Published online Jan 7, 2013. doi: 10.3748/wjg.v19.i1.78
Revised: September 17, 2012
Accepted: September 29, 2012
Published online: January 7, 2013
AIM: To identify factors associated with prognosis of hepatocellular carcinoma (HCC) after initial therapy.
METHODS: A total of 377 HCC patients who were newly treated at Katsushika Medical Center, Japan from January 2000 to December 2009 and followed up for > 2 years, or died during follow-up, were enrolled. The factors related to survival were first analyzed in 377 patients with HCC tumor stage T1-T4 using multivariate Cox proportional hazards regression analysis. A similar analysis was performed in 282 patients with tumor stage T1-T3. Additionally, factors associated with the period between initial and subsequent therapy were examined in 144 patients who did not show local recurrence. Finally, 214 HCC stage T1-T3 patients who died during the observation period were classified into four groups according to their alcohol consumption and postprandial glucose levels, and differences in their causes of death were examined.
RESULTS: On multivariate Cox proportional hazards regression analysis, the following were significantly associated with survival: underlying liver disease stage [non-cirrhosis/Child-Pugh A vs B/C, hazard ratio (HR): 0.603, 95% CI: 0.417-0.874, P = 0.0079], HCC stage (T1/T2 vs T3/T4, HR: 0.447, 95% CI: 0.347-0.576, P < 0.0001), and mean postprandial plasma glucose after initial therapy (< 200 vs≥ 200 mg/dL, HR: 0.181, 95% CI: 0.067-0.488, P = 0.0008). In T1-T3 patients, uninterrupted alcohol consumption after initial therapy (no vs yes, HR: 0.641, 95% CI: 0.469-0.877, P = 0.0055) was significant in addition to underlying liver disease stage (non-cirrhosis/Child-Pugh A vs B/C, HR: 0649, 95% CI: 0.476-0.885, P = 0.0068), HCC stage (T1 vs T2/T3, HR: 0.788, 95% CI: 0.653-0.945, P = 0.0108), and mean postprandial plasma glucose after initial therapy (< 200 mg/dL vs≥ 200 mg/dL, HR: 0.502, 95% CI: 0.337-0.747, P = 0.0005). In patients without local recurrence, time from initial to subsequent therapy for newly emerging HCC was significantly longer in the “postprandial glucose within 200 mg/dL group” than the “postprandial glucose > 200 mg/dL group” (log-rank test, P < 0.05), whereas there was no difference in the period between the “non-alcohol group” (patients who did not drink regularly or those who could reduce their daily consumption to < 20 g) and the “continuation group” (drinkers who continued to drink > 20 g daily). Of 214 T1-T3 patients who died during the observation period, death caused by other than HCC progression was significantly more frequent in “group AL” (patients in the continuation and postprandial glucose within 200 mg/dL groups) than “group N” (patients in the non-alcohol and postprandial glucose within 200 mg/dL groups) (P = 0.0016).
CONCLUSION: This study found that abstinence from habitual alcohol consumption and intensive care for diabetes mellitus were related to improved prognosis in HCC patients.
- Citation: Abe H, Aida Y, Ishiguro H, Yoshizawa K, Miyazaki T, Itagaki M, Sutoh S, Aizawa Y. Alcohol, postprandial plasma glucose, and prognosis of hepatocellular carcinoma. World J Gastroenterol 2013; 19(1): 78-85
- URL: https://www.wjgnet.com/1007-9327/full/v19/i1/78.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i1.78
Hepatocellular carcinoma (HCC) is one of the most important diseases, with increasing numbers of patients worldwide in recent years[1]. The number of deaths from HCC has also increased steadily. In Japan, chronic viral infection, such as hepatitis C virus (HCV) or hepatitis B virus (HBV), is considered to be the most significant risk factor for the development of HCC[2]. It has been recognized that chronic infection with these viruses induces continuous or recurrent necroinflammatory reactions in the liver that lead to cirrhosis; in most cases, the cirrhotic stage facilitates the development of HCC[3].
In Japan, hepatitis B surface antigen (HBs-Ag)-positive or anti-HCV (HCV-Ab)-positive subjects have accounted for > 80% of HCC patients in the last decade[4]. Meanwhile, 5%-15% of patients with HCC have neither HBs-Ag nor HCV-Ab (non-B non-C)[5-7], and the proportion of non-B non-C HCC has been increasing in newly diagnosed subjects[8].
Alcoholic liver disease[9] and nonalcoholic fatty liver disease[10] have been proposed as possible major backgrounds associated with non-B non-C HCC. Moreover, diabetes mellitus (DM), which is frequently present in association with nonalcoholic steatohepatitis or alcoholic liver disease, has been identified as a risk factor for developing HCC[11-15].
As mentioned above, factors associated with the development of HCC have been well recognized. However, factors affecting the prognosis after treatment for HCC have not been extensively examined, except for HCC tumor stage and underlying liver function.
A large majority of HCC patients have been treated according to the “Japanese Guidelines for HCC”[16]. There have been numerous therapeutic strategies against HCC, including surgical resection, percutaneous ethanol injection therapy (PEIT), percutaneous radiofrequency ablation (RFA), and transhepatic arterial chemoembolization (TACE). The therapeutic strategy is determined based on liver function and HCC tumor stage, and aims to achieve the best prognosis[16]. However, there have often been obvious differences in clinical course even when HCC has been treated according to the “Japanese Guidelines for HCC”[16].
A relationship between HCC recurrence and the presence of DM in patients who are negative for markers of HBV and HCV[17] has been reported. The prognostic factors of HCC other than HCC tumor stage and underlying liver function have been evaluated[18]. However, there is no information about the relationship between HCC prognosis and general health management, including abstinence from alcohol drinking and intensive control of plasma glucose levels after therapy for HCC.
In the present study, the impact of plasma glucose levels and continuation of alcohol consumption on the prognosis after initial therapy for HCC was investigated.
Of 475 consecutive HCC patients who were newly diagnosed and treated at the Jikei University Katsushika Medical Center from January 2000 to December 2009, we enrolled 377 who were treated based on the Japanese Guidelines for HCC[16]. Initial therapy consisted of surgical resection in five of 377 HCC patients, PEIT in 46 patients, RFA in 111 patients, TACE in 146 patients, transhepatic arterial infusion in 54 patients, and supportive care without therapy for HCC in 15 patients. Only 1.3% of patients underwent surgical resection, because patients who did not wish to undergo an operation tended to consult our department. Patients who received liver transplantation were not included in this study, because the number of deceased donors is small in Japan.
The diagnosis of HCC was based on the presence of characteristic imaging findings on computed tomography, magnetic resonance imaging, or ultrasonography. In subjects in whom the definite diagnosis of HCC could not be made by imaging, the diagnosis was confirmed by ultrasonography-guided tumor biopsy.
The characteristics of the 377 patients at the time of initial diagnosis of HCC, including age, sex, serum aspartate aminotransferase (AST)/alanine aminotransferase/α-fetoprotein (AFP)/protein induced by vitamin K absence or antagonist-II (PIVKA-II) level at the time of diagnosis, stage of underlying liver disease (cirrhotic or non-cirrhotic, Child-Pugh staging in cirrhotic patients), obesity [body mass index (BMI) > 25 kg/m2], presence of DM, existence of malignant tumors other than HCC, habitual use of alcohol, and HCC tumor stage according to the tumor-node-metastasis staging classification system[19], are summarized in Table 1.
| Characteristics | Data |
| Age (yr) | 67 ± 9.4 |
| Sex (M:F) | 276:101 |
| Stage of liver disease | |
| Non LC | 57 (15.1) |
| LC | |
| Child A | 213 (56.5) |
| Child B | 86 (22.8) |
| Child C | 21 (5.6) |
| T stage in the TNM system | |
| 1 | 101 (26.8) |
| 2 | 125 (33.2) |
| 3 | 56 (14.9) |
| 4 | 95 (25.2) |
| HBs-Ag-positive | 38 (10.1) |
| HCV-Ab-positive | 256 (67.9) |
| Serum AST (IU/L) | 88 ± 119.1 |
| Serum ALT (IU/L) | 66 ± 62.9 |
| Serum AFP (ng/mL) | 11 623 ± 48 270 |
| Serum PIVKA-II (mAU/mL) | 15 270 ± 96 781 |
| Habitual alcohol drinkers | 203 (53.8) |
| Obesity (BMI > 25 kg/m2) | 80 (21.1) |
| Presence of DM | 132 (35.0) |
The presence or absence of serum HCV-Ab and HBs-Ag was determined in all patients using an enzyme immunoassay kit (AxSYM Abbott HCV; Axsym Co. Ltd., Tokyo, Japan, for HCV-Ab and AxSYM Abbott HBs-Ag; Axsym, for HBs-Ag).
The diagnosis of cirrhosis was made based on imaging findings, histological findings of liver biopsy specimens, and a combination of laboratory data[20]. Cirrhotic patients were then subdivided into A-C according to the Child-Pugh classification[21].
Habitual alcohol consumption was defined as cumulative consumption ≥ 320 kg ethanol in females or ≥ 480 kg in males, with daily alcohol consumption ≥ 20 g ethanol. A history of alcohol consumption was obtained from interviews with patients, their families, or neighbors.
The definition of the presence of DM was as follows: fasting plasma glucose level > 126 mg/dL or postprandial plasma glucose > 200 mg/dL on at least two occasions, or the need for subcutaneous injection of insulin or oral hypoglycemic drugs to control glucose levels.
Enrolled patients were followed up after initial therapy for HCC for at least 2 years or until death during follow-up. No patients took drugs or foods containing carcinogenic substances. HCV-positive HCC patients did not undergo antiviral therapy after therapy for HCC, while all of the HBs-Ag-positive HCC patients were treated with nucleoside and/or nucleotide analogs including lamivudine, adefovir, dipivoxil, and entecavir.
Survival after initial therapy and the period from initial therapy to subsequent therapy were recorded. Continuation of alcohol consumption and the mean postprandial plasma glucose values during almost monthly follow-up were examined.
The manner of drinking after initial therapy for HCC was classified into two groups: patients who did not drink regularly or habitual alcohol drinkers who were able to reduce their quantity of daily alcohol consumption to < 20 g ethanol (non-alcohol group), and habitual alcohol drinkers who continued to drink > 20 g ethanol daily (continuation group).
Patients defined as having DM were classified into the “postprandial glucose within 200 mg/dL group” or the “postprandial glucose > 200 mg/dL group”. The postprandial glucose within 200 mg/dL group was defined as having a mean monthly postprandial plasma glucose level (plasma glucose measured about 2 h after taking the meal) ≤ 200 mg/dL during follow-up. Patients without DM were included in the postprandial glucose within 200 mg/dL group. The other HCC patients whose mean postprandial plasma glucose level was > 200 mg/dL despite treatment for DM were included in the postprandial glucose > 200 mg/dL group.
Education about abstinence from drinking was given to all habitual drinkers. For all patients with DM, education about appropriate caloric intake and standard drug therapy for DM, including insulin injection, was provided. This study complied with the standards of the Declaration of Helsinki and current ethical guidelines.
Data are expressed as means ± SD, and the parameters were categorized into two groups by the median value, except for the stage of underlying liver disease and the T stage of HCC. Factors associated with survival were examined by Cox proportional hazards regression analysis to elucidate factors associated with HCC prognosis. The differences in the survival period and in the period from initial therapy to subsequent therapy were evaluated by the Kaplan-Meier method with the log-rank test. Differences in the causes of death were evaluated by the χ2 test with Yates’s correction. All tests of significance were two-tailed, and a P < 0.05 was considered significant. All statistical analyses were carried out using Statistica for Windows Version 6.
The patients’ median age was 69 years. The most common stage of underlying liver disease was Child-Pugh class A cirrhosis. The frequencies of HBs-Ag and HCV-Ab were 10.1% and 67.9%, respectively. The prevalence of habitual drinkers was 53.8%, and 21.1% had a BMI > 25 kg/m2. DM was present in 35.0% of HCC patients (Table 1). The median survival period after initial therapy for HCC was 23 mo.
The factors associated with survival after initial therapy for HCC were investigated in 377 patients. On univariate analysis, the following 11 factors were significantly related to survival: stage of underlying liver disease (non-cirrhosis, Child A vs B, C cirrhosis, P = 0.0001), T stage of HCC (T1, 2 vs 3, 4, P < 0.0001), number of HCCs (solitary vs multiple, P < 0.0001), maximum size of HCCs (< 20 vs≥ 20 mm, P < 0.0001), vascular invasion of HCCs (no vs yes, P < 0.0001), method of therapy (curative: surgical resection, PEIT, and RFA vs non-curative: other than surgical resection, PEIT, and RFA, P = 0.0004), serum AST (< 63 vs≥ 63 IU/L, P = 0.0089), serum AFP (< 41 vs≥ 41 ng/mL, P < 0.0001), serum PIVKA-II (< 83 vs≥ 83 mAU/mL, P < 0.0001), uninterrupted habitual drinking after initial therapy for HCC (no vs yes, P = 0.0135), and the mean postprandial plasma glucose level after initial therapy for HCC (< 200 vs≥ 200 mg/dL, P < 0.0001).
These parameters were entered into the multivariate Cox proportional hazards regression analysis. Stage of underlying liver disease (non-cirrhosis, Child A vs B, C cirrhosis, P = 0.0079), T stage of HCC (T1, 2 vs 3, 4; P < 0.0001), and the mean postprandial plasma glucose level after initial therapy for HCC (< 200 vs≥ 200 mg/dL, P = 0.0008) were extracted as independent factors affecting survival. However, uninterrupted habitual alcohol consumption was not related to survival (Table 2).
| HR | 95% CI | P value | |
| Age (< 69/≥ 69 yr) | 0.899 | 0.587-1.376 | 0.6232 |
| Sex (M/F) | 1.218 | 0.760-1.952 | 0.4132 |
| Stage of underlying liver disease (non-cirrhosis, Child-Pugh A/Child-Pugh B, C) | 0.537 | 0.394-0.733 | 0.0001 |
| T stage in the TNM system (T1,T2/T3, T4) | 0.419 | 0.330-0.531 | < 0.0001 |
| Number of HCCs (solitary/multiple) | 0.192 | 0.119-0.309 | < 0.0001 |
| Maximum size of HCCs (< 20/≥ 20 mm) | 0.403 | 0.260-0.623 | < 0.0001 |
| Vascular invasion of HCCs (no/yes) | 0.105 | 0.051-0.216 | < 0.0001 |
| Method of therapy (curative/non curative)1 | 0.432 | 0.272-0.687 | 0.0004 |
| HBs-Ag (negative/positive) | 1.078 | 0.538-2.163 | 0.8317 |
| HCV-Ab (negative/positive) | 1.362 | 0.858-2.162 | 0.1907 |
| AST (< 63/≥ 63 IU/L) | 0.554 | 0.357-0.861 | 0.0089 |
| ALT (< 49/≥ 49 IU/L) | 0.939 | 0.609-1.448 | 0.7750 |
| AFP (< 41/≥ 41 ng/mL) | 0.260 | 0.155-0.439 | < 0.0001 |
| PIVKA-II (< 83/≥ 83 mAU/mL) | 0.200 | 0.103-0.386 | < 0.0001 |
| Habitual alcohol drinkers (yes/no) | 1.130 | 0.740-1.726 | 0.5722 |
| Uninterrupted alcohol consumption after initial therapy for HCC (no/yes) | 0.489 | 0.278-0.861 | 0.0135 |
| BMI (≥ 25/< 25 kg/m2) | 0.885 | 0.530-1.476 | 0.6398 |
| Presence of DM (yes/no) | 1.141 | 0.731-1.782 | 0.5623 |
| Mean postprandial plasma glucose level after initial therapy for HCC (< 200/≥ 200 mg/dL) | 0.131 | 0.051-0.336 | < 0.0001 |
The survival period is quite short in T4 stage HCC, therefore, T4 stage HCC was excluded to clarify the factors related to survival (Table 3). Univariate analysis of 282 patients with T1-T3 stage HCC identified the following seven factors as related to survival after initial therapy for HCC: stage of underlying liver disease (non-cirrhosis, Child A vs B, C, P = 0.0009), T stage of HCC (T1 vs 2, 3, P = 0.0023), number of HCCs (solitary vs multiple, P = 0.0082), serum AFP (< 41 vs≥ 41 ng/mL, P = 0.0007), serum PIVKA-II (< 83 vs≥ 83 mAU/mL, P = 0.0101), uninterrupted alcohol consumption after initial therapy for HCC (no vs yes, P = 0.0002), and the mean postprandial plasma glucose level (< 200 vs≥ 200 mg/dL, P = 0.0001).
| T stage of hepatocellular carcinoma | Survival period (mo) |
| T1 | 43.3 ± 31.3 |
| T2 | 39.8 ± 30.8 |
| T3 | 23.6 ± 17.2 |
| T4 | 8.7 ± 11.8 |
On multivariate regression analysis using a stepwise Cox’s hazard model, stage of underlying liver disease (non-cirrhosis, Child A vs B, C cirrhosis, P = 0.0068), T stage of HCC (T1 vs 2, 3, P = 0.0108), uninterrupted habitual alcohol consumption (no vs yes, P = 0.0055), and the mean postprandial plasma glucose level (< 200 vs≥ 200 mg/dL, P = 0.0005) were extracted as independent factors related to survival after initial therapy for HCC (Table 4).
| HR | 95% CI | P value | |
| Univariate analysis | |||
| Age (< 69/≥ 69 yr) | 0.846 | 0.527-1.356 | 0.4854 |
| Sex (male/female) | 1.148 | 0.687-1.919 | 0.5975 |
| Stage of underlying liver disease (non cirrhosis, Child-Pugh A/Child-Pugh B, C) | 0.363 | 0.200-0.658 | 0.0009 |
| T stage in the TNM system (T1/T2, T3 ) | 0.352 | 0.181-0.687 | 0.0023 |
| Number of HCCs (solitary/multiple) | 0.397 | 0.232-0.677 | 0.0082 |
| Maximum size of HCCs (< 20/≥ 20 mm) | 0.833 | 0.520-1.335 | 0.4469 |
| Vascular invasion of HCCs (no/yes) | 0.382 | 0.119-1.220 | 0.1039 |
| Method of therapy (curative/non-curative)1 | 0.770 | 0.472-1.255 | 0.2918 |
| HBs-Ag (negative/positive) | 1.596 | 0.696-3.664 | 0.2686 |
| HCV-Ab (negative/positive) | 0.972 | 0.568-1.664 | 0.9157 |
| AST (< 63/≥ 63 IU/L) | 0.683 | 0.418-1.115 | 0.1269 |
| ALT (< 49/≥ 49 IU/L) | 0.968 | 0.596-1.570 | 0.8935 |
| AFP (< 41/≥ 41 ng/mL) | 0.365 | 0.205-0.649 | 0.0007 |
| PIVKA-II (< 83/≥ 83 mAU/mL) | 0.381 | 0.183-0.792 | 0.0101 |
| Habitual alcohol drinkers (yes/no) | 1.065 | 0.665-1.704 | 0.7939 |
| Uninterrupted alcohol consumption after initial therapy for HCC (no/yes) | 0.319 | 0.172-0.589 | 0.0002 |
| BMI (< 25/≥ 25 kg/m2) | 0.826 | 0.473-1.441 | 0.4989 |
| Presence of diabetes mellitus (yes/no) | 0.681 | 0.416-1.115 | 0.1258 |
| Mean postprandial plasma glucose level after initial therapy for HCC (< 200/≥ 200 mg/dL) | 0.143 | 0.054-0.381 | 0.0001 |
| Multivariate regression analysis | |||
| A stepwise Cox's hazard model | |||
| Stage of underlying liver disease | |||
| Non-cirrhosis, Child-Pugh A | 1.0 | ||
| Child-Pugh B, C | 0.649 | 0.476-0.885 | 0.0068 |
| T stage in the TNM system | |||
| T1 | 1.0 | ||
| T2/T3 | 0.788 | 0.653-0.945 | 0.0108 |
| Uninterrupted alcohol consumption after initial therapy for HCC | |||
| No | 1.0 | ||
| Yes | 0.641 | 0.469-0.877 | 0.0055 |
| Mean postprandial plasma glucose level after initial therapy for HCC | |||
| < 200 mg/dL | 1.0 | ||
| ≥ 200 mg/dL | 0.502 | 0.337-0.747 | 0.0005 |
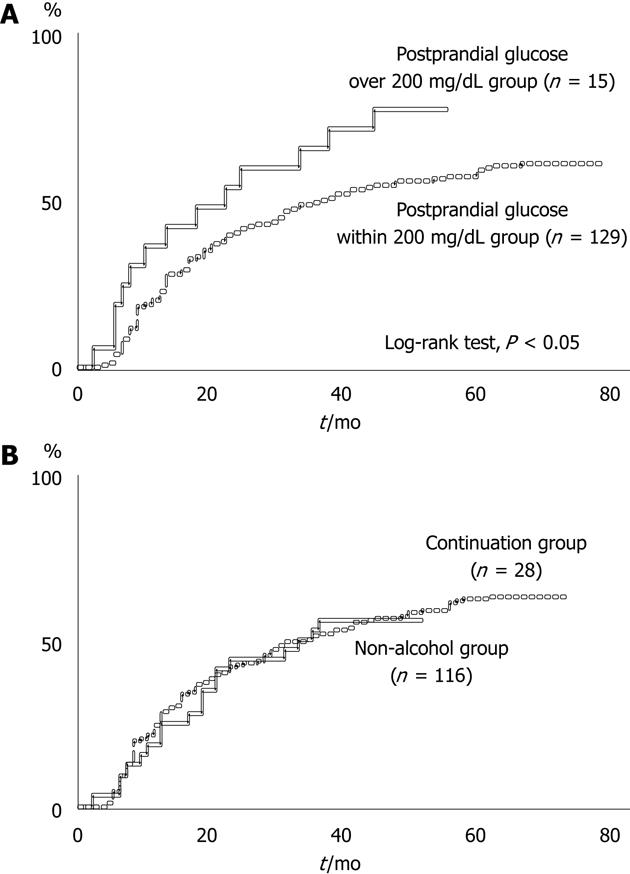
Of the 377 HCC patients, 144 (38.2%) patients did not show local recurrence after initial curative therapy for HCC. Of the 144 HCC patients who did not show local recurrence, 100 (69.4%) died during the observation period. Of the patients who did not show local recurrence, the period from initial curative therapy to subsequent therapy for newly emerging HCC was significantly longer in the postprandial glucose within 200 mg/dL group than the postprandial glucose > 200 mg/dL group (log-rank test, P < 0.05), although there was no difference in the period to second therapy between the non-alcohol group and continuation group (Figure 1).
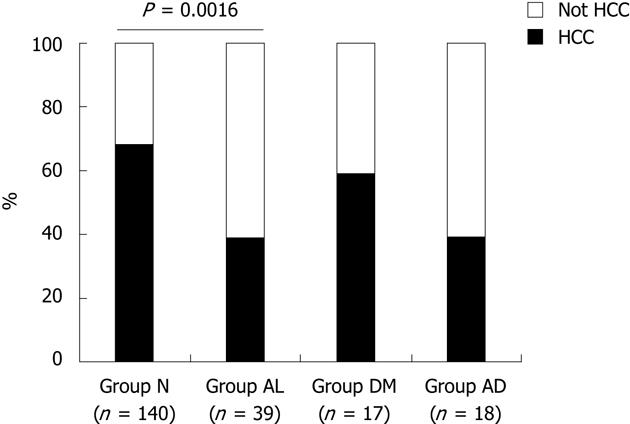
A total of 214 T1-T3 stage HCC patients who died during the observation period were classified into four groups: “Group N”, patients belonging to both the nonalcohol group and the postprandial glucose within 200 mg/dL group; “Group AL”, patients belonging to both the continuation group and the postprandial glucose within 200 mg/dL group; “Group DM”, patients belonging to both the daily alcohol < 20 g ethanol and the postprandial glucose > 200 mg/dL group; and “Group AD”, patients belonging to both the continuation group and the postprandial glucose > 200 mg/dL group (Table 5). Death caused by other than HCC progression (hepatic failure, gastrointestinal bleeding, ischemic heart disease, cerebral vessel disease, bacterial infection, etc.) was significantly more frequent in “Group AL” than in “Group N” (P = 0.0016). On the other hand, the cause of death in “Group DM” was the same as that in “Group N”. Similarly, the cause of death was not different between “Group AD” and “Group AL” (Figure 2).
| Group N | Group AL | Group DM | Group AD | |
| Age (yr) | 69.1 ± 8.1 | 68.2 ± 8.8 | 68.9 ± 6.5 | 67.4 ± 9.6 |
| Sex (M:F) | 88:52 | 38:1 | 7:10 | 1:17 |
| Stage of liver disease | ||||
| Non LC | 13 | 6 | 2 | 2 |
| LC child | ||||
| A | 91 | 23 | 6 | 8 |
| B | 31 | 7 | 9 | 8 |
| C | 5 | 3 | 0 | 0 |
| T stage in the TNM system | ||||
| 1 | 54 | 10 | 5 | 4 |
| 2 | 60 | 18 | 5 | 8 |
| 3 | 26 | 11 | 7 | 6 |
| Habitual alcohol drinkers | 47 (33.6) | 39 (100) | 0 (0.0) | 18 (100) |
| Presence of diabetes mellitus | 41 (29.3) | 8 (20.5) | 17 (100) | 18 (100) |
In the present study, stage of underlying liver disease, T stage of HCC, and the mean postprandial plasma glucose level after initial therapy for HCC despite standard treatment for DM were extracted as independent factors related to survival after initial therapy in 377 HCC patients who were newly diagnosed and treated according to the Japanese Guidelines for HCC[16]. Then, after T4 stage HCC patients were excluded, uninterrupted excess alcohol consumption was extracted as an independent factor along with the three factors described above.
Alcohol abuse provokes inflammatory and innate immune responses of Kupffer cells due to elevation of gut-derived endotoxin and increases reactive-oxygen-species-induced damage and profibrogenic factors such as acetaldehyde or lipid peroxidation products, which contribute to activation of hepatic stellate cells and progression of liver fibrosis[22]. Therefore, uninterrupted alcohol abuse may induce rapid worsening of hepatic dysfunction. Meanwhile, the presence of DM is related to HCC recurrence after potentially curative therapy in patients with non-B non-C HCC[17]. Furthermore, type 2 DM has consistently been identified as an independent predictor of hepatic fibrosis[10,23,24], faster fibrosis progression[24], and increased mortality[25]. In type 2 DM, insulin resistance may be observed in the majority of patients. Therefore, high levels of circulating insulin in type 2 DM may stimulate the proliferation of hepatic stellate cells and induce production of collagen, resulting in hepatic fibrosis, as well as carcinogenesis of hepatocytes[26]. Therefore, the presence of DM may cause deterioration of hepatic function and carcinogenesis of the liver.
In the present study, the methods of initial therapy for HCC were varied, including surgical resection, PEIT, RFA and TACE. Therefore, selection bias of the therapeutic method could have affected the prognosis of HCC. However, we chose the therapeutic method according to the Japanese Guidelines for HCC[16], which guaranteed the equality of the therapeutic value of the various methods. Thus, it is not difficult to elucidate the factors related to the prognosis of HCC even though patients were treated with different methods, as long as the treatment was performed based on the Japanese Guidelines for HCC[16]. Therefore, patients treated in accordance with these guidelines were included in this study.
Although HCV and/or HBV infection is considered to be a strong carcinogenic factor, it was not extracted as a factor related to the period of survival after initial therapy for HCC. This finding suggested that the prognosis of non-B non-C HCC is no better than that of virus-related HCC, and that the prognosis is influenced by the T stage of HCC or the stage of underlying liver disease rather than the etiology of the liver disease. Non-B non-C HCC would be discovered at an advanced stage[7] compared with virus-related HCC because of the lack of periodic medical assessments[8]. Therefore, detection by means of periodic medical assessments in patients with chronic liver damage, even without HCV and/or HBV infection to diagnose early stage non-B non-C HCC, is crucial for long-term survival. Recently, the prevalence of non-B non-C HCC has been increasing. Thus, along with intensive surveillance of HCC, investigation of factors affecting the prognosis of non-B non-C HCC is urgently needed.
In the present study, the cause of death differed between patients with uninterrupted habitual alcohol consumption and those with higher postprandial plasma glucose levels. The most frequent cause of death in the postprandial glucose > 200 mg/dL group was recurrence of HCC in other locations of the liver, while hepatic failure without HCC recurrence was the most frequent cause of death in the continuation group among habitual alcohol drinkers. However, the rate of recurrence of HCC in other locations of the liver as a cause of death in the postprandial glucose > 200 mg/dL group was similar to that in Group N. These findings suggest that abstinence from alcohol did not affect the prevalence of new HCC but was related to rapid deterioration of hepatic function. Meanwhile, higher postprandial plasma glucose levels are considered to lead to both deterioration of liver function and enhanced carcinogenesis of the liver.
As shown in the period from initial curative therapy to subsequent therapy for HCC in patients without local recurrence, the period was significantly longer in the postprandial glucose within 200 mg/dL group than in the postprandial glucose > 200 mg/dL group. Therefore, the potential for carcinogenesis was considered to be enhanced in the postprandial glucose > 200 mg/dL group. In the postprandial glucose > 200 mg/dL group, the dose of insulin and the use of various oral hypoglycemic drugs, including drugs stimulating insulin secretion, tended to increase. Therefore, a higher level of circulating insulin may have an effect on the emergence of new HCC.
Control of glucose levels after therapy for HCC may be an important factor to improve the prognosis of HCC. However, it is unclear whether increasing the dose of insulin or the use of various drugs for good control of plasma glucose levels improves the prognosis of HCC. Large prospective studies are needed to establish whether the prognosis of HCC after initial therapy is associated with the postprandial plasma glucose level in itself or other factors related to plasma glucose, including the type of hypoglycemic drug used, because the use of hypoglycemic drugs stimulating insulin secretion may affect the prognosis of HCC[27].
In conclusion, the present study provided clear evidence that uninterrupted alcohol drinking and high postprandial glucose levels after initial therapy for HCC worsen the prognosis of HCC patients. Education about abstinence from alcohol in the group having a history of habitual drinking and achieving good control of plasma glucose in the group having DM may improve the clinical course of HCC.
There are many factors other than hepatitis C virus or hepatitis B virus identified as risk factors for developing hepatocellular carcinoma (HCC). Among them, diabetes mellitus (DM) and habitual alcohol consumption have been considered significant risk factors for developing HCC. Meanwhile, factors affecting the prognosis after initial therapy for HCC have not been extensively examined, except for HCC tumor stage and underlying liver function.
In T1-T3 stage HCC patients, uninterrupted alcohol consumption and the mean postprandial plasma glucose level (< 200 vs≥ 200 mg/dL) after initial therapy for HCC were independent significant factors affecting HCC prognosis. Uninterrupted alcohol consumption was associated with rapid deterioration of hepatic function, while the postprandial plasma glucose level was related to both early development of new HCC and rapid deterioration of hepatic function.
It is known from experience that continuation of alcohol consumption and postprandial plasma glucose levels after initial therapy for HCC greatly affect the prognosis of HCC. To the best of our knowledge, however, there have been few studies that have shown these findings.
Education about abstinence from alcohol in the group having a history of habitual drinking, and achieving good control of plasma glucose in the group having DM after HCC diagnosis may be critical for improving the clinical course of HCC.
The authors investigated whether continuation of alcohol consumption and postprandial plasma glucose levels after initial therapy for HCC were related to the prognosis of HCC. The outcome of the study is interesting and important for the care of HCC patients after initial therapy for HCC.
| 1. | Seeff LB, Hoofnagle JH. Epidemiology of hepatocellular carcinoma in areas of low hepatitis B and hepatitis C endemicity. Oncogene. 2006;25:3771-3777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Ikeda K, Saitoh S, Koida I, Arase Y, Tsubota A, Chayama K, Kumada H, Kawanishi M. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 402] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3258] [Article Influence: 130.3] [Reference Citation Analysis (1)] |
| 4. | Ikai I, Itai Y, Okita K, Omata M, Kojiro M, Kobayashi K, Nakanuma Y, Futagawa S, Makuuchi M, Yamaoka Y. Report of the 15th follow-up survey of primary liver cancer. Hepatol Res. 2004;28:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Noguchi K, Nakashima O, Nakashima Y, Shiota K, Nawata H, Kojiro M. Clinicopathologic study on hepatocellular carcinoma negative for hepatitis B surface antigen and antibody to hepatitis C virus. Int J Mol Med. 2000;6:661-665. [PubMed] |
| 6. | Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, Hamamura K, Imai Y, Yoshida H, Shiina S. Risk factors for recurring hepatocellular carcinoma differ according to infected hepatitis virus-an analysis of 236 consecutive patients with a single lesion. Hepatology. 2000;32:1216-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Yotsuyanagi H, Shintani Y, Moriya K, Fujie H, Tsutsumi T, Kato T, Nishioka K, Takayama T, Makuuchi M, Iino S. Virologic analysis of non-B, non-C hepatocellular carcinoma in Japan: frequent involvement of hepatitis B virus. J Infect Dis. 2000;181:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Abe H, Yoshizawa K, Kitahara T, Aizawa R, Matsuoka M, Aizawa Y. Etiology of non-B non-C hepatocellular carcinoma in the eastern district of Tokyo. J Gastroenterol. 2008;43:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Horie Y, Yamagishi Y, Kajihara M, Kato S, Ishii H. National survey of hepatocellular carcinoma in heavy drinkers in Japan. Alcohol Clin Exp Res. 2003;27:32S-36S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1093] [Article Influence: 40.5] [Reference Citation Analysis (1)] |
| 11. | Lagiou P, Kuper H, Stuver SO, Tzonou A, Trichopoulos D, Adami HO. Role of diabetes mellitus in the etiology of hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:1096-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Fujino Y, Mizoue T, Tokui N, Yoshimura T. Prospective study of diabetes mellitus and liver cancer in Japan. Diabetes Metab Res Rev. 2001;17:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96:2462-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 191] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Tazawa J, Maeda M, Nakagawa M, Ohbayashi H, Kusano F, Yamane M, Sakai Y, Suzuki K. Diabetes mellitus may be associated with hepatocarcinogenesis in patients with chronic hepatitis C. Dig Dis Sci. 2002;47:710-715. [PubMed] |
| 15. | Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 539] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 16. | Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, Matsuyama Y, Okazaki M, Okita K, Omata M. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 17. | Kawamura Y, Ikeda K, Arase Y, Yatsuji H, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Saitoh S, Suzuki F. Diabetes mellitus worsens the recurrence rate after potentially curative therapy in patients with hepatocellular carcinoma associated with nonviral hepatitis. J Gastroenterol Hepatol. 2008;23:1739-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 269] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 19. | Fleming ID, Cooper JS, Henson DE, Hutter RVP, Kennedy BJ, Murphy GP, editors . AJCC cancer staging manual. 5th ed. Philadelphia: Lippincott-Raven 1997; 225-230. |
| 20. | Ikeda K, Saitoh S, Kobayashi M, Suzuki Y, Tsubota A, Suzuki F, Arase Y, Murashima N, Chayama K, Kumada H. Distinction between chronic hepatitis and liver cirrhosis in patients with hepatitis C virus infection. Practical discriminant function using common laboratory data. Hepatol Res. 2000;18:252-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5826] [Article Influence: 109.9] [Reference Citation Analysis (2)] |
| 22. | Cubero FJ, Urtasun R, Nieto N. Alcohol and liver fibrosis. Semin Liver Dis. 2009;29:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | de Lédinghen V, Ratziu V, Causse X, Le Bail B, Capron D, Renou C, Pilette C, Oules V, Gelsi E, Oberti F. Diagnostic and predictive factors of significant liver fibrosis and minimal lesions in patients with persistent unexplained elevated transaminases. A prospective multicenter study. J Hepatol. 2006;45:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 664] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 25. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2092] [Cited by in RCA: 2148] [Article Influence: 102.3] [Reference Citation Analysis (1)] |
| 26. | Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, Macarri G, Perego L, Benedetti A. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 254] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 27. | Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, Javle M, Moghazy DM, Lozano RD, Abbruzzese JL. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938-1946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
P- Reviewers Kietzmann T, TakumaY S- Editor Gou SX L- Editor Kerr C E- Editor Xiong L