Published online Apr 14, 2013. doi: 10.3748/wjg.v19.i14.2249
Revised: February 6, 2013
Accepted: February 8, 2013
Published online: April 14, 2013
Processing time: 153 Days and 3.7 Hours
AIM: To investigate the effects of Lizhong Tang, an herbal product used in traditional Chinese medicine, on mouse small intestine interstitial cells of Cajal (ICCs).
METHODS: Enzymatic digestions were used to dissociate ICCs from mouse small intestine tissues. The ICCs were morphologically distinct from other cell types in culture and were identified using phase contrast microscopy after verification with anti c-kit antibody. A whole-cell patch-clamp configuration was used to record potentials (current clamp) from cultured ICCs. All of the experiments were performed at 30-32 °C.
RESULTS: ICCs generated pacemaker potentials, and Lizhong Tang produced membrane depolarization in current-clamp mode. The application of flufenamic acid (a nonselective cation channel blocker) abolished the generation of pacemaker potentials by Lizhong Tang. Pretreatment with thapsigargin (a Ca2+-ATPase inhibitor in the endoplasmic reticulum) also abolished the generation of pacemaker potentials by Lizhong Tang. However, pacemaker potentials were completely abolished in the presence of an external Ca2+-free solution, and under this condition, Lizhong Tang induced membrane depolarizations. Furthermore, When GDP-β-S (1 mmol/L) was in the pipette solution, Lizhong Tang still induced membrane depolarizations. In addition, membrane depolarizations were not inhibited by chelerythrine or calphostin C, which are protein kinase C inhibitors, but were inhibited by U-73122, an active phospholipase C inhibitors.
CONCLUSION: These results suggest that Lizhong Tang might affect gastrointestinal motility by modulating pacemaker activity in interstitial cells of Cajal.
Core tip: The gastroprokinetic effects of Lizhong Tang are mediated by the induction of pacemaker potentials in interstitial cells of Cajal (ICCs). Taken together, our data suggest that the gastroprokinetic effects of Lizhong Tang are mediated by the induction of pacemaker potentials in ICCs. Considering the effects of this drug on ICCs, further research is required to identify the compounds responsible for the effects of Lizhong Tang and to determine their mechanisms of action.
- Citation: Hwang MW, Kim JN, Song HJ, Lim B, Kwon YK, Kim BJ. Effects of Lizhong Tang on cultured mouse small intestine interstitial cells of Cajal. World J Gastroenterol 2013; 19(14): 2249-2255
- URL: https://www.wjgnet.com/1007-9327/full/v19/i14/2249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i14.2249
Lizhong Tang, was first reported 1800 years ago in “Shanghan Lun”, and it remains a classical herbal product in traditional Chinese medicine. Lizhong Tang is composed of Radix Ginseng (Panax ginseng C.A. Meyer), Rhizoma Zingiberis (Zingiber officinale Roscoe), Rhizoma Atractylodis Macrocephalae (Atractylodes macrocephala Koidz.) and Radix Glycyrrhizae (Glycyrrhiza uralensis Fisch)[1], and it is widely used in traditional medicine to treat spleen deficiency patterns in many diseases with common symptoms, such as vomiting, diarrhea, stomach pain, poor appetite, cold limbs, and stomach bleeding which, are caused by cold and weak organs[1,2]. However, little is known of the molecular basis of the effects of Lizhong Tang on gastrointestinal (GI) motility.
Interstitial cells of Cajal (ICCs) are pacemaker cells in GI muscles that generate rhythmic oscillations in membrane potentials known as slow waves[3-5]. Slow waves propagate within ICC networks and are conducted into smooth muscle cells via gap junctions. Furthermore, they initiate phasic contractions by activating Ca2+ entry through L-type Ca2+ channels. Pacemaker activity in the murine small intestine is mainly due to periodic activations of nonselective cation channels[6,7] or Cl- channels[8,9]. ICCs also mediate or transduce inputs from the enteric nervous system. However, the effects of Lizhong Tang in mouse small intestine ICCs have not been investigated, and therefore, we undertook this study to investigate the characteristics of Lizhong Tang in mouse small intestine ICCs.
Balb/c mice (3-7 d old) of either sex were anesthetized with ether and sacrificed by cervical dislocation. The small intestines, from 1 cm below the pyloric ring to the cecum, were removed and opened along the mesenteric border. The luminal contents were removed by washing with Krebs-Ringer bicarbonate solution. The tissues were then pinned to the base of a Sylgard dish, and the mucosae were removed by sharp dissection. Small tissue strips of intestinal muscle (consisting of both circular and longitudinal muscles) were equilibrated in Ca2+-free Hanks solution (containing the following in mmol/L: KCl 5.36, NaCl 125, NaOH 0.34, Na2HCO3 0.44, glucose 10, sucrose 2.9, and HEPES 11) for 30 min, and then, the cells were dispersed using an enzyme solution containing collagenase (Worthington Biochemical Co., Lakewood, NJ, United States) 1.3 mg/mL, bovine serum albumin (Sigma Chemical Co., St. Louis, MO, United States) 2 mg/mL, trypsin inhibitor (Sigma) 2 mg/mL and ATP 0.27 mg/mL. The cells were plated onto sterile glass coverslips coated with murine collagen (2.5 μg/mL, Falcon/BD, Franklin Lakes, NJ, United States) in a 35-mm culture dish and then cultured at 37 °C in a 95% O2, 50 mL/L CO2 incubator in smooth muscle growth medium (Clonetics Corp., San Diego, CA, United States) supplemented with 2% antibiotics/antimycotics (Gibco, Grand Island, NY, United States) and murine stem cell factor (SCF 5 ng/mL, Sigma). ICCs were identified immunologically using an anti-c-kit antibody (phycoerythrin-conjugated rat anti-mouse c-kit monoclonal antibody; eBioscience, San Diego, CA, United States) at a dilution of 1:50 for 20 min[10]. The ICCs were morphologically distinct from other cell types in culture and were identified using phase contrast microscopy after verification with anti c-kit antibody.
A whole-cell patch-clamp setup was used to record the membrane potentials (current clamp) of cultured ICCs. An axopatch ID (Axon Instruments, Foster, CA, United States) was used to amplify membrane currents and potentials. The command pulse was applied using an IBM-compatible personal computer running pClamp software (version 6.1; Axon Instruments). The data obtained were filtered at 5 kHz displayed on an oscilloscope and a computer monitor, and printed using a pen recorder (Gould 2200, Gould, Valley View, OH, United States). The results were analyzed using pClamp and Origin (version 6.0) software. All of the experiments were performed at 30-32 °C.
The physiological salt solution used to bathe cells (Na+-Tyrode) contained the following (in mmol/L): KCl 5, NaCl 135, CaCl2 2, glucose 10, MgCl2 1.2 and HEPES 10, adjusted to pH 7.4 with NaOH. The pipette solution contained the following (in mmol/L): KCl 140, MgCl2 5, K2ATP 2.7, NaGTP 0.1, creatine phosphate disodium 2.5, HEPES 5 and EGTA 0.1, adjusted to pH 7.2 with KOH. Lizhong Tang was purchased from I-WORLD Pharmaceuticals (South Korea). Lizhong Tang is composed of Radix Ginseng (Panax ginseng C.A. Meyer), Rhizoma Zingiberis (Zingiber officinale Roscoe), Rhizoma Atractylodis Macrocephalae (Atractylodes macrocephala Koidz.) and Radix Glycyrrhizae (Glycyrrhiza uralensis Fisch.). The adult dosage is 10-15 g (crude material) per day. More information about Lizhong Tang can be obtained at the I-WORLD Pharmaceuticals Homepage (http://i-pharm.koreasme.com). The pills were dissolved with distilled water at a concentration of 0.5 g of crude drug/ml and stored in the refrigerator. All of the drugs were obtained from Sigma (Sigma Chemical Co., United States). The drugs were dissolved in distilled water, and added to the bathing solution to the desired concentrations immediately prior to use. The addition of these chemicals to the bathing solution did not alter its pH. Thapsigargin, U-73122, and U-73343 were dissolved in dimethyl sulfoxide (DMSO) to produce 50 and 100 mmol/L stock solutions and added at 1000 times dilutions to the bathing solution on the day of the experiment. The final concentration of DMSO in the bathing solution was always < 0.1%, and we confirmed that this concentration did not affect the results.
All of the data are expressed as the mean ± SE. Student’s t-test for unpaired data was used to compare the control and experimental groups. Statistical significance was accepted for P values < 0.05.
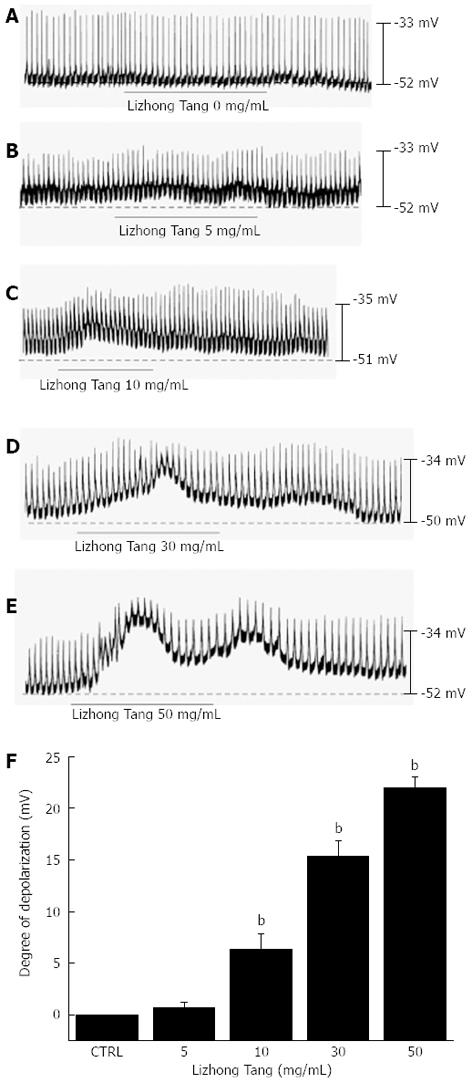
The patch-clamp technique was tested on ICCs, which had formed network-like structures in culture after 2-4 d. Spontaneous rhythms were routinely recorded from cultured ICCs under current- and voltage-clamp conditions; ICCs within networks displayed more robust electrical rhythms. Tissue-like spontaneous slow waves have been previously recorded from these cells[11]. To understand the relationship between Lizhong Tang and the modulation of pacemaker activity in ICCs, we examined the effects of Lizhong Tang on pacemaker potentials. Recordings from cultured ICCs under current-clamp mode (I = 0) showed spontaneous pacemaker potentials. The mean resting membrane potential was -52 ± 1.3 mV, and the mean amplitude was 23 ± 2 mV. In the presence of Lizhong Tang (0-50 mg/mL), the membrane potentials were depolarized to 0.6 ± 0.5 mV at 5 mg/mL, 6.3 ± 1.5 mV at 10 mg/mL, 15.2 ± 1.3 mV at 30 mg/mL, and 22.1 ± 1.2 mV at 50 mg/mL (Figure 1A-E). Summarized values and a bar graph of the effects of Lizhong Tang on pacemaker potentials are provided in Figure 1F (n = 4).
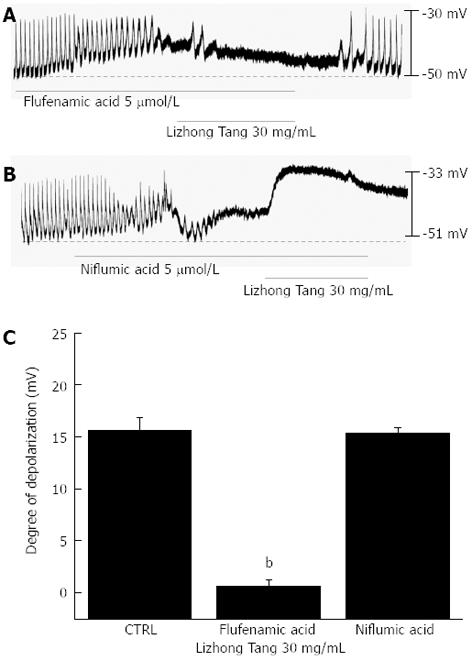
To determine the characteristics of the membrane depolarizations induced by Lizhong Tang, flufenamic acid (a non-selective cation channel blocker)[12,13] and niflumic acid (a Cl-channel blocker)[12,14] were used. In the presence of flufenamic acid (5 μmol/L), pacemaker potentials were abolished and the subsequent application of Lizhong Tang (30 mg/mL) did not produce membrane depolarization (Figure 2A). In the presence of flufenamic acid, the membrane depolarizations produced by Lizhong Tang were 0.6 ± 0.4 mV, which was significantly different from the control values obtained in the absence of flufenamic acid (n = 4, Figure 2C). Pacemaker potentials were also abolished in the presence of niflumic acid (5 μmol/L), but Lizhong Tang still produced membrane depolarization (Figure 2B). In the presence of niflumic acid, the mean membrane depolarization produced by Lizhong Tang was 15.3 ± 0.4 mV, which was not significantly different from the control condition (n = 4, Figure 2C).
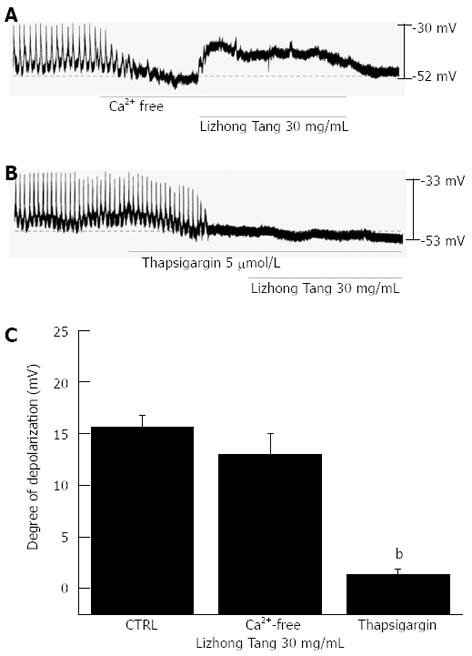
External Ca2+ influx is necessary for GI smooth muscle contractions and is essential for the generation of pacemaker potentials by ICCs. The generation of pacemaker currents is known to be dependent on intracellular Ca2+ oscillations[15]. To investigate the roles of external and of internal Ca2+, Lizhong Tang was tested under external Ca2+-free conditions and in the presence of thapsigargin, an inhibitor of Ca2+-ATPase in the endoplasmic reticulum[6,12]. Pacemaker potentials were completely abolished in the presence of an external Ca2+-free solution, and under this condition, Lizhong Tang induced membrane depolarizations (n = 4, Figure 3A). However, under external Ca2+-free conditions, membrane depolarizations by Lizhong Tang (30 mg/mL) were not significantly different from the depolarizations induced by Lizhong Tang (30 mg/mL) under normal Ca2+ conditions (n = 4, Figure 3C). In addition, Lizhong Tang-induced membrane depolarizations were inhibited by thapsigargin pretreatment (Figure 3B). Furthermore, the membrane depolarizations induced by Lizhong Tang were significantly affected by the presence of thapsigargin (n = 4, Figure 3C).
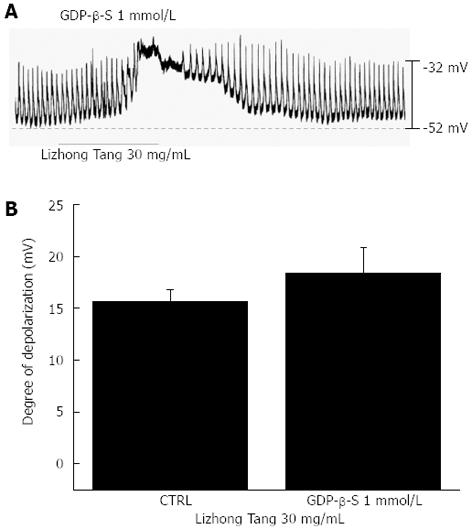
The effects of GDP-β-S (a non-hydrolysable guanosine 5’-diphosphate analogue that permanently inactivates G-protein binding proteins[16]) were examined to determine whether G-proteins are involved in the effects of Lizhong Tang on ICCs. When GDP-β-S (1 mmol/L) was in the pipette solution, Lizhong Tang (30 mg/mL) still induced membrane depolarizations (Figure 4A). However, the membrane depolarizations induced by Lizhong Tang were not significantly affected by the presence of GDP-β-S (1 mmol/L) in the pipette solution (n = 4, Figure 4C).
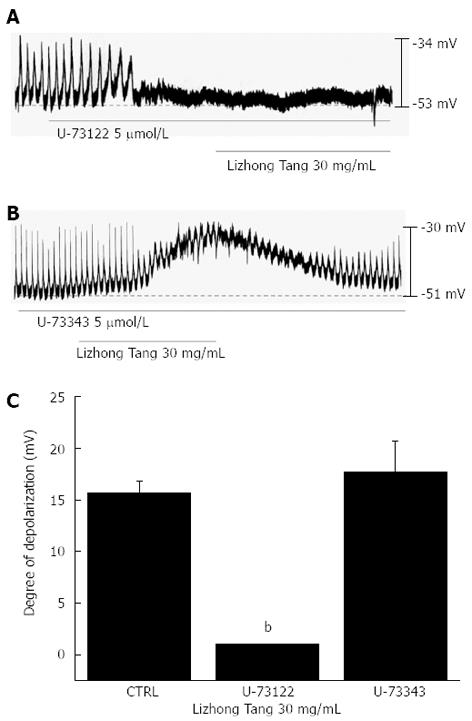
Because membrane depolarizations induced by Lizhong Tang are related to intracellular Ca2+ mobilization, we examined whether the effects of Lizhong Tang on pacemaker potentials required phospholipase C (PLC) activation. To test this possibility, Lizhong Tang-induced membrane depolarizations were measured in the absence or presence of U-73122 (an active PLC inhibitor[17]). Pacemaker membrane depolarizations currents were completely abolished by U-73122 (5 μmol/L), and under these conditions, Lizhong Tang-induced (30 mg/mL) membrane depolarizations were suppressed (n = 4, Figure 5A). In the presence of U-73122, the mean membrane depolarization produced by Lizhong Tang was 0.5 ± 0.3 mV, and this was significantly different than the depolarization observed in the absence of U-73122 (n = 4, Figure 5C). Treatment with U-73343 (5 μmol/L; an inactive analog of U-73122) had no influence on Lizhong Tang-induced pacemaker potentials, and Lizhong Tang-induced (30 mg/mL) membrane depolarizations were not suppressed by U-73343 (Figure 5C).
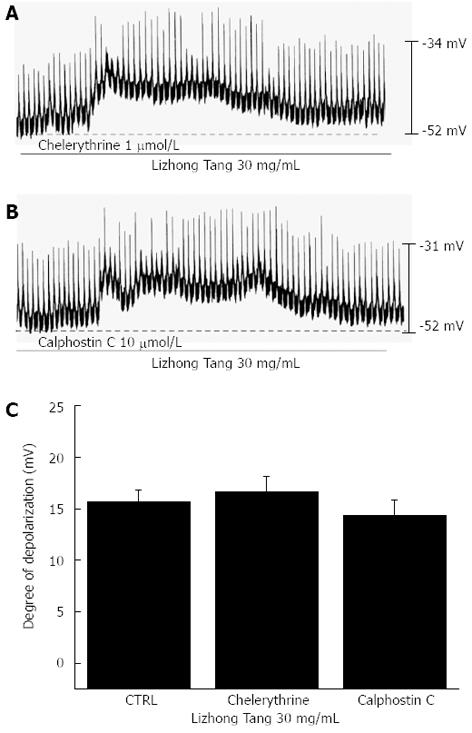
We tested the effects of chelerythrine and of calphostin C (both inhibitors of protein kinase C (PKC)[12,18]) to investigate whether Lizhong Tang-induced pacemaker potential responses are mediated by the activation of PKC. Neither chelerythrine (1 μmol/L) nor calphostin C (1 μmol/L) had any effect on membrane depolarizations induced by Lizhong Tang (30 mg/mL; Figure 6) and the value was also not significantly different when compared with the membrane depolarizations induced by Lizhong Tang in the absence of chelerythrine or calphostin C (n = 5, Figure 6C).
The GI tract exhibits spontaneous mechanical contractions that are mediated by the periodic generation of electrical pacemaker potentials, which are the basic determinant of GI smooth muscle activity[3]. Recent studies have shown that the ICCs act as the pacemakers and conductors of electrical slow waves in GI smooth muscles[3-5]. Moreover, evidence indicates that endogenous agents, such as, neurotransmitters, hormones, and paracrine substances modulate GI tract motility by influencing ICCs[5-7,19,20]. Therefore, one of the best ways to investigate the role of GI motility is to use ICCs. Many types of ICCs with different immunohistochemical and electrical properties, including myenteric ICCs (ICC-MY), intramuscular ICCs (ICC-IM), deep muscular plexus ICCs (ICC-DMP), and submucosal ICCs (ICC-SM), are distributed throughout the GI tract[16]. In animal models lacking ICC-MY, slow waves in the small intestine are strongly attenuated, which shows that these cells are indeed essential for pacemaker activity in the GI tract[21]. Furthermore, ICCs are involved in physiological GI motility and are therefore clinically important in many bowel disorders, including inflammatory bowel disease, chronic idiopathic intestinal pseudo-obstruction, intestinal obstruction with hypertrophy, achalasia, Hirschsprung’s disease, juvenile pyloric stenosis, juvenile intestinal obstruction, and anorectal malformation[16].
Lizhong Tang warms the liver and spleen and stren-gthens the spleen and stomach. It has been widely used as treatment from deficiency, diarrhea with watery stool, nausea and vomiting. In addition, it also has ameliorative effects on loss of appetite, abdominal pain, acute or chronic gastritis gastric or duodenal ulcers, irritable bowel syndrome, chronic colitis, chronic bronchitis, oral herpes, and functional uterine bleeding[1,22,23]. However, the effects of Lizhong Tang on GI tract motility and ICCs have not been investigated.
In this study, Lizhong Tang produced membrane depolarization in current-clamp mode, and the application of flufenamic acid (a non-selective cation channel blocker), but not niflumic acid (a Cl- channel blocker), abolished the generation of the pacemaker potentials induced by Lizhong Tang, suggesting that the Lizhong Tang-induced membrane depolarizations may be mediated by non-selective cationic channels. In addition, pretreatment with a Ca2+-free solution or with thapsigargin (a Ca2+-ATPase inhibitor in the endoplasmic reticulum), abolished the generation of pacemaker potentials. Under Ca2+-free conditions, Lizhong Tang also showed membrane depolarization; however, in the presence of thapsigargin, Lizhong Tang did not show membrane depolarization, suggesting that intracellular calcium release is necessary. Furthermore, pacemaker membrane depolarizations were inhibited by U-73122 (PLC inhibitor), but not by GDP-β-S, which permanently binds G-binding proteins. In addition, the PKC inhibitors chelerythrine and calphostin C did not block Lizhong Tang-induced pacemaker potentials, suggesting that PLC is involved in the induction of the pacemaker potentials, but that PKC is not. In summary, Lizhong Tang affects GI motility by modulating pacemaker activity in ICCs, and this activation is associated with non-selective cationic channels via phospholipase C activation, and Ca2+ release from internal storage in an external Ca2+-, G-protein-, and PKC-independent manner.
Taken together, our data suggest that the gastroprokinetic effects of Lizhong Tang are mediated by the induction of pacemaker potentials in ICCs. Considering the effects of this drug on ICCs, further research is required to identify the compounds responsible for the effects of Lizhong Tang and to determine their mechanisms of action.
Interstitial cells of Cajal (ICCs) are the pacemaker cells that generate slow waves in the gastrointestinal (GI) tract. Lizhong Tang is a classic herbal product in traditional Chinese medicine. However, the effects of Lizhong Tang in mouse small intestine ICCs have not been investigated.
The gastroprokinetic effects of Lizhong Tang are mediated by the induction of pacemaker potentials in ICCs.
Lizhong Tang affects GI motility by modulating pacemaker activity in ICCs, and this activation is associated with non-selective cationic channels via phospholipase C activation, and Ca2+ release from internal storage in an external Ca2+-, G-protein-, and protein kinase C-independent manner.
Lizhong Tang may be a new target for pharmacological treatment of GI motility disorders.
In their manuscript, authors studies the effect of Lizhong Tang, an herbal product used in traditional Chinese medicine, on the pacemaking activity of mouse small ICCs. It was well written.
| 1. | Zhao N, Zhang W, Guo Y, Jia H, Zha Q, Liu Z, Xu S, Lu A. Effects on neuroendocrinoimmune network of Lizhong Pill in the reserpine induced rats with spleen deficiency in traditional Chinese medicine. J Ethnopharmacol. 2011;133:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Cheng WJ. Shanghan Lun Zhujie. 2nd ed. Beijing: The People’s Medical Publishing House 2005; . |
| 3. | Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91-97. [PubMed] |
| 4. | Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1082] [Article Influence: 34.9] [Reference Citation Analysis (4)] |
| 5. | Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Koh SD, Jun JY, Kim TW, Sanders KM. A Ca(2+)-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002;540:803-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY, Park CS, So I, Stanfield PR, Kim KW. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005;129:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Huizinga JD, Zhu Y, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology. 2002;123:1627-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca(2+)-activated Cl(-) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587:4905-4918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Goto K, Matsuoka S, Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol. 2004;559:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 1998;513:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 223] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Choi S, Choi JJ, Jun JY, Koh JW, Kim SH, Kim DH, Pyo MY, Choi S, Son JP, Lee I. Induction of pacemaker currents by DA-9701, a prokinetic agent, in interstitial cells of Cajal from murine small intestine. Mol Cells. 2009;27:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Sanders KM, Koh SD, Ordög T, Ward SM. Ionic conductances involved in generation and propagation of electrical slow waves in phasic gastrointestinal muscles. Neurogastroenterol Motil. 2004;16 Suppl 1:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Kuriyama H, Kitamura K, Itoh T, Inoue R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol Rev. 1998;78:811-920. [PubMed] |
| 15. | Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525 Pt 2:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 251] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Sanders KM, Ordög T, Koh SD, Torihashi S, Ward SM. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil. 1999;11:311-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 250] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Sakamoto T, Unno T, Matsuyama H, Uchiyama M, Hattori M, Nishimura M, Komori S. Characterization of muscarinic receptor-mediated cationic currents in longitudinal smooth muscle cells of mouse small intestine. J Pharmacol Sci. 2006;100:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Aiello EA, Clément-Chomienne O, Sontag DP, Walsh MP, Cole WC. Protein kinase C inhibits delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1996;271:H109-H119. [PubMed] |
| 19. | Kim BJ, Chang IY, Choi S, Jun JY, Jeon JH, Xu WX, Kwon YK, Ren D, So I. Involvement of Na(+)-leak channel in substance P-induced depolarization of pacemaking activity in interstitial cells of Cajal. Cell Physiol Biochem. 2012;29:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Kim BJ, Chang IY, So I. Pharmacological differences of endothelin receptors-mediated modulation in cultured interstitial cells of Cajal from the murine small and large intestine. Cell Physiol Biochem. 2012;30:359-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369-375. [PubMed] |
| 22. | Jung YP, Hwang YH, Lee JH, Yim NH, Cho WK, Ma JY. A Study on the Acute Toxicity of Leejung tang (Lizhong-tang) and Fermented Leejung-tang (Lizhong-tang) Extract in ICR Mice. Kor J Herbol. 2012;27:95-100. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Seo HY, Han JK, Kim YH. Therapeutic Effects of Yijungtang on Atopic Dermatitis-like Skin Lesions of NC/Nga Mouse Induced by Mite Antigen. J Korean Oriental Pediatrics. 2011;25:1-27. |
P- Reviewer Rampoldi L S- Editor Wen LL L- Editor A E- Editor Zhang DN