HEPATIC ENCEPHALOPATHY
Introduction and division
Hepatic encephalopathy (HE) continues to be a major clinical problem. In subjects suffering from acute/chronic liver failure, HE can lead to coma and death. This medical state causes a broad range of neuropsychiatric disturbances with variable intensities, including poor concentration, psychomotor retardation, decreased reaction time, short-term memory impairment and sensory dysfunction[1,2]. According to the newest data, HE occurs as one of four types. Therefore, this encephalopathy syndrome might be classified into four groups: A, B, C and D[3-5]. Type A HE is inseparably associated with acute liver failure resulting from severe inflammatory and/or necrotic rapid onset liver disease (e.g., paracetamol overdose, excessive alcohol intake or acute fatty liver caused by pregnancy or Reye’s syndrome). Type B HE is associated with portosystemic bypass in the absence of parenchymal liver disorders and is mainly connected with congenital abnormalities. Type C HE accompanies chronic liver failure (cirrhosis) and portal hypertension with portosystemic shunts. Type C has three subcategories: episodic (precipitated, spontaneous, or recurrent), persistent (mild, severe, or treatment-dependent) and minimal. In 2009, the International Society for the Study of Hepatic Encephalopathy and Nitrogen Metabolism completed these classifications and added the experimental models associated with each type[6]. Finally, type D is associated with disorders of the urea cycle. Typically, these syndromes are rare hereditary disorders of urea biosynthesis caused by genetic defects in the urea cycle and have been described for each of the six enzymes that participate in urea formation. They occur mainly in neonates and more rarely in adults[7-16]. Alcohol, viral infections, drugs or toxins and biliary obstruction are several reasons for the development of cirrhosis. Portal hypertension resulting from cirrhosis stimulates the opening of embryonic venous channels. Portal-systemic shunts then allow intestinal toxins (ammonia, manganese and cytokines) to bypass the liver in the systemic circulation. A classification based on HE severity enables the division of HE into two types: (1) minimal hepatic encephalopathy (MHE); and (2) overt hepatic encephalopathy (OHE). MHE affects patients who have normal mental and neurological status; however, abnormalities appear in specific psychometric tests[17-20]. OHE is characterized by neurological and neuropsychiatric disruptions, which can be detected by bedside clinical tests[21].
Pathogenesis
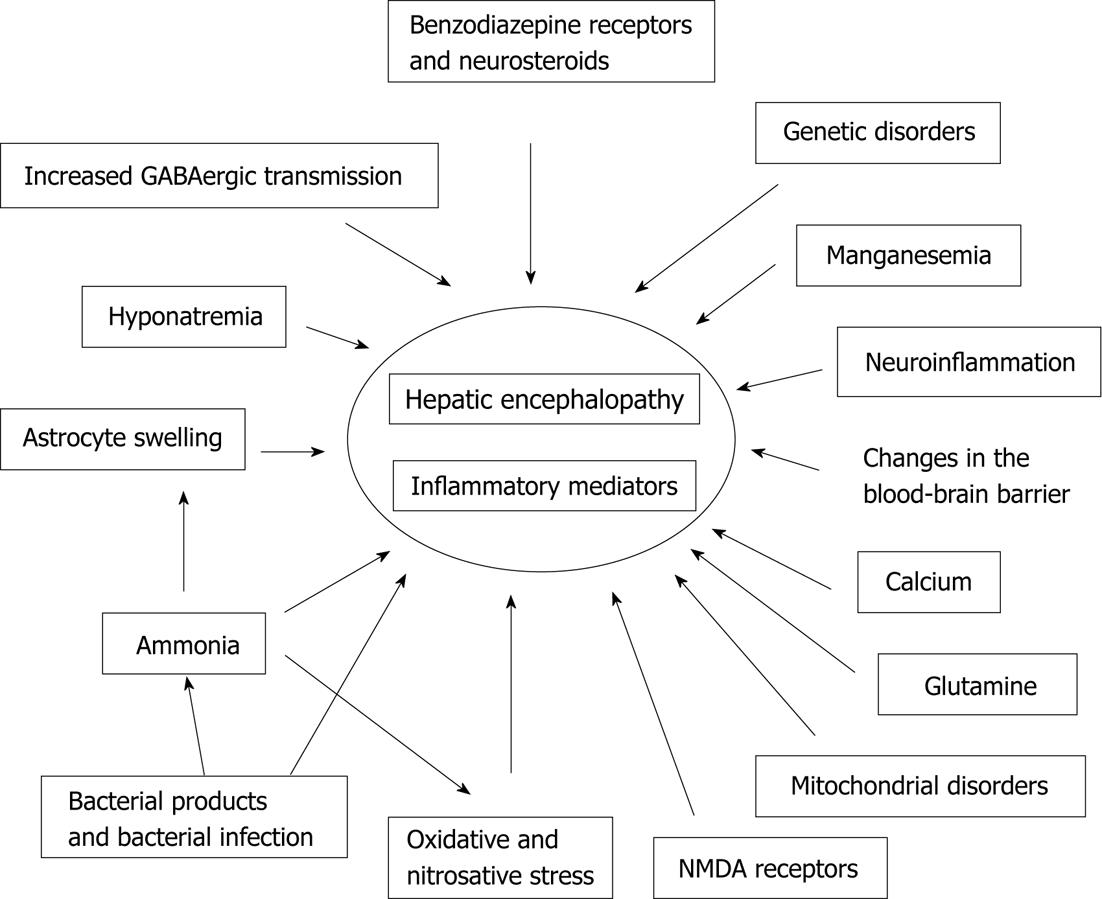
Although the etiology of HE has not been conclusively established, it is widely agreed to be the result of numerous pathological processes. Research on the pathogenesis of HE is still being conducted, and several theories have been reported (Figure 1).
Figure 1 The different factors and mechanisms involved in the pathogenesis of hepatic encephalopathy.
GABA: Gamma-aminobutyric acid; NMDA: N-methyl-D-aspartate.
The ammonia hypothesis
The neurotoxicity of ammonia is well recognized and was first thought to play a significant role in the development of HE in the 1890s. In one study, creating a surgical shunt from the portal vein to the vena cava of dogs mitigated their aggression and irritability during the experiment[22]. Similar investigations led to the description of HE. In HE, complications from the hepatic detoxification of ammonia expose the brain to high ammonia concentrations. Consequently, these complications lead to neurotoxicity, which results in neuropsychiatric syndromes and abnormal cerebral blood flow[23,24]. The human body has several sources of ammonia. Primarily, ammonia is created in the colon as a byproduct of proteins and urea, which are metabolized by bacteria. Moreover, ammonia is produced in the small intestine as the result of the bacterial degradation of glutamine (an amino acid)[6,25]. Muscles and kidneys can also produce ammonia. However, ammonia appears to play a negligible role in organs that are beyond the gastrointestinal tract. In HE, the muscles and the kidneys are involved in the metabolism of ammonia to a greater extent than normal. In chronic liver failure, the skeletal muscle tissue (because of its large size) becomes a key organ that detoxifies ammonia in organisms. Liver disease often results in a condition requiring a hypoproteic diet. However, in the context of the role of muscles in hyperammonemia, this diet is not recommended for HE; a hypoproteic diet might decrease both muscle mass and ammonia detoxification. This proceeding is supported by the latest research proving that diet supplementation with branched-chain amino acids reduces MHE and improves muscle mass[26,27]. The kidneys also interact with the liver to metabolize ammonia. Under normal circumstances, 30% of produced ammonia is excreted in the urine. In liver disease, 70% of the synthesized ammonia is eliminated by this means. As a result, renal disturbances may intensify cognitive impairment during HE. Note that a healthy liver (periportal hepatocytes) protects the brain from ammonia by converting it into urea, which is excreted through the kidneys and into the colon. Ammonia is also detoxified by the formation of glutamine from glutamate, which occurs in the liver (the hepatocytes near the central vein) and the brain[28-30]. Therefore, HE, hepatocellular failure and portosystemic shunting disable the ability of the liver to neutralize ammonia, and the arterial levels of this compound become elevated. Because of the role it plays, ammonia is sometimes described as a key toxin in the etiology of HE. First, ammonia can induce astrocyte swelling as the result of osmotic imbalance. This phenomenon is thought to play a major role in the pathogenesis of HE. Astrocytes are the only brain cells that are able to metabolize ammonia. This metabolism occurs because of the presence of glutamine synthetase (a special enzyme) in the astrocytes, which transforms glutamate and ammonia into glutamine. Therefore, the intracellular level of glutamine rapidly increases in conjunction with the concentration of ammonia in liver disease. Glutamine is an osmocyte and causes the movement of water into the astrocytes. As the result, the astrocytes swell, and this process leads to cerebral edema and intracranial hypertension. In support of this theory, the administration of methionine sulfoximine (a glutamine synthetase inhibitor) prevents astrocytes from swelling in animal models[31-33]. Astrocytes that are exposed to ammonia for prolonged periods can undergo a morphologic transformation into Alzheimer’s type II astrocytes, which are defined by large nuclei, prominent nucleoli and marginated chromatin[34]. Hyperammonemia among neural cells also produces reactive oxygen species (ROS). These oxygen species are able to trigger astrocyte swelling and modulate Zn2+ mobilization, which results in the stimulation of the peripheral benzodiazepine receptor. Furthermore, ammonia increases the resting membrane potential in the brain, inactivates the neuronal chloride extrusion pumps, suppresses inhibitory postsynaptic potential formation and causes neuron depolarization[35]. This neuroinhibitory state is characteristic of HE. The molecular effects of ammonia reflect the behavioral changes in patients with hyperammonemia.
Bacterial products and bacterial infections in HE
Ammonia is an important factor in the pathogenesis of HE. However, approximately 10% of patients with significant encephalopathy have normal serum ammonia levels; this statistic supports the argument against the prevalence of the ammonia hypothesis. Furthermore, many cirrhosis patients have elevated ammonia levels and no evidence of encephalopathy. In addition to ammonia, other bacteria-produced substances may cause neurotoxicity in HE. Mercaptans exert their neurotoxic influence on human organisms as the result of the bacterial metabolism of amino acids. The phenols synthesized by bacteria from aromatic amino acids are also dangerous. Elevated levels of both mercaptans and phenols have been found in patients with chronic liver disease. These endogenous toxic substances, which are produced in the intestines, are absorbed into the portal venous flow. The toxins escape from liver catabolism because of the impaired liver function and the presence of portosystemic shunts. Finally, the toxins reach the brain through the blood-brain barrier (BBB) and cause cerebral function disorders[29]. Coexisting gastrointestinal bleeding enhances the flow of toxins into the systemic bloodstream. Bacterial flora might also be the source of benzodiazepine-like compounds, which are produced during the metabolism of amino acids. These compounds can impair the function of the astrocytes and disrupt gamma-aminobutyric acid (GABA)ergic neurotransmission. Similarly to bacterial flora, bacterial pathogens are also involved in the etiology of HE. Recent investigations underline the potential importance of acute infections caused by bacterial pathogens and the resulting inflammation in the neuropsychiatric symptoms of HE[36]. Moreover, the inflammatory response might modulate the reaction to hyperammonemia. In such a situation, cytokine-induced changes in ammonia diffusion can occur in the brain, and the expression of benzodiazepine receptors might increase.
The GABAergic neurotransmission theory
GABA is a principle inhibitory neurotransmitter. GABA-mediated transmission is based on the GABAA/benzodiazepine receptor/chloride ionophore complex. This binding opens the ion channel and increases the amount of chloride in the neuron. Increased GABA-mediated neurotransmission contributes to motor function impairment and decreased consciousness, which are the main manifestations of HE[37,38]. Mechanisms leading to the increased GABAergic transmission in liver failure are not well known. Nevertheless, there is evidence suggesting that the amount of natural benzodiazepine receptor agonists in the liver increases with impaired function. Two observations confirm this theory. First, increased levels of these agonists have been found in the brain and body fluids of HE patients[3,21]. Furthermore, treatment based on flumazenil, a benzodiazepine receptor antagonist, ameliorates HE. The role of neurosteroids (e.g., tetrahydro-progesterone and tetrahydrodeoxycorticosterone) in the problem of GABAergic transmission should be noted[39]. Neurosteroids are potent agonists of the GABAA receptor, acting through a unique binding site to enhance GABAergic neurotransmission. Animal research revealed the ability of pregnenolone (an endogenous neurosteroid “antagonist”) to decrease mortality in hyperammonemic models. The results demonstrate the important role of GABAergic enhancers in the pathology of HE. The enhancers are normally synthesized in astrocytes and appear in elevated concentrations in hyperammonemic mice or mice with acute liver failure. The elevated concentrations are caused by the glial cell activity in ammonia-induced disorders. The specific path leading to neurosteroid synthesis is not yet clear; however, this phenomenon appears to be connected with ammonia-induced increases in the density of peripheral-type benzodiazepine receptors[40-42]. GABAergic neurotransmission might be enhanced by the increased access of GABA in the synaptic clefts. This phenomenon is caused by the loss of presynaptic feedback inhibition from the GABA release, which is the result of decreased GABAB receptor values. Finally, recent research suggests that ammonia itself might directly increase GABAergic transmission[43].
The idea of neuroinflammation
Ammonia dysmetabolism does not exist as the sole factor of the neurological changes observed in patients with HE. It is well known that sepsis is precipitating state that plays significant role in the decompensation of liver diseses[44-46]. The peripheral immune system communicates with the brain in response to infection and inflammation. This response results in neutrophil adhesion, migration across the BBB and release of chemokines, proinflammatory cytokines, proteases and ROS as well as inflammatory gene transcription[47]. Simultaneously, astrocytes and microglial cells release cytokines in response to injury or inflammation. Researchers have proven that the increased blood level of tumor necrosis factor (TNF), which occurs during inflammation, stimulates the glial cells to excrete the cytokines [interleukin (IL)-1, IL-6] and influences the permeability of the BBB[21,22,48-50]. In conclusion, the diffusion of ammonia into the astrocytes increases in vitro. Moreover, a recent study has revealed that TNF or IL-1 receptor gene deletion retards the process of encephalopathy and decreases the intensity of brain edema in mice with acute liver failure. In addition, treatment based on a TNF receptor antagonist (etanercept) similarly reduces the severity of encephalopathy and prevents brain edema[49]. The concept of neuroinflammation (microglial activation) is defined as a wide variety of neurological disorders including multiple sclerosis, Alzheimer’s disease, stroke and acquired immune deficiency syndrome dementia complex. Microglial activation is followed by highly increased levels of proinflammatory cytokines (TNF, IL-1 and IL-6) in the brain[51,52]. This discovery revealed a new aspect of HE, provided a new look at the pathophysiology of HE and opened a new gate into the pharmacotherapy of HE. This neuroinflammatory concept is also supported by the therapeutic effect of mild hypothermia and indomethacin, which reduces the activation of microglial cells and simultaneously prevents the central proinflammatory process in mild HE[27,51,53,54]. Moreover, mild hypothermia remarkably decreases brain edema and the duration of HE. Intracranial pressure (ICP) may be reduced by hypothermia even in patients who are unresponsive to mannitol. The additional benefits of indomethacin depend on how it affects cognitive function via modulation of the glutamate-nitric oxide cyclic GMP pathway. Indomethacin might reduce ICP and cerebral edema independently of changing the cerebral blood flow (excepting the brain function modulation). Numerous studies have revealed the beneficial role of anti-inflammatory agents in the course of HE. Ibuprofen reportedly reduces locomotor disorders and increases the learning abilities of rats with portacaval shunts. Ibuprofen also normalizes blood and brain ammonia levels[46,55]. Consequently, anti-inflammatory therapy inhibits microglial activation during HE. The findings in different experimental models provide new therapeutic targets for HE and require further study.
Oxidative and nitrosative stress
The metabolism of astrocytes changes after exposure to ammonia, hyponatremia, benzodiazepines or inflammatory cytokines; under such circumstances, the production of reactive nitrogen species and ROS increases in the astrocytes. N-methyl-D-aspartate (NMDA) receptors play an important role in the production of free radicals. The stimulation of ammonia-induced NMDA receptors reduces antioxidant enzyme activity and increases the production of superoxide anions[56,57]. The presence of ammonia inside the astrocyte mitochondria (with the coexistence of calcium) initiates the process. Moreover, the ROS source (neutrophil metabolism) constitutes a systemic/local inflammatory state as well. Glutamine exposure is known to increase the oxidative stress among astrocytes (the “Trojan horse hypothesis”)[32,58]. The other area of research interest involves RNA oxidation and the resulting impairment in protein synthesis. Molecular disorders lead to cognitive abnormalities in HE. ROS are involved in the nitration of tyrosine (the component of intracellular proteins). Nitrated tyrosine tends to selectively reduce the permeability of the BBB, which promotes astrocyte swelling and cerebral edema[59-61].
The ‘two-hit hypothesis’ is another pathological path leading to ROS output, and it is connected inseparably to the activity of ammonia. Liver disease followed by hyperammonemia constitutes a starting point (an ‘initial hit’) in this theory. Astrocyte swelling and oxidative stress appear next. Finally, there is a ‘second hit’, such as an ammonia load caused by an upper gastrointestinal bleed, dehydration, hyponatremia or infection. Astrocyte damage occurs, and the level of ROS increases further. This close connection between astrocyte swelling and oxidative stress generates an auto-amplifying signaling loop that results in the deterioration of neurocognitive abilities[22].
Changes in the blood-brain barrier
The BBB constitutes a highly specialized mechanism of brain microvasculature, and it is indispensable for the proper function of the central nervous system (CNS)[26]. The BBB consists of pericytes, capillary basement membranes, endothelial capillary cells and astrocytes, which encase the vessels[62]. There is evidence of BBB dysfunction in the pathogenesis of many CNS disorders (e.g., Alzheimer’s disease and multiple sclerosis). The pathological appearance of HE is closely related to BBB changes. Swollen astrocytes constitute a well-known phenomenon in the course of HE[22,63]. The phenomenon is primarily caused by cellular and vasogenic edema and aquaporins (AQP). In CNS structures, elevated values of AQP 1, 4 and 9 are characteristic of brain edema[26]. The increased expression of these water channels serves an important role in the movement of water through the BBB and its pathological result. Moreover, AQPs have been reported to participate in apoptosis in the CNS[64,65]. Astrocyte disorders in HE result in changes to the BBB, reflected by the loss of ion homeostasis and the accumulation of intracellular water. Nevertheless, increased BBB ammonia permeability during hyperammonemia or HE is still a matter of controversy among scientists[66]. There are investigations that support and refute this theory. Several positron emission tomography studies have revealed elevated brain ammonia concentrations in HE. In addition, transmission electron microscopy demonstrated a disruption in the junction of BBB capillaries in the hippocampal area in a MHE model[26,35]. According to the presented data, the structure of the BBB and its connection to systemic hyperammonemia in HE require further exploration. The large number of studies conducted in this area indicates that it might be essential for the pathological appearance of HE.
Death of CNS cells
Brains exposed to high ammonia concentrations undergo several serious changes, including metabolic and neurophysiological disorders with pathological synaptic inhibition and excitation. As a result, alterations in cerebral energy metabolism appear and neurotransmitter-related processes begin to falter[59]. The mechanisms leading to cell death in the CNS are associated with changes in the neuronal mitochondria[67,68]. First, a reduction in the ATP concentration occurs in the course of HE. The two basic paths responsible for this modified energy metabolism are the NMDA receptors and the inhibition of the tricarboxylic acid cycle. The NMDA receptors are able to mediate mitochondrial swelling, neurotoxicity and neuron death during acute liver failure[57,69]. Furthermore, it is suggested that calcium plays a key role in the pathology of cell death in the CNS[22,26,70]. In HE, the mitochondrial calcium content becomes elevated because of acute ammonia intoxication. Calcium is able to enter the mitochondria via NMDA receptors[71,72]. This mechanism results in the release of glutamate and ROS and induces an apoptotic cascade among the neurons. Peripheral benzodiazepine receptors located in the mitochondrial membrane cells also cause the programmed death of neurons in association with calcium[21,40-42,73]. In conclusion, the loss of neuronal cells depends on the neurotoxicity of ammonia and mitochondrial dysfunction. The above-mentioned pathological mechanisms are reflected in the cerebral atrophy and mental retardation observed in patients suffering from HE[26].
Other pathogenic elements in HE
It is clear that the etiology of HE is complex and multifactorial. In addition, theories different from those presented above have been postulated to explain the pathogenesis of HE. The opioid, serotonergic and dopaminergic systems are likely involved. Furthermore, ‘false’ neurotransmitters (e.g., phenylethanolamine and octopamine) produced from aromatic amino acids are elevated in individuals with HE. These substances compete with real neurotransmitters and impair neurological functions[43]. The genetic background of HE is underlined. Genetic analyses showed that particular haplotypes in glutaminase (an enzyme that converts glutamine into glutamate) in patients suffering from cirrhosis affect the risk of HE. A characteristic section in the promoter region of the gene sequence for glutaminase has been detected that contains repeated base pairs. Studies have shown higher luciferase activity in the cells transfected with this doubled region. This research indicates that those repeated elements increase glutaminase transcriptional activity. As the result, genetic disorders appear to be responsible for the development of HE in patients with cirrhosis[74].
In addition to ammonia, manganese participates in the development of HE[75,76]. Manganese is an essential metal that is involved in the metabolism of carbohydrates, lipids and proteins. Manganese is an important cofactor of numerous enzymes. It is a neurotoxin that may be transported into the brain across the BBB and the brain-cerebrospinal fluid barriers. In such circumstances, it is preferentially accumulated in the basal ganglia. Manganese enhances neurosteroid synthesis via the activation of translocator proteins on astrocyte membranes[3]. Manganese affects the operations of certain proteins that interact with dopamine (a neurotransmitter), NMDA toxicity or oxidative and nitrosative stress. Three mechanisms of manganese neurotoxicity have been described[26,77,78]. The first is mitochondrial dysfunction and the disruption of energy metabolism; the second is connected with the inflammatory activation of the glial cells; and the third mechanism constitutes the disruption of synaptic transmission and neuroglial communication. This mechanism induces the phosphorylation of c-Jun and SEK1/MKK4 and the tyrosine phosphorylation of several proteins[79]. Manganese produces active oxygen species (superoxide hydrogen peroxide and hydroxyl radical) and toxic catecholamines (6-hydroxydopamine). Moreover, manganese is the first metal reported to induce apoptosis in the CNS[26]. Furthermore, manganese deposition has been detected by magnetic resonance imaging in the caudate nucleus and in the globus pallidus of cirrhotic patients. This detection might explain the presence of Parkinson symptoms (such as tremors) observed in certain patients with HE. Researchers have indicated that manganese enhances neurosteroid synthesis and promotes the formation of Alzheimer’s type II astrocytes[21].
The study conducted by Guevara et al[80] revealed that hyponatremia (serum sodium < 130 mEq/L) is an independent and serious predictor of overt HE. This hypothesis may open new strategies for HE treatment. The reduction of hyponatremia might be a novel therapeutic approach in preventing HE in liver cirrhosis[52].
Neuroendocrine correlations in the course of HE are not well recognized. Nevertheless, studies conducted on rats demonstrated decreased levels of prolactin in models suffering from HE. This disorder might be caused by a hyperammonemic state. Endocrinal curiosity reveals the necessity of further research in this area[26].
NONCIRRHOTIC HYPERAMMONEMIC ENCEPAHALOPATHY
Research shows that approximately 90% of adult hyperammonemia cases are caused by severe liver disease. A small number of cases have other causes and can be divided into two groups: (1) cases that are connected with increased ammonia production; and (2) cases that are caused by decreased ammonia elimination[81].
Increased ammonia production
Hyperammonemic encephalopathy may develop in patients with progressive multiple myeloma[82]. Several studies have demonstrated that malignant cells are able to produce excess ammonia as the result of increased amino acid metabolism. During intensive chemotherapy, leukemia patients tend to struggle with hyperammonemia[83]. Idiopathic hyperammonemia is also described among bone marrow recipients. The other reason for noncirrhotic hyperammonemic encephalopathy might be the presence of infections caused by urea-producing bacteria (Proteus mirabilis, Escherichia coli, Klebsiella and Providencia rettgeri). In extreme cases, the protein load and increased metabolism might cause the elevated ammonia level. Therefore, seizures, severe exercise, starvation, total parenteral nutrition or gastrointestinal bleeding can (rarely) result in encephalopathy.
Decreased ammonia elimination
Metabolic disorders and problems with ammonia elimination might be the source of encephalopathy. Such disorders include organic acidurias, urea-cycle disorders, dibasic aminoaciduria, impaired fatty acid oxidation and mistakes in pyruvate metabolism[7,11,12]. Noncirrhotic congenital portosystemic shunts can also cause an elevated ammonia level in the blood stream, and the intake of certain drugs (e.g., valproic acid, glycine, ribavirin and carbamazepine) may induce noncirrhotic hyperammonemic encephalopathy[84-87].