Published online Apr 14, 2013. doi: 10.3748/wjg.v19.i14.2270
Revised: December 24, 2012
Accepted: January 17, 2013
Published online: April 14, 2013
Processing time: 201 Days and 19.3 Hours
AIM: To study non-cardiac chest pain (NCCP) in relation to ineffective esophageal motility (IEM) and rapid food intake.
METHODS: NCCP patients with a self-reported habit of fast eating underwent esophageal manometry for the diagnosis of IEM. Telephone interviews identified eating habits of additional IEM patients. Comparison of manometric features was done among IEM patients with and without the habit of rapid food intake and healthy controls. A case study investigated the effect of 6-mo gum chewing on restoration of esophageal motility in an IEM patient. The Valsalva maneuver was performed in IEM patients and healthy controls to assess the compliance of the esophagus in response to abdominal pressure increase.
RESULTS: Although most patients diagnosed with NCCP do not exhibit IEM, remarkably, all 12 NCCP patients who were self-reporting fast eaters with a main complaint of chest pain (75.0%) had contraction amplitudes in the mid and distal esophagus that were significantly lower compared with healthy controls [(23.45 mmHg (95%CI: 14.06-32.85) vs 58.80 mmHg (95%CI: 42.56-75.04), P < 0.01 and 28.29 mmHg (95%CI: 21.77-34.81) vs 50.75 mmHg (95%CI: 38.44-63.05), P < 0.01, respectively)]. In 7 normal-eating IEM patients with a main complaint of sensation of obstruction (42.9%), the mid amplitude was smaller than in the controls [30.09 mmHg (95%CI: 19.48-40.70) vs 58.80 mmHg (95%CI: 42.56-75.04), P < 0.05]. There was no statistically significant difference in manometric features between the fast-eating and normal-eating groups. One NCCP patient who self-reported fast eating and was subsequently diagnosed with IEM did not improve with proton-pump inhibition but restored swallow-induced contractions upon 6-mo gum-chewing. The Valsalva maneuver caused a markedly reduced pressure rise in the mid and proximal esophagus in the IEM patients.
CONCLUSION: Habitual rapid food intake may lead to IEM. A prospective study is needed to validate this hypothesis. Gum-chewing might strengthen weakened esophageal muscles.
- Citation: Li KL, Chen JH, Zhang Q, Huizinga JD, Vadakepeedika S, Zhao YR, Yu WZ, Luo HS. Habitual rapid food intake and ineffective esophageal motility. World J Gastroenterol 2013; 19(14): 2270-2277
- URL: https://www.wjgnet.com/1007-9327/full/v19/i14/2270.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i14.2270
Non-cardiac chest pain (NCCP) and ineffective esophageal motility (IEM) are often associated with gastroesophageal reflux disease (GERD). Although esophageal dysmotility is considered an uncommon cause of non-GERD-related NCCP[1,2], in our practice it is not infrequent. In recent years, we noted that some patients with a primary complaint of chest pain or discomfort had a life long habit of rapid food intake. Their esophageal manometry exhibited low esophageal contraction amplitudes during wet swallows. This initiated the current investigation into a possible relationship between habitual rapid food intake, symptoms and motility dysfunction.
In a recent study, a possible association was investigated between self-reported eating behavior and metabolic risk factors (overweight, hypertension, hyperglycemia, hypertriacylglycerolemia, low levels of high-density lipoprotein (HDL) cholesterol, hyperuricemia and fatty liver)[3]. The conclusion was that rapid eating increases metabolic risk factors although the mechanism was not investigated. In the present study, we analyzed the clinical and manometric characteristics of IEM patients with and without a habit of rapid eating. Our main objective was to investigate a possible correlation between rapid food intake and IEM. Our hypothesis was that rapid eating is associated with less swallow-induced contractions, contributing to IEM through disuse of the esophageal musculature; hence we predicted that patients with IEM and rapid eating should have more severe ineffective esophageal motility compared to IEM patients without the habit of rapid eating. We also report a case-study of an IEM patient whose symptoms were improved by 6 mo of gum-chewing exercise.
Data were collected from patients in our department with various symptoms including chest pain or discomfort, dysphagia, heartburn that lasted from 1 mo to 30 years who underwent esophageal manometry. Some patients volunteered information about their fast eating habits as a cause of their symptoms. We collected information about the manometry tests of all patients whose eating habits were recorded at the first visit, and in addition, we obtained information about eating habits by telephone interview of 9 additional patients.
Two groups of volunteers participated in the study: Group V1 as healthy controls in the manometric analysis; Group V2 recruited to record healthy Chinese people’s daily meal duration. Group V1 were without any digestive or systemic symptoms and the volunteers underwent the same manometric procedures as the patients. Group V2 were sent out to canteens, fast-food restaurants, Chinese restaurants and local families to time the duration of the meal intake.
Written informed consent was provided by all the participants. This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University.
The patients’ current symptoms, medical history and basic information including age, gender, body mass index (BMI) was obtained and standard esophageal manometry was performed. During the manometry testing, the patients were instructed to perform the Valsalva maneuver. Volunteers in Group V1 underwent the same manometric procedure after their basic information was obtained. Each of them performed the Valsalva maneuver. Group V2 was assigned to the above-mentioned dining locations to record the meal duration.
Following an overnight fast and 48-h discontinuation of any medication that may interfere with esophageal motility, conventional stationary esophageal manometry was performed using a 3.5 mm diameter, eight-lumen, sleeve sensor catheter assembly (Mui Scientific, Mississauga, Ontario, Canada) with eight side-holes arranged in radial form and located 2-7 cm apart. Manometric data were recorded and analyzed by means of the Polygram 98 and the Polygram Net Esophageal Manometry Testing Application Software (Medtronic A/S, Tonsbakken, Skovlunde, Denmark). The catheter was inserted transnasally into the stomach and intragastric pressure (GP) was obtained in a supine position. The lower esophageal sphincter (LES) resting pressure was defined as the mid-respiratory LES pressure compared with GP. Patients or healthy volunteers were then instructed to perform ten wet swallows (10 mL water each, separated by an interval of 30 s) to measure and calculate the contraction amplitude, duration and velocity in the proximal, mid and distal esophagus. When calculating the velocity, we did not incorporate data indicative of simultaneous (i.e., velocity > 8 cm/s) contractions. The existence of double-peaked or multi (≥ 3) -peaked waves was also noted.
The manometric criteria for the diagnosis of IEM were no fewer than 30% of the wet swallows featuring one or more of the following characteristics: (1) contraction amplitude < 30 mmHg at either or both of the distal points 5 and 10 cm above the LES; (2) simultaneous contraction (distal velocity between 5 and 10 cm above the LES > 8 cm/s) with amplitude < 30 mmHg; and (3) absent or non-transmitted peristalsis[4,5].
After wet swallows, 12 patients and all the volunteers in Group V1 were instructed to perform the Valsalva maneuvers in the supine position, exhaling forcibly with the mouth closed and the nose pinched shut[6,7]. Data on the pressure changes in the esophageal body and the LES were collected.
We used the length of time it took for a patient to finish an average meal as the indicator of the speed of eating. Meal lengths of the healthy population were recorded when they had regular Chinese meals with or without water. None of them took alcohol or had chat time included.
Except for age which was presented as median and range, the other data were expressed as means and 95%CI:. Kolmogorov-Smirnov analysis was applied to determine data distribution. Student’s t test was employed for the comparison of data. Statistical significance was acknowledged if P < 0.05.
Ten NCCP patients mentioned their eating habits specifically during initial evaluation, six of whom reported a habit of rapid eating and were all diagnosed with IEM according the manometric criteria. We managed to obtain information from 9 other IEM patients by telephone calls. Among these 19 patients, 12 (63.2%) (7 males and 5 females, median age 44.5 years, range 18-57 years, mean BMI 22.52; 95%CI: 20.45-24.59) volunteered the fact that they had been eating much faster than normal speed for a long time from 5 to 31 years. The other seven (36.8%) patients (2 males and 5 females, median age 52 years, range 45-74 years, mean BMI 22.48 (95%CI: 20.56-24.39) reported no habit of rapid eating.
Ten [4 males and 6 females, median age 22 years, range 20-33 years, mean BMI 20.69 (95%CI: 19.39-21.87)] healthy volunteers were recruited into Group V1. Group V2 consisted of 91 (50 males and 41 females) healthy volunteers.
For the self-reporting fast-eaters, meals all lasted no more than 8 min (3 min in one patient; 4 min in one; 5 min in five; 6 min in three; 7 min in one and 8 min in one). Their average meal duration was significantly shorter than that of the healthy volunteers (5.42 min, 95%CI: 4.58-6.25 vs 16.58 min, 95%CI: 14.21-18.94, P < 0.01). The meal lengths in the IEM patients with normal eating habits ranged from 10 to 30 min and their mean meal length was not statistically different from healthy volunteers (18.86 min, 95%CI: 12.31-25.41 vs 16.58 min, 95%CI: 14.21-18.94, P > 0.05). We found that the meal lengths of all IEM patients with normal eating habits were longer than those of the self-reporting rapidly eating patients.
Some fast-eating patients reported that while eating fast they spent shorter time chewing. They also swallowed more rapidly and frequently though they did not quantify it.
The predominant clinical manifestation in the fast-eating group was chest pain or discomfort (9/12, 75.0%), followed by sensation of obstruction (5/12, 41.7%), heartburn (2/12, 16.7%), acid reflux (1/12, 8.3%), dysphagia (1/12, 8.3%), chest tightness (1/12, 8.3%), food regurgitation (1/12, 8.3%), abdominal discomfort (1/12, 8.3%), nausea (1/12, 8.3%) and eructation (1/12, 8.3%). In the normal-eating group, sensation of obstruction was the most common (3/7, 42.9%), followed by heartburn (2/7, 28.6%), acid reflux (2/7, 28.6%) and chest pain or discomfort (1/7, 14.3%).
Table 1 shows the IEM patients’ manometric features. The contraction amplitudes in the distal and mid esophagus of the fast-eating IEM patients were significantly lower (P < 0.01) than in the control group. The amplitude in the mid esophagus of the normal-eating IEM patients was also significantly lower (P < 0.05) than in the controls. There was no statistically significant difference in manometric features between the IEM patients with and without the habit of rapid food intake.
| IEM patients with the habit of fast eating (n = 12) | IEM patients without the habit of fast eating (n = 7) | Healthy controls (n = 10) | |
| LES pressure (mmHg) | 12.71 (6.80-18.62) | 11.08 (-0.59-22.76) | 14.94 (10.38-19.49) |
| Distal esophagus | |||
| Amplitude (mmHg) | 28.29 (21.77-34.81)b | 33.78 (19.56-48.00) | 50.75 (38.44-63.05) |
| Duration (s) | 3.04 (2.44-3.65) | 2.74 (1.32-4.15) | 3.09 (2.30-3.88) |
| Velocity (cm/s)1 | 1.42 (1.14-1.70) | 3.33 (0.79-5.86) | 1.57 (0.89-2.24) |
| Mid esophagus | |||
| Amplitude (mmHg) | 23.45 (14.06-32.85)b | 30.09 (19.48-40.70)a | 58.80 (42.56-75.04) |
| Duration (s) | 3.12 (2.32-3.91) | 3.18 (1.81-4.55) | 2.45 (2.13-2.79) |
| Velocity (cm/s)2 | 2.18 (1.23-3.12) | 3.76 | 2.35 (1.38-3.33) |
| Proximal esophagus | |||
| Amplitude (mmHg) | 36.75 (22.93-50.57) | 41.47 (8.79-74.15) | 49.96 (36.28-63.64) |
| Duration (s) | 2.42 (1.84-3.00) | 3.13 (1.48-4.77) | 2.25 (1.80-2.71) |
| Velocity (cm/s)3 | 3.66 (1.71-5.60) | 2.56 | 2.42 (1.75-3.09) |
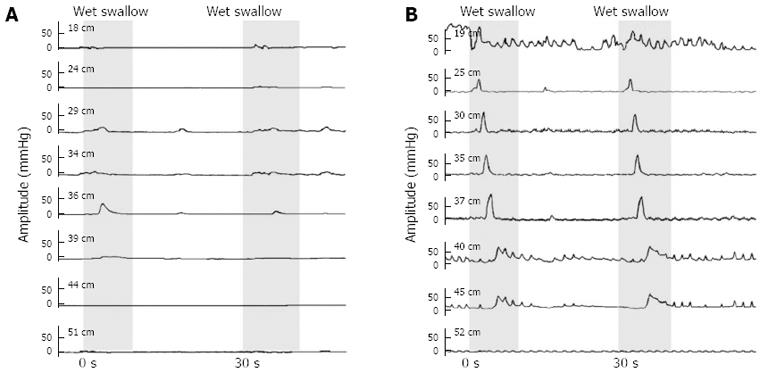
Simultaneous contractions were observed in 11 fast-eating and 6 normal-eating IEM patients (91.7% and 85.7% respectively vs 30% in healthy controls) and non-propulsive (but not simultaneous) contractions in 1 fast-eating patient (8.3% vs 0% in controls). Seven fast-eating and 6 normal-eating patients (58.3% and 85.7% vs 20% in controls) exhibited double-peaked waves and 3 fast-eating and 3 normal-eating patients (25.0% and 42.9% vs 20% in controls) had multi-peaked waves during certain wet swallows. A typical manometric tracing from one of the fast-eating IEM patients is shown in Figure 1.
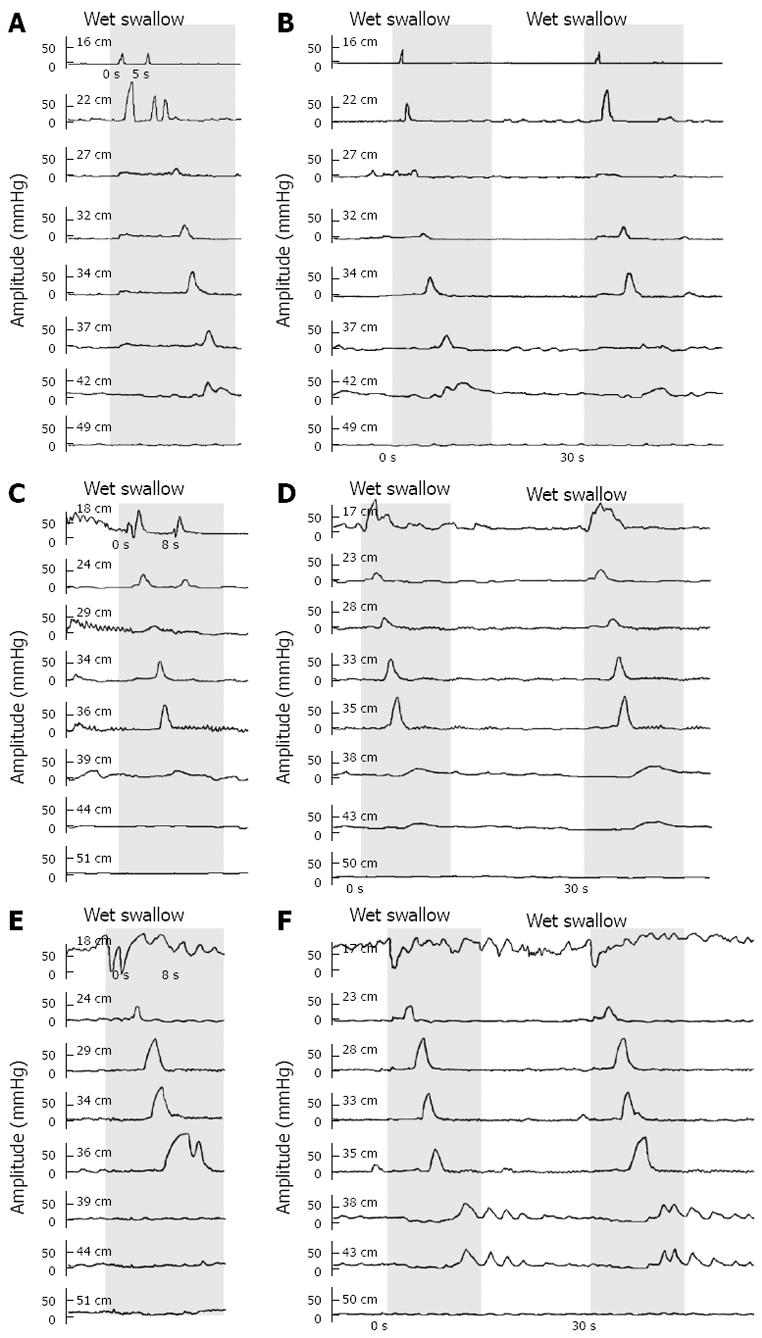
According to our protocol, wet swallows should be separated by an interval of 30 s. However, in some patients and healthy controls, the interval between certain swallows happened to be shorter than 10 s or even near zero. We observed that in pairs of short-interval swallows, only one peristalsis appeared in response to the first or the second swallow while the response to the other swallows was only contraction in the proximal esophagus, and the contraction in the distal part was prevented, as shown in Figure 2.
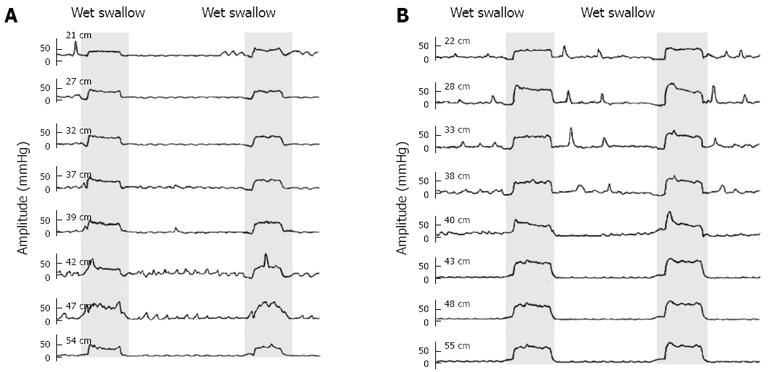
Pressure alterations in the LES and distal, mid and proximal esophagus during the Valsalva maneuver between IEM patients and healthy controls were compared (Table 2), and the manometry tracings are illustrated in Figure 3. IEM patients showed a much lower increase in esophageal pressure due to the Valsalva maneuver compared with controls. Mean changes in LES pressure of IEM patients were not statistically different from that of healthy volunteers.
| Increase in LES pressure (mmHg) | Increase in distal pressure (mmHg) | Increase in mid pressure (mmHg) | Increase in proximal pressure (mmHg) | |
| IEM patients (n = 12) | 11.56 (0.57-22.54) | 21.73 (15.46-27.99) | 21.18 (12.28-30.08)a | 19.07 (11.41-26.74)a |
| Control (n = 10) | 7.81 (-0.86-16.48) | 39.43 (15.37-63.49) | 43.44 (22.85-64.03) | 34.18 (23.41-44.95) |
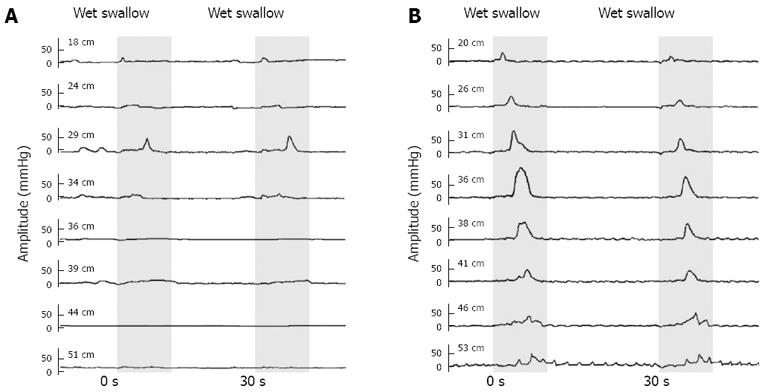
A 57-year-old male with a history of rapid food intake for more than 30 years, with each meal lasting less than 5 min, presented to our outpatient department with 2 years of moderate retrosternal chest pain, sensation of obstruction and occasional dysphagia. The initial esophageal manometry revealed that his swallow-induced esophageal contraction amplitude was extremely low (distal amplitude 10.42 mmHg on average). He was advised to slow down his speed of eating and to take proton-pump inhibitor (PPI) for 4 mo, but resulting in no benefit. The drug was discontinued. Then a gum-chewing exercise (about 10 times a day, 15 min each time, for 6 mo) was recommended. The patient returned to the hospital 6 mo later, reporting that his symptoms had been relieved. The contraction amplitude of his repeat manometry was improved (distal amplitude 58.03 mmHg on average). His manometric tracings before and after the gum-chewing exercise are shown in Figure 4. A repeat manometry after another 6 mo revealed continued normalized esophageal motility (distal amplitude 60.07 mmHg on average), though he had reduced the frequency of gum-chewing exercise since the previous manometry. During the manometry this time, the patient was also asked to perform 10 pairs of wet swallows at the interval of 2 s, 8 of which failed to initiate any peristalsis and only 2 of which were observed with peristaltic contraction at the end of the second pair of wet swallows.
Of the 19 IEM patients whose eating habits were investigated, 12 were fast eaters. The main presenting symptom of the fast eaters was chest pain or discomfort; the main symptom of the normal-eating patients was sense of obstruction. Although the average values of all swallow-induced contraction amplitudes were lower in the fast-eating group, there was no statistically significant difference compared with the normal-eating IEM patients. There are two possible explanations for this result. One is that factors other than fast eating were the dominant cause of weakened esophageal muscle in both groups. The other is that the weakened esophageal muscle could be due to fast eating (disuse of musculature) in fast-eating patients while other causes may contribute to the similar weakening in normal-eating patients. The other causes likely include acid reflux since 57% of the patients in this group reported heartburn or acid reflux, whereas only 25% of the fast eating group reported this symptom. The present study cannot distinguish between these two possibilities although it is very striking that all fast eaters showed dramatic weakening of the esophageal muscle. When NCCP patients are evaluated for esophageal dysmotility, only a few are subsequently diagnosed with IEM. The fact that all NCCP patients who self-reported fast eating were diagnosed with IEM suggests but does not prove a causal relationship. The case study suggests, but does not yet prove, that gum-chewing strengthens the esophageal muscle and it is consistent with the hypothesis that the weakening of the musculature was due to non-use of the musculature because of reduced swallow-induced contractions, although the weakened musculature may have been caused by other factors. In summary, although it is possible that fast eating is associated with weakening of the musculature, the present study does not provide direct evidence for it.
Habitual fast eating associated with rapid swallowing may limit the number of swallow-induced contractions since only the first or the last bolus are associated with a propulsive contraction. We have observed this phenomenon in this study, which is consistent with previous reports[8-11]. The contractions may become weaker with time. Another feature of rapid eating is insufficient mastication. Reduced duration of chewing prevents the optimization of the size, the softness and the lubrication of food boluses ready for swallowing[12]. Vagus nerve activity, which plays a vital role in the regulation of salivation[13] and esophageal peristalsis[14] and is enhanced by mastication[13], may also be less activated by inadequate chewing. To provide further evidence for or against the hypothesis that fast eating contributes to IEM, a prospective study is needed where the meal composition is standardized and the actual timing of swallows is measured.
The case report suggests that gum-chewing may strengthen the esophageal musculature. It would be important to find out if this is true independent of the cause of IEM. In this patient, PPI treatment did not relieve symptoms, and regular daily gum-chewing restored muscle contractile activity. Chewing gum on a regular basis is a stimulus that induces mastication-associated vagal activation[15] and swallow-associated propulsive contractions.
In the distal and mid esophagus, the contraction amplitudes in the fast-eating IEM patients were significantly reduced. However, their proximal manometric features were not statistically different from controls. This was probably due to the special musculature of the human esophagus, whose upper one-third is composed of striated muscle whereas the lower one-third is made up of smooth muscle and in between both types exist. Peristalsis in the striated muscle portion is induced by the sequential activation of neurons in the ambiguous nucleus which is solely a central mechanism; while in the smooth muscle portion, the peripheral intramural and central mechanisms cooperate to control peristalsis[14]. Considering the different manometric presentation of the distal and proximal esophagus in IEM, it is probable that a disorder in the peripheral neural control of esophageal smooth muscle contributes to the development of IEM in these patients.
Consensus on a causal relationship between NCCP and IEM has not hitherto been reached. Heartburn, dysphagia and regurgitation, reported by our patients, are possible risk factors for NCCP, in addition to psychological factors such as anxiety and depression[16] which often haunt our patients and aggravate their symptoms. Hence, NCCP in IEM is a result of many complex interactions and evidence is insufficient to assert that NCCP is caused by IEM. NCCP is often associated with GERD and IEM is the most common form of dysmotility in GERD and is correlated with more GERD episodes and prolonged acid clearance in a posture-dependent manner[17]. Although 24 h pH monitoring was not carried out, most of our patients did not suffer from GERD and the LES pressure was normal in our patients. Nevertheless, a contribution of gastroesophageal reflux to the symptoms of our IEM patients cannot be excluded.
The habit of rapid eating is a common phenomenon in China and may originate from periods in China when food supply was limited and collective dining was the main form of meal, so to ensure that sufficient food could be secured, many people developed the habit of rapid eating that eventually persisted for years. In addition, certain occupations in China, such as waiters/waitresses in restaurants and sales assistants in shops may not get sufficient free time to eat meals relaxed and hence quick eating may become a habit. We now investigate eating habits routinely in association with IEM and recommend changes in life style and exercise to alleviate their symptoms by strengthening their esophageal musculature.
The Valsalva maneuver increases the intrathoracic[18] and intra-abdominal pressure and leads to the activation of the diaphragm muscle[19]. Both the LES musculature and the crural diaphragm can contribute to the increase in LES pressure in response to an increased intra-abdominal pressure although evidence suggests that no active contraction of the smooth muscle is involved[20,21]. Most of our patients did not show decreased LES, but those who did might benefit from the Valsalva maneuver since it does increase the pressure of the esophageal junction. Previous studies in humans and animals showed that adjusted respiration could increase the pressure around the LES[22-25]. The effect of the Valsalva maneuver on esophageal muscle contraction is rarely mentioned. Our IEM patients showed a dramatic reduction in the proximal and mid esophageal response to the Valsalva, suggesting a weakened adaptive response of the esophageal musculature, at least the skeletal muscle.
In summary, inquiry into eating behavior is an important part of examination of patients with NCCP. Eating fast increases metabolic risk and should be discouraged. Eating fast may lead to ineffective esophageal motility, but more studies are needed to prove a direct causal relationship.
Esophageal dysmotility is considered an uncommon cause of non-cardiac chest pain (NCCP), but in our practice it is not infrequent. Previous studies have reported the correlation between eating behaviors and development of diseases, but the role of rapid eating in ineffective esophageal motility (IEM) and related symptoms has not been investigated.
Both IEM and NCCP are often associated with gastroesophageal reflux disease, but the pathophysiological mechanisms underlying IEM and NCCP are still poorly understood.
This study raises the possibility that rapid eating leads to IEM and attaches importance to inquiry into eating behavior as part of the examination of patients with NCCP.
Clinicians can take into account rapid eating as a potential cause of IEM, and the test in esophageal function. Further studies are needed to prove a direct causal relationship between rapid food intake and IEM.
IEM: IEM is defined manometrically as esophageal body contractions with ≥ 30% of wet swallows at an amplitude < 30 mmHg in the distal esophagus.
The concept is interesting, as the next step the authors should approach it prospectively, applying an objective definition of eating patterns rather than self-reporting.
| 1. | Fass R, Dickman R. Non-cardiac chest pain: an update. Neurogastroenterol Motil. 2006;18:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Cheung TK, Lim PW, Wong BC. The view of gastroenterologists on non-cardiac chest pain in Asia. Aliment Pharmacol Ther. 2007;26:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Hsieh SD, Muto T, Murase T, Tsuji H, Arase Y. Eating until feeling full and rapid eating both increase metabolic risk factors in Japanese men and women. Public Health Nutr. 2011;14:1266-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 467] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 5. | Haack HG, Hansen RD, Malcolm A, Kellow JE. Ineffective oesophageal motility: manometric subsets exhibit different symptom profiles. World J Gastroenterol. 2008;14:3719-3724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Yale SH. Antonio Maria Valsalva (1666 - 1723). Clin Med Res. 2005;3:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Wong LF, Taylor DM, Bailey M. Vagal response varies with Valsalva maneuver technique: a repeated-measures clinical trial in healthy subjects. Ann Emerg Med. 2004;43:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Ask P, Tibbling L. Effect of time interval between swallows on esophageal peristalsis. Am J Physiol. 1980;238:G485-G490. [PubMed] |
| 9. | Meyer GW, Gerhardt DC, Castell DO. Human esophageal response to rapid swallowing: muscle refractory period or neural inhibition? Am J Physiol. 1981;241:G129-G136. [PubMed] |
| 10. | Brito EM, Camacho-Lobato L, Paoletti V, Gideon M, Katz PO, Castell DO. Effect of different swallow time intervals on the nutcracker esophagus. Am J Gastroenterol. 2003;98:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Gidda JS, Goyal RK. Influence of successive vagal stimulations on contractions in esophageal smooth muscle of opossum. J Clin Invest. 1983;71:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Matsuo K, Palmer JB. Coordination of Mastication, Swallowing and Breathing. Jpn Dent Sci Rev. 2009;45:31-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Shiba Y, Nitta E, Hirono C, Sugita M, Iwasa Y. Evaluation of mastication-induced change in sympatho-vagal balance through spectral analysis of heart rate variability. J Oral Rehabil. 2002;29:956-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008;42:610-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Jang SY, Ju EY, Kim DE, Kim JH, Kim YH, Son M, Jang M, Jeong JH, Kim KS. First flatus time and xerostomia associated with gum-chewing after liver resection. J Clin Nurs. 2012;21:2188-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Kachintorn U. How do we define non-cardiac chest pain? J Gastroenterol Hepatol. 2005;20 Suppl:S2-S5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Leite LP, Johnston BT, Barrett J, Castell JA, Castell DO. Ineffective esophageal motility (IEM): the primary finding in patients with nonspecific esophageal motility disorder. Dig Dis Sci. 1997;42:1859-1865. [PubMed] |
| 18. | Looga R. The Valsalva manoeuvre--cardiovascular effects and performance technique: a critical review. Respir Physiol Neurobiol. 2005;147:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Thompson JA, O’Sullivan PB, Briffa NK, Neumann P. Differences in muscle activation patterns during pelvic floor muscle contraction and Valsalva maneuver. Neurourol Urodyn. 2006;25:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Dodds WJ, Hogan WJ, Miller WN, Stef JJ, Arndorfer RC, Lydon SB. Effect of increased intraabdominal pressure on lower esophageal sphincter pressure. Am J Dig Dis. 1975;20:298-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 53] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Mittal RK, Fisher M, McCallum RW, Rochester DF, Dent J, Sluss J. Human lower esophageal sphincter pressure response to increased intra-abdominal pressure. Am J Physiol. 1990;258:G624-G630. [PubMed] |
| 22. | Whitehead WE. Biofeedback treatment of gastrointestinal disorders. Biofeedback Self Regul. 1992;17:59-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Shaker R, Bardan E, Gu C, Massey BT, Sanders T, Kern MK, Hoffmann RG, Hogan WJ. Effect of lower esophageal sphincter tone and crural diaphragm contraction on distensibility of the gastroesophageal junction in humans. Am J Physiol Gastrointest Liver Physiol. 2004;287:G815-G821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Mittal RK, Shaffer HA, Parollisi S, Baggett L. Influence of breathing pattern on the esophagogastric junction pressure and esophageal transit. Am J Physiol. 1995;269:G577-G583. [PubMed] |
| 25. | Boyle JT, Altschuler SM, Nixon TE, Tuchman DN, Pack AI, Cohen S. Role of the diaphragm in the genesis of lower esophageal sphincter pressure in the cat. Gastroenterology. 1985;88:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
P- Reviewer Alvarez F S- Editor Wen LL L- Editor Ma JY E- Editor Zhang DN