcribed and the mRNA translated following the induction of cytokines such as IL-2 and TNF-α, resulting in immediate activation of coagulation[17,23,26,27]. fgl2 functions as a bridge molecule between immune and coagulation reactions. fgl2 is highly expressed in endothelial cells due to the action of TNF-α[27,28]. Otherwise, interferon-γ is necessary for macrophage induction of fgl2[27]. In the present study, fgl2 was clearly up-regulated and localized in inflammatory regions of the pancreas sections, suggesting that fgl2 as an effector molecule may contribute to SAP pathogenesis by initiating and promoting coagulation through the induction of proinflammatory cytokines such as TNF-α.
fgl2/fibroleukin is a new procoagulant that belongs to the fibrinogen-related protein superfamily, which has a potent capability of inducing microthrombosis[16,17,29]. fgl2 is expressed in activated macrophages, T cells, and endothelial cells[30]. fgl2 expression and the subsequent fibrin deposition account for microthrombus formation in situ[18], which occurs via a novel way by directly producing thrombin in addition to the classic extrinsic and intrinsic coagulant pathway[16,17]. Researches suggest that microcirculatory disturbance is an important aspect of the mechanism of SAP[4,8]. Our data show that microthrombi generated during pancreatitis (due to increased fgl2 expression) led to ischemia/hemorrhage injury and consequently resulted in necrosis and dysfunction of the pancreas. Moreover, we found that fgl2 plays a contributing role in pancreatic microthrombus formation in rats with SAP. Our study also shows that fgl2 has procoagulant activity in the pancreatic endothelial cells of microvessels in rats with SAP, and fgl2 expression correlates strongly with the severity of pancreatic injury.
We observed that both fgl2 mRNA and protein levels were higher in rats with SAP and that the levels gradually increased in parallel with the progression of SAP. We also observed that fgl2 expression was associated with microthrombus formation and that microthrombus formation in situ may be caused by fgl2, leading to partial impairment of the pancreatic tissues and the functions involved. We propose that fgl2 functions similarly as in other diseases[26,31-33]: microthrombi form as a consequence of fgl2 expression in the pancreas, leading to microcirculatory disturbance and consequent hemorrhage/ischemia injury in rats with SAP, thus aggravating the pancreatic injury.
To evaluate the relevance of fgl2 expression and the severity of pancreatic disease, a Pearson’s correlation coefficient was calculated. Evaluation of fgl2 expression levels in both the pancreas and PBMCs (containing lymphocytes, monocytes, dendritic cells, and other cell types) revealed a strong correlation with the severity of pancreatic disease as illustrated by the pathological score. Thus, fgl2 expression may serve as a promising marker for predicting the occurrence of SAP in early stages.
Injection of a neutralizing antibody or genetic therapy against fgl2 in diseases involving fgl2, such as murine hepatitis virus 3-induced hepatitis and graft rejection, has been beneficial in terms of attenuating fibrin deposition and pathology and preventing death in mice[18,34-36]. Thus, we will perform an in-depth investigation to see whether inhibiting fgl2 activity or applying fgl2 antibodies will delay or ameliorate the disease course of SAP.
In summary, fgl2 functions as a novel prothrombinase and may initiate the coagulation reaction, finally leading to microthrombosis in microvessels of pancreatic tissues in an experimental model of rats with SAP, and resulting in ischemia/hemorrhage injury as well as necrosis and dysfunction of the pancreas. fgl2 expression is closely correlated with the severity of pancreatic disease, and thus fgl2 may serve as a useful biomarker for predicting SAP at the onset of disease. Whether inhibiting fgl2 or using antibodies against fgl2 will delay or ameliorate SAP requires further exploration.
We thank Rong-Rong Wang and Guo-Rong Chen, who are experienced pathologists in the First Affiliated Hospital, Wenzhou Medical College, Wenzhou, China for the pathological studies.
Fibrinogen-like protein 2 (fgl2)/fibroleukin (also termed fgl2 prothrombinase) is a recently discovered member of the fibrinogen-related protein superfamily. fgl2 is a direct prothrombinase with serine protease activity and can cleave prothrombin to thrombin via a noncanonical pathway, resulting in fibrin deposition. Several studies have demonstrated that fgl2 leads to pathology by mediating “immune coagulation”, fibrin deposition, and microthrombosis in murine hepatitis virus 3-induced fulminant hepatitis, spontaneous abortion, xenograft rejection, and type 2 diabetic nephropathy.
Intravascular coagulation and thromboembolism play a pivotal role in the pathogenesis of severe acute pancreatitis (SAP) and are related to its severity. Acute inflammatory events during SAP may result in dysregulation of the coagulation cascade. However, whether fgl2 is involved in the pathogenesis of SAP has not been studied. In this study, the authors demonstrate that increased expression of fgl2 contributes to pancreatic impairment in rats with SAP by mediating microthrombosis. Thus, fgl2 level may serve as a useful biomarker at early stages of disease.
The mechanism(s) responsible for the microcirculatory disturbances and coagulation abnormalities during the SAP process remains to be elucidated. This is the first study to report that fgl2 is highly expressed in microvascular endothelial cells of pancreatic tissues in rats with SAP. Furthermore, microthrombosis due to flg2 contributes to pancreatic impairment in rats with SAP, and fgl2 level may serve as a useful biomarker at disease onset.
In the present study, the authors investigated fgl2 expression and localization in the pancreas of rats with SAP, which will provide new insight into the pathogenesis of SAP and efficacious anticoagulant therapy for SAP treatment.
SAP is principally caused by autodigestion of the pancreas and is a potentially fatal pathogenic condition characterized by rapid progression and high mortality. fgl2 is a new member of the fibrinogen-related protein superfamily, which includes fibrinogen, tenascin, ficolin, and angiopoietin.
The authors of this study investigated the pathogenesis of acute pancreatitis. Their results provide insight into the contribution of microthrombosis to the development of pathological changes during the progression of acute pancreatitis. The authors have demonstrated increased expression of fgl2 mRNA in the pancreas and peripheral blood mononuclear cells with a subsequent increase in the levels of the corresponding proteins. The anticipated pathological changes were further substantiated by Masson staining. Stringency of the proposed hypothesis was validated, revealing a strong correlation between induced coagulation disorders and pathological changes in pancreatic tissues. It is an interesting subject, and the results are clearly described.
| 1. | Zhang XP, Zhang J, Ma ML, Cai Y, Xu RJ, Xie Q, Jiang XG, Ye Q. Pathological changes at early stage of multiple organ injury in a rat model of severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2010;9:83-87. [PubMed] |
| 2. | Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 594] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 3. | Mayerle J, Hlouschek V, Lerch MM. Current management of acute pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2005;2:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Kakafika A, Papadopoulos V, Mimidis K, Mikhailidis DP. Coagulation, platelets, and acute pancreatitis. Pancreas. 2007;34:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Cappell MS. Acute pancreatitis: etiology, clinical presentation, diagnosis, and therapy. Med Clin North Am. 2008;92:889-923, ix-x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 6. | Bhatia M. Inflammatory response on the pancreatic acinar cell injury. Scand J Surg. 2005;94:97-102. [PubMed] |
| 7. | Levi M, ten Cate H, van der Poll T, van Deventer SJ. Pathogenesis of disseminated intravascular coagulation in sepsis. JAMA. 1993;270:975-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Ranson JH, Lackner H, Berman IR, Schinella R. The relationship of coagulation factors to clinical complications of acute pancreatitis. Surgery. 1977;81:502-511. [PubMed] |
| 9. | Zhou ZG, Chen YD. Influencing factors of pancreatic microcirculatory impairment in acute panceatitis. World J Gastroenterol. 2002;8:406-412. [PubMed] |
| 10. | Hagiwara S, Iwasaka H, Shingu C, Matsumoto S, Uchida T, Noguchi T. Antithrombin III prevents cerulein-induced acute pancreatitis in rats. Pancreas. 2009;38:746-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Machała W, Wachowicz N, Komorowska A, Gaszyński W. The use of drotrecogin alfa (activated) in severe sepsis during acute pancreatitis - two case studies. Med Sci Monit. 2004;10:CS31-CS36. [PubMed] |
| 12. | Dobosz M, Mionskowska L, Hac S, Dobrowolski S, Dymecki D, Wajda Z. Heparin improves organ microcirculatory disturbances in caerulein-induced acute pancreatitis in rats. World J Gastroenterol. 2004;10:2553-2556. [PubMed] |
| 13. | Rüegg CR, Chiquet-Ehrismann R, Alkan SS. Tenascin, an extracellular matrix protein, exerts immunomodulatory activities. Proc Natl Acad Sci USA. 1989;86:7437-7441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887-2894. [PubMed] |
| 15. | Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511-2514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1047] [Cited by in RCA: 1014] [Article Influence: 37.6] [Reference Citation Analysis (3)] |
| 16. | Levy GA, Liu M, Ding J, Yuwaraj S, Leibowitz J, Marsden PA, Ning Q, Kovalinka A, Phillips MJ. Molecular and functional analysis of the human prothrombinase gene (HFGL2) and its role in viral hepatitis. Am J Pathol. 2000;156:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Chan CW, Chan MW, Liu M, Fung L, Cole EH, Leibowitz JL, Marsden PA, Clark DA, Levy GA. Kinetic analysis of a unique direct prothrombinase, fgl2, and identification of a serine residue critical for the prothrombinase activity. J Immunol. 2002;168:5170-5177. [PubMed] |
| 18. | Ning Q, Sun Y, Han M, Zhang L, Zhu C, Zhang W, Guo H, Li J, Yan W, Gong F. Role of fibrinogen-like protein 2 prothrombinase/fibroleukin in experimental and human allograft rejection. J Immunol. 2005;174:7403-7411. [PubMed] |
| 19. | Ding JW, Ning Q, Liu MF, Lai A, Peltekian K, Fung L, Holloway C, Yeger H, Phillips MJ, Levy GA. Expression of the fgl2 and its protein product (prothrombinase) in tissues during murine hepatitis virus strain-3 (MHV-3) infection. Adv Exp Med Biol. 1998;440:609-618. [PubMed] |
| 20. | Clark DA, Arck PC, Chaouat G. Why did your mother reject you? Immunogenetic determinants of the response to environmental selective pressure expressed at the uterine level. Am J Reprod Immunol. 1999;41:5-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Marsden PA, Ning Q, Fung LS, Luo X, Chen Y, Mendicino M, Ghanekar A, Scott JA, Miller T, Chan CW. The Fgl2/fibroleukin prothrombinase contributes to immunologically mediated thrombosis in experimental and human viral hepatitis. J Clin Invest. 2003;112:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Mendicino M, Liu M, Ghanekar A, He W, Koscik C, Shalev I, Javadi M, Turnbull J, Chen W, Fung L. Targeted deletion of Fgl-2/fibroleukin in the donor modulates immunologic response and acute vascular rejection in cardiac xenografts. Circulation. 2005;112:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Knackstedt MK, Zenclussen AC, Hertwig K, Hagen E, Dudenhausen JW, Clark DA, Arck PC. Th1 cytokines and the prothrombinase fgl2 in stress-triggered and inflammatory abortion. Am J Reprod Immunol. 2003;49:210-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Schmidt J, Lewandrowski K, Fernandez-del Castillo C, Mandavilli U, Compton CC, Warshaw AL, Rattner DW. Histopathologic correlates of serum amylase activity in acute experimental pancreatitis. Dig Dis Sci. 1992;37:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Eşrefoğlu M, Gül M, Ates B, Batçioğlu K, Selimoğlu MA. Antioxidative effect of melatonin, ascorbic acid and N-acetylcysteine on caerulein-induced pancreatitis and associated liver injury in rats. World J Gastroenterol. 2006;12:259-264. [PubMed] |
| 26. | Su K, Chen F, Yan WM, Zeng QL, Xu L, Xi D, Pi B, Luo XP, Ning Q. Fibrinogen-like protein 2/fibroleukin prothrombinase contributes to tumor hypercoagulability via IL-2 and IFN-gamma. World J Gastroenterol. 2008;14:5980-5989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 27. | Liu M, Mendicino M, Ning Q, Ghanekar A, He W, McGilvray I, Shalev I, Pivato D, Clark DA, Phillips MJ. Cytokine-induced hepatic apoptosis is dependent on FGL2/fibroleukin: the role of Sp1/Sp3 and STAT1/PU.1 composite cis elements. J Immunol. 2006;176:7028-7038. [PubMed] |
| 28. | Clark DA, Foerster K, Fung L, He W, Lee L, Mendicino M, Markert UR, Gorczynski RM, Marsden PA, Levy GA. The fgl2 prothrombinase/fibroleukin gene is required for lipopolysaccharide-triggered abortions and for normal mouse reproduction. Mol Hum Reprod. 2004;10:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Doolittle RF. The structure and evolution of vertebrate fibrinogen. Ann N Y Acad Sci. 1983;408:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Schwartz BS, Levy GA, Fair DS, Edgington TS. Murine lymphoid procoagulant activity induced by bacterial lipopolysaccharide and immune complexes is a monocyte prothrombinase. J Exp Med. 1982;155:1464-1479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Su GH, Liu K, Wang Y, Wang J, Li XW, Li WZ, Liao YH, Wang ZH. Fibrinogen-like protein 2 expression correlates with microthrombosis in rats with type 2 diabetic nephropathy. J Biomed Res. 2011;25:120-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Ding Y, Liu K, Wang Y, Su G, Deng H, Zeng Q, Liao Y, Wang Z. Expression and significance of fgl2 prothrombinase in cardiac microvascular endothelial cells of rats with type 2 diabetes. J Huazhong Univ Sci Technolog Med Sci. 2010;30:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Melnyk MC, Shalev I, Zhang J, Bartczak A, Gorczynski RM, Selzner N, Inman R, Marsden PA, Phillips MJ, Clark DA. The prothrombinase activity of FGL2 contributes to the pathogenesis of experimental arthritis. Scand J Rheumatol. 2011;40:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Li C, Fung LS, Chung S, Crow A, Myers-Mason N, Phillips MJ, Leibowitz JL, Cole E, Ottaway CA, Levy G. Monoclonal antiprothrombinase (3D4.3) prevents mortality from murine hepatitis virus (MHV-3) infection. J Exp Med. 1992;176:689-697. [PubMed] |
| 35. | Gao S, Wang M, Ye H, Guo J, Xi D, Wang Z, Zhu C, Yan W, Luo X, Ning Q. Dual interference with novel genes mfgl2 and mTNFR1 ameliorates murine hepatitis virus type 3-induced fulminant hepatitis in BALB/cJ mice. Hum Gene Ther. 2010;21:969-977. [PubMed] |
| 36. | Zhu C, Sun Y, Luo X, Yan W, Xi D, Ning Q. Novel mfgl2 antisense plasmid inhibits murine fgl2 expression and ameliorates murine hepatitis virus type 3-induced fulminant hepatitis in BALB/cJ mice. Hum Gene Ther. 2006;17:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
P- Reviewer Barauskas G S- Editor Gou SX L- Editor Ma JY E- Editor Xiong L