Published online Mar 7, 2012. doi: 10.3748/wjg.v18.i9.991
Revised: September 22, 2011
Accepted: September 29, 2011
Published online: March 7, 2012
AIM: To evaluate the prophylaxis of chronic kidney disease (CKD) after liver transplantation (LT) with low-dose calcineurin inhibitor (CNI) and mycophenolate mofetil (MMF).
METHODS: From March 1999 to December 2009, a total of 572 patients (478 males and 94 females) underwent LT enrolled in the study. Initial immunosuppression was by triple-drug regimens that included a CNI, MMF, and prednisone. The initial dose of CNI was 0.05-0.10 mg/kg per day for tacrolimus (TAC) and 5-10 mg/kg per d for cyclosporine A (CSA) respectively, and was gradually reduced based on a stable graft function. The serum trough level of CNI was 6-8 ng/mL for TAC and 120-150 ng/mL for CSA 3-mo post-operation, 4-6 ng/mL for TAC and 80-120 ng/mL for CSA 1-year after transplantation was expected with stable liver function. MMF was personalized between 1.0-1.5 g/d. Glomerular filtration rate (GFR) was estimated by an abbreviated Modification of Diet in Renal Disease formula. Risk factors of CKD were examined by univariate and multivariate logistic regression.
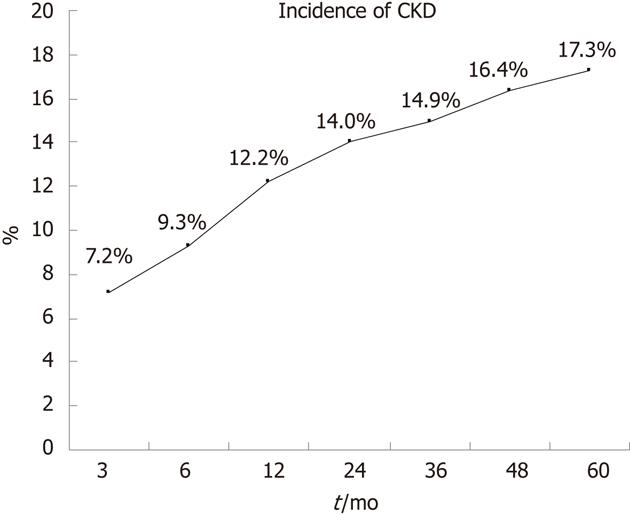
RESULTS: With a definition of GFR < 60 mL/min per 1.73 m2, the incidence of CKD was 17.3% 5-year after LT. There were 68.3% (293 of 429 cases) patients managed to control their TAC trough concentrations within 8 ng/mL and 58.0% (83 of 143 cases) patients’ CSA trough concentrations within 150 ng/mL. Of the 450 recipients followed-up over 1 year, 55.5% (183 of 330 cases) of which were treated with TAC had a trough concentration ≤ 6 ng/mL while 65.8% (79 of 120 cases) of which were treated with CSA had a concentration ≤ 120 ng/mL. The incidence of CKD in the groups of lower CNI trough concentrations was significantly lower than the groups with CNI concentrations above the ideal range. Patients with CKD had much higher CNI trough concentrations than that of patients without CKD. MMF was adopted in 359 patients (62.8%). Patients administrated with MMF had a relatively low CNI trough concentrations but with no significant difference. The graft function remained stable during follow-up. No difference was found between different groups of CNI trough concentrations. Pre-LT renal dysfunction, ages, acute kidney injury, high blood trough concentrations of CNI in 3 mo (TAC > 8 ng/mL, CSA > 150 ng/mL) and hypertension after operation were associated with CKD progression, while male gender and adoption of MMF were protection factors.
CONCLUSION: Low dose of CNI combined with MMF managed to prevent CKD after LT with stable graft function.
- Citation: Shao ZY, Yan LN, Wang WT, Li B, Wen TF, Yang JY, Xu MQ, Zhao JC, Wei YG. Prophylaxis of chronic kidney disease after liver transplantation - experience from west China. World J Gastroenterol 2012; 18(9): 991-998
- URL: https://www.wjgnet.com/1007-9327/full/v18/i9/991.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i9.991
Since the introduction of calcineurin inhibitor (CNI) in the 1980s, its use in clinical solid organ has greatly increased transplant recipient survival rates and reduced graft rejection rates[1]. More and more patients with end stage liver disease are benefiting from liver transplantation (LT) in the last three decades. However, although the number of long-term surviving recipients has increased, many of them suffer from chronic complications. Chronic kidney disease (CKD) is one such complication that has severely affected the quality of life and survival of organ recipients[2-7]. Cohen et al[8] reported that 27.5% of 191 patients had progressive renal dysfunction [glomerular filtration rate (GFR) < 40 mL/min] 5 years after LT. Ojo et al[9] found that GFR < 29 mL/min was in up to 18% of patients by 5 years post LT, and that chronic renal failure elevated the risk of death after transplantation (relative risk 4.55). Long duration of CNI-taken is one of the many factors adversely affecting renal function after transplantation[2,4-7]. Its nephrotoxicity is seen by kidney biopsy, which includes severe tubular atrophy, interstitial fibrosis and focal hyalinosis of small renal arteries and arterioles[10,11]. Lee et al[12] pointed out that rapid progression of kidney disease was associated with CNI nephrotoxicity which significantly increased the risk by a factor of 4.24.
There are two main strategies for CNI induced CKD, one is CNI withdrawal and conversion to a non-nephrotoxic immunosuppressant, such as sirolimus, mycophenolate mofetil (MMF) and azathioprine; the other is dose reduction in combination with an auxiliary immunosuppressant[4,6,7,13,14]. Shenoy et al[13] found no significant improvement in renal function after 12 mo’ follow-up in a prospective trial of CNI withdrawal and replacement with sirolimus for renal insufficiency in liver transplant recipients. Cantarvich et al[15], on the other hand, found significant improvement in the renal function of long-term liver-transplant recipients with renal dysfunction by introducing MMF and tapering cyclosporine A (CSA) to a very low dose (50 mg/d), however, this strategy may increase the risk of acute rejection (AR). No agreement has been reached on this issue. Since it has been proven that the nephrotoxicity was associated with the dosage and duration of CNI, we can expect that administration of initial low-dose CNI and maintaining low blood concentrations after would prevent the progression of renal dysfunction. However, such a conclusion cannot be drawn yet because most researches were based on patients who had pre-existing renal dysfunction. Moreover, there is no consensus on the minimum CNI dose which is considered to be safe for LT recipients.
In our center, a protocol of combining CNI [tacrolimus (TAC) or CSA] with MMF was adopted after LT. The CNI initial dose and blood concentrations after were kept at a relatively low level. The purpose of this study was to delineate the risk factors for developing CKD, and more important, to find out whether the strategy of combination low-dose CNI and MMF can make a successfully prophylaxis of CKD after LT.
Data from the clinical records of 772 consecutive Chinese patients who underwent LT from March 1999 until December 2009 were retrospectively analysed. Patients were monitored till August 2010 or to their death. Recipients with a short follow-up (less than 3 mo), died within 3 mo after transplantation and younger than 18 years old were excluded. All the liver grafts were from brain-dead donors or living donors. Living and deceased donations were voluntary in all cases, approved by the West China Hospital Ethics Committee, and in accordance with the ethical guidelines of the Declaration of Helsinki.
Renal function was assessed by estimated glomerular filtration rate (eGFR) using the abbreviated Modification of Diet in Renal Disease formula: eGFR = 186 × creatinine (mg/dL)−1.154× (age)−0.203× (0.742 if female). Acute kidney injury (AKI) was defined as more than 25% decrease of GFR in the first post-operative week compared with the pre-operative level by the RIFLE (risk, injury, failure, loss and end-stage renal failure) criteria[16]. CKD was defined as GFR < 60 mL/min per 1.73 m2 for at least 3 consecutive months. Hepatorenal syndrome was defined as Salerno et al[17] reported: cirrhosis with ascites, serum creatinine > 1.5 mg/dL, no improvement of serum creatinine after at least 2 d with diuretic withdrawal and volume expansion with albumin, no current or recent treatment with nephrotoxic drugs, absence of parenchyma kidney disease. Renal dysfunction before LT was also defined as eGFR < 60 mL/min per 1.73 m2.
According to the latest guideline of prevention and treatment of plasma lipid abnormality for Chinese adults, hypercholesterolemia was defined as total plasma cholesterol ≥ 6.22 mmol/L, hypertriglyceridemia as triglyceride ≥ 2.26 mmol/L[18]. Diabetes mellitus (DM) was diagnosed if random blood glucose ≥ 11.1 mmol/L or fasting plasma glucose ≥ 7.0 mmol/L. AR was confirmed either by liver biopsy or recovery from high-dose methylprednisolone. If chronic rejection (CR) was suspected, liver biopsy was also carried out. Hypertension was defined as a systolic blood pressure over 140 mmHg or diastolic pressure over 90 mmHg twice at different time. Mayo end-stage liver disease (MELD) scores were calculated for each patient.
Initial immunosuppression was by triple-drug regimens that included a CNI (TAC or CSA), MMF and prednisone. The initial dose of CNI was 0.05-0.10 mg/kg per day for TAC and 5-10 mg/kg per day for CSA respectively. MMF was personalized between 1.0-1.5 g/d. At the early phase in our center, patients were administrated with MMF only when they were diagnosed hypertension and DM; however, all recipients in the late period were administrated with it unless severe gastrointestinal side effects or myelosuppression happened. Prednisone was generally discontinued within 3 mo after transplantation.
Observations of clinical indices including CNI trough concentrations were checked daily for the first week and weekly for the next three in the first month post-operation, monthly within 3-mo and every three months thereafter. The ideal serum trough level of CNI was 6-8 ng/mL for TAC and 120-150 ng/mL for CSA 3-mo post-operation. Liver function was monitored intensely while adjusting the CNI dose. If AR happened, prior dosage was restarted, together with the prednisone increase or high-dose methylprednisolone administration. Dose reduction was more carefully and slowly carried out. A trough level of 4-6 ng/mL for TAC and 80-120 ng/mL for CSA one year after transplantation was expected with stable liver function.
SPSS 17.0 statistical software (SPSS Company, Chicago, IL) was used to analyse the relevant data. Numerical data are presented as the mean ± SD or as the median. Continuous data were compared using the independent t-test if data were normally distributed, or using the rank-sum test if data were non-normally distributed. Categorical data were compared using the χ2 test. Univariate logistic regression analysis was used to discover risk factors for CKD. Variables reaching statistical significance were then included for multivariate analysis. Results were reported as odds ratios with 95% confidence intervals.
The medical records of 572 patients [male:female 478:94, mean age 44 (20-69) years old] meeting the inclusion criteria were reviewed retrospectively. Mean follow-up duration was 28 (3-125) mo. Pre-operation baseline included DM in 36 (6.29%) patients, hypertension in 13 (2.27%) patients, renal dysfunction in 54 (9.44%) patients, and hepatorenal syndrome in 27 (4.72%) patients, 19 (3.32%) were given hemodialysis therapy within a 2-wk period before surgery. The main indications for LT were tumors and end stage liver diseases, with 268 (46.9%) and 276 (48.2%) patients respectively. More than 80% patients were found to be hepatitis B virus (HBV) related. The deceased donor transplantation rate was 75.5%.
The eGFR was calculated after each visit of a patient. And once met the criterion of CKD, they were registered into the CKD group. As shown in Figure 1, 17.3% of the whole population (99 cases) developed CKD during the 5-year’s follow-up.
Our analysis of the difference in over 20 clinical indices between patients with and without CKD showed that the CKD group had older age, higher MELD scores, more female, more patients with pre-operative renal dysfunction, more with hepatorenal syndrome and more received pre-operative hemodialysis. There was also a between-group difference in immunosuppression protocols, TAC and MMF was preferred in the non-CKD group. Of the 85 cases of AKI (14.9%), 32 progressed to CKD. In addition, the CKD group had more patients with post-operative DM, hypertension and hypertriglyceridemia (Table 1).
| Clinical features | CKD group (n = 99) | Non-CKD group (n = 473) | P value |
| Age (median years) | 49 | 42 | 0.001 |
| Sex (male/female) | 70/29 | 408/65 | 0.001 |
| Donor type (DDLT/LDLT) | 82/17 | 350/123 | NS |
| Indications for LT | |||
| Cirrhosis | 38 (38.4) | 160 (33.8) | NS |
| Chronic active hepatitis | 13 (13.1) | 39 (8.2) | NS |
| Tumors | 34 (34.3) | 234 (49.5) | 0.006 |
| Others | 14 (14.1) | 40 (8.5) | NS |
| HBV infection | 80 (80.8) | 401 (85.0) | NS |
| Complications pre-LT | |||
| DM | 10 (10.1) | 26 (5.5) | NS |
| Renal dysfunction | 22 (22.2) | 32 (6.8) | 0.001 |
| HRS | 11 (11.1) | 16 (3.4) | 0.003 |
| Hemodialysis | 9 (9.1) | 10 (2.1) | 0.002 |
| Hypertension | 5 (5.1) | 8 (1.7) | NS |
| MELD scores | 14 | 11 | 0.001 |
| CNI type (TAC/CSA) | 64/35 | 365/108 | 0.009 |
| MMF adoption | 49 (49.5) | 310 (65.5) | 0.003 |
| Complications post-LT | |||
| DM | 27 (27.3) | 85 (18.0) | 0.034 |
| Hypertension | 19 (19.2) | 35 (7.4) | 0.001 |
| Hypercholesterolemia | 19 (19.2) | 57 (12.1) | NS |
| Hypertriglyceridemia | 26 (26.3) | 81 (17.1) | 0.034 |
| AKI | 32 (32.3) | 53 (11.2) | 0.001 |
| AR | 18 (18.2) | 56 (11.8) | NS |
| CR | 4 (4.0) | 11 (2.3) | NS |
| Graft failure | 6 (6.1) | 11 (2.3) | NS |
| Re-transplantation | 5 (5.1) | 8 (1.7) | NS |
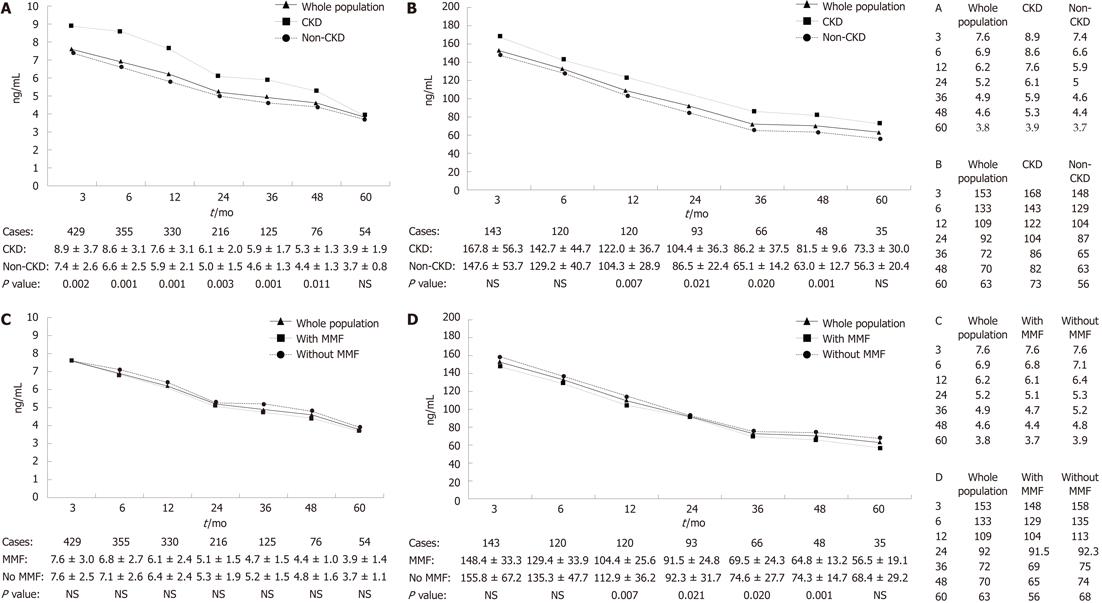
The CNI trough concentrations were recorded in each visit too. Mean concentrations of both TAC and CSA were calculated at different time points. A decreasing trend was discovered with lengthening of follow-up time (Figure 2).
As we mentioned before, an ideal concentration was expected at 3-mo and 1-year post LT. The results showed that there were 68.3% (293 of 429 cases) patients managed to control their TAC trough concentrations within 8 ng/mL and 58.0% (83 of 143 cases) patients’ CSA trough concentrations within 150 ng/mL. Of the 450 recipients followed-up over 1 year, 55.5% (183 of 330 cases) of which were treated with TAC had a trough concentration ≤ 6 ng/mL while 65.8% (79 of 120 cases) of which were treated with CSA had a concentration ≤ 120 ng/mL. The incidence of CKD in the groups of lower CNI trough concentrations was significantly lower than the groups with CNI concentrations above the ideal range (Table 2). At the same time, we compared the CNI trough concentrations between patients with and without CKD. As shown in Figure 2A and B, the CKD group had much higher CNI trough concentrations than that of patients without CKD.
| Groups | Cases (CKD incidence %) | P value |
| Three months after LT | ||
| CSA trough concentrations | ||
| > 150 ng/mL | 20/60 (32.3) | 0.036 |
| ≤ 150 ng/mL | 15/83 (18.1) | |
| TAC trough concentrations | ||
| > 8 ng/mL | 32/136 (23.5) | 0.001 |
| ≤ 8 ng/mL | 32/293 (10.9) | |
| One year after LT | ||
| CSA trough concentrations | ||
| > 120 ng/mL | 19/41 (46.3) | < 0.001 |
| ≤ 120 ng/mL | 12/79 (15.2) | |
| TAC trough concentrations | ||
| > 6 ng/mL | 36/147 (24.5) | < 0.001 |
| ≤ 6 ng/mL | 17/183 (9.3) |
Also, recipients were grouped by whether MMF was used. We found its adoption in 359 patients (62.8%). It was used in 49.5% of the CKD group and 65.5% of the non-CKD group (P = 0.003). Although patients administrated with MMF had a relatively low CNI trough concentrations, but no significant difference was found between groups (Figure 2C and D).
To assess the impact of CNI concentrations on the chronic complications and graft function post transplantation, patients were still grouped according to the CNI trough concentrations 3-mo post transplantation. The analysis showed between-group differences in these parameters were without statistical significance (Table 3).
| Complications | Cases (CKD incidence %) | Group 1 (%) | Group 2 (%) | P value |
| DM | 112 (19.6) | 17.6 | 23.5 | NS |
| Hypertriglyceridemia | 107 (18.7) | 18.1 | 19.9 | NS |
| Hypercholesterolemia | 76 (13.3) | 12.8 | 14.3 | NS |
| Hypertension | 54 (9.4) | 8.2 | 11.7 | NS |
| AR | 74 (12.9) | 11.7 | 15.3 | NS |
| CR | 15 (2.6) | 2.1 | 3.8 | NS |
| Graft failure | 17 (3.0) | 2.7 | 3.6 | NS |
| Re-transplantation | 13 (2.3) | 2.1 | 2.6 | NS |
Together with the different CNI concentrations and whether uses of MMF, over twenty parameters were examined by univariate logistic analysis to identify the risk factors of CKD (Table 4). All the factors with statistical significance were chosen for multivariate logistic analysis, and seven of them were singled out. Age at LT, pre-operative renal dysfunction, AKI, high CNI concentration 3 mo after LT (TAC > 8 ng/mL or CSA > 150 ng/mL), post-operative hypertension were risk factors of CKD; male, use of MMF were protective factors of CKD (Table 5).
| Clinical factors | P value | OR | 95% CI |
| Factors before LT | |||
| Gender (female = 0, male = 1) | 0.001 | 0.385 | 0.232-0.638 |
| Ages at LT | 0.001 | 1.048 | 1.025-1.072 |
| Liver cirrhosis | 0.303 | 1.590 | 0.658-3.840 |
| End stage liver disease | 0.014 | 1.740 | 1.120-2.703 |
| HBV infection | 0.327 | 0.756 | 0.432-1.323 |
| DM | 0.091 | 1.932 | 0.900-4.147 |
| Hypertension | 0.052 | 3.092 | 0.990-9.659 |
| Renal dysfunction | 0.001 | 3.937 | 2.173-7.134 |
| HRS | 0.002 | 3.570 | 1.603-7.953 |
| Hemodialysis | 0.001 | 4.630 | 1.830-11.716 |
| TB | 0.014 | 1.001 | 1.000-1.003 |
| MELD | 0.000 | 1.049 | 1.023-1.075 |
| Factors after LT | |||
| Re-operation | 0.454 | 1.324 | 0.635-2.761 |
| Use of TAC | 0.010 | 0.541 | 0.340-0.861 |
| Use of MMF | 0.003 | 0.520 | 0.336-0.805 |
| CNI trough concentrations | 0.001 | 2.528 | 1.627-3.927 |
| AKI | 0.001 | 3.785 | 2.275-6.296 |
| Hypertension | 0.001 | 2.972 | 1.619-5.455 |
| DM | 0.035 | 1.712 | 1.037-2.824 |
| AR | 0.509 | 1.107 | 0.820-1.494 |
| CR | 0.338 | 0.565 | 0.176-1.814 |
| Graft failure | 0.055 | 2.710 | 0.978-7.510 |
| Re-transplantation | 0.052 | 3.092 | 0.990-9.659 |
| Hypertriglyceridemia | 0.036 | 1.724 | 1.038-2.863 |
| Hypercholesterolemia | 0.059 | 1.733 | 0.979-3.070 |
| Clinical factors | P value | OR | 95% CI |
| Age at LT | 0.001 | 1.048 | 1.020-1.076 |
| Pre-operative renal dysfunction | 0.049 | 2.300 | 1.005-5.260 |
| AKI | 0.001 | 4.435 | 2.404-8.182 |
| CNI trough concentration | 0.001 | 3.233 | 1.923-5.438 |
| Post-operative hypertension | 0.035 | 2.230 | 1.059-4.696 |
| Female | 0.018 | 0.464 | 0.245-0.877 |
| Use of MMF | 0.002 | 0.435 | 0.255-0.741 |
The incidence of CKD increases with survival time after LT. As its nephrotoxicity proved by more researches, every center realized the importance of CNI dose adjustment. But how and when to adjust it still remains a question. There are two mainly strategies for CNI induced CKD, one is CNI withdrawal and conversion to a non-nephrotoxic immunosuppressant, such as sirolimus, MMF and azathioprine; the other is CNI dose reduction in combination with an auxiliary immunosuppressant[4,6,7,13,14]. Both were used. However, because of the CNI withdrawal time and dose reduction level differed among different centers, no agreement was reached.
Unlike the strategies mentioned above, in our center, an initial low CNI dose was administered. And by intensely monitoring of the graft function and gradually CNI dose reduction, a low blood concentration of CNI was maintained thereafter. The results displayed that over half the patients managed to maintain a CNI level within the target range in 3-mo and 1-year post LT and graft function remained stable compared with the high CNI level group. Moreover, groups of CNI concentrations within target range had significantly lower CKD incidence than the rest.
It was reported that CKD incidence varied to a great distance. Data from different centers varies partly because of different definitions of CKD[2-7]. We defined CKD as GFR < 60 mL/min per 1.73 m2 in this research, a level at which the prevalence of complications of CKD begins to increase[19,20]. By this criterion, the incidence of CKD 5 years after LT was 17.3%, lower than many reports. Five factors have been incriminated as etiologic factors of CKD as demonstrated by the multivariate logistic analysis.
An important risk factor for CKD was CNI trough concentrations 3 mo after LT (OR = 2.935). Dose of CNI varies between different centers. Nevertheless, there is a consensus that the CNI concentration should be as low as possible to avoid CKD. Morard et al[21] identified trough levels of CSA ≥ 150 ng/mL or TAC ≥ 10 ng/mL at 1 year and CSA ≥ 100 ng/mL or TAC ≥ 8 ng/mL at 5 years as independent risk factors for impaired renal function. However, no agreement has yet been reached on what is the minimum and safe CNI dose for LT recipients.
Pre-LT baseline renal function has a major impact on that post-transplantation[3,6,7,22]. In this study, both renal dysfunction pre-operation and AKI post-operation proved to be important risk factors for CKD. Velidedeoglu et al[23] suggested that a combination of events during the first postoperative week after LT serve as a physiologic “stress test” for the kidneys. Patients who failed the test (peak creatinine > 2 mg/dL) were at increased risk of chronic renal disease. Although both pre-operative renal dysfunction and AKI were considered to be reversible, with persistently nephrotoxication of CNI, the chance of recovery for kidney function is small and the injury could become irreversible and chronic finally. Induction therapy with both lymphocyte-depleting and non lymphocyte-depleting antibodies and delayed introduction of CNI (3-7 d) may preserve or ameliorate renal function in LT recipients with pre-transplant renal dysfunction without increasing the risk of rejection or compromising patient and graft survival[24-26].
Hypertension commonly causes renal disease in general population, so it was not surprising that post-operative hypertension became risk factor of CKD. Once confirmed, recipients should receive active treatment. Administration of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers were recommended, for they could theoretically protect these patients from both the acute hemodynamic component and chronic vascular and tubulointerstitial injury. The benefit of the former class of agents is conferred predominantly via reduction in CNI-induced afferent arteriolar vasoconstriction and that of the latter is conferred via the inhibition of angiotensin II effects of transforming growth factor-β and other profibrotic mediators[27].
Our finding showed use of MMF as a protective factor of CKD. And as an accessory immunosuppressant, MMF does not have nephrotoxicity and could reduce the CNI dosage. Strategies to alleviate CNI nephrotoxicity include use of MMF with CNI dose reduction or CNI-withdrawal and conversion to MMF[4,6,7,13,14,28]. We found that, with an 1.0-1.5 g/d administration, most people had no severe side effects, which proved to be safe and effective.
Another protective factor of CKD was male gender. In the general population, however, male gender is more closely associated with renal disease progression, but the reason for this inconsistency is unclear[5,29].
HBV infection was not a significant risk factor compared to other causes in this research. This finding was similar to previous studies based on Asian people[12]. HCV infection was also not a significant risk factor for CKD progression. In western countries, HCV is the most common cause of liver failure, and it increases CKD risk in liver transplantation primarily because it may cause glomerulonephritis[9,30]. Nevertheless, only 7 patients had HCV related cirrhosis in this cohort. Lamivudine combined with individualized low-dose hepatitis B immunoglobulin was used as a prophylaxis against HBV recurrence after LT in our center. Adefovir, which is known to be nephrotoxic, was administrated with only a few patients[31]. This could partly explain why our center has a relatively low incidence of CKD.
Posttransplantation DM is prevalent among LT recipients. It was reported earlier that the incidence of posttransplantation DM could reach 14.9% in the living donor liver transplantation[32]. However, in this study we found no relationship between DM and CKD. The proper explanation maybe that insulin was widely accepted and used among LT recipients. Most people had his blood sugar under the ideal range. Complications of DM were not as popular as usual.
While there is conflicting evidence in the literature on whether a TAC or CSA use is more beneficial[33,34], we found a lower incidence of CKD in the TAC group and identified CSA as a risk factor for CKD by univariate logistic regression analysis. However, the analysis did not take into account a small number of patients (n = 20) who switched CNIs during the follow-up. Therefore, the benefit of TAC over CSA remains inconclusive. Randomized, prospective studies with a large number of patients will be needed to resolve this issue.
In conclusion, many factors have been associated with CKD progression. With few practical and validate strategy, we have shown that administration of low-dose CNI in combination with MMF could lower CKD incidence and did not increase AR rate, which provided as a successful experience for Chinese LT recipients. As mentioned above, there was part of the population left whose CNI concentration was above the target range. So the low dose was not adaptable for everyone. Closely monitoring of liver function during tapering the CNI dose was needed. Dose reduction must be based on a stable liver function. Limitation of this study is that data were collected retrospectively and that GFR was evaluated rather than measured. Study of prospective designed and CKD defined by measured GFR would be more convincing.
Use of calcineurin inhibitor (CNI) has greatly increased liver transplant recipient survival rates and reduced graft rejection rates in recent years. However, long duration of its use may cause chronic complications, like chronic kidney disease (CKD), which has severely affected the quality of life and survival of organ recipients.
There are two main strategies for CNI induced CKD, one is CNI withdrawal and conversion to a non-nephrotoxic immunosuppressant, such as sirolimus, mycophenolate mofetil (MMF) and azathioprine; the other is dose reduction combined with an auxiliary immunosuppressant. Both strategies were used, but no agreement has been reached.
It has been proven that CNI nephrotoxicity was associated with its dosage and duration. However, no consensus was reached on the minimum CNI dose which is considered to be safe for liver transplantation (LT) recipients. Different with other centers, the authors carried out a strategy of initial low-dose CNI and maintaining low blood concentrations after. By closely monitoring of liver function and gradually tapering the CNI dose, the result was favorable with low incidence of CKD and acceptable graft functions.
Administration of low-dose CNI in combination with MMF could lower CKD incidence and did not increase acute rejection rate, which provided as a successful experience for Chinese LT recipients. Limitation of this study is that data were collected retrospectively and that glomerular filtration rate (GFR) was evaluated rather than measured. Study of prospective designed and CKD defined by measured GFR is needed in the future.
The research proved a successful strategy to prevent CKD liver transplantation with ample data and strict design, which should be popularized by more centers.
| 1. | Hong JC, Kahan BD. Immunosuppressive agents in organ transplantation: past, present, and future. Semin Nephrol. 2000;20:108-125. [PubMed] |
| 2. | Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Yalavarthy R, Edelstein CL, Teitelbaum I. Acute renal failure and chronic kidney disease following liver transplantation. Hemodial Int. 2007;11 Suppl 3:S7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Wilkinson A, Pham PT. Kidney dysfunction in the recipients of liver transplants. Liver Transpl. 2005;S47-S51. [PubMed] |
| 5. | Bloom RD, Doyle AM. Kidney disease after heart and lung transplantation. Am J Transplant. 2006;6:671-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Bahirwani R, Reddy KR. Outcomes after liver transplantation: chronic kidney disease. Liver Transpl. 2009;15 Suppl 2:S70-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Pham PT, Pham PC, Wilkinson AH. Management of renal dysfunction in the liver transplant recipient. Curr Opin Organ Transplant. 2009;14:231-239. [PubMed] |
| 8. | Cohen AJ, Stegall MD, Rosen CB, Wiesner RH, Leung N, Kremers WK, Zein NN. Chronic renal dysfunction late after liver transplantation. Liver Transpl. 2002;8:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW et al. Chronic renal failure after transplantation of a norenal organ. N Engl J Med. 2003;349:931-940. |
| 10. | Mihatsch MJ, Kyo M, Morozumi K, Yamaguchi Y, Nickeleit V, Ryffel B. The side-effects of ciclosporine-A and tacrolimus. Clin Nephrol. 1998;49:356-363. [PubMed] |
| 11. | Porayko MK, Gonwa TA, Klintmalm GB, Wiesner RH. Comparing nephrotoxicity of FK 506 and cyclosporine regimens after liver transplantation: preliminary results from US Multicenter trial. U.S. Multicenter Liver Study Group. Transplant Proc. 1995;27:1114-1116. [PubMed] |
| 12. | Lee JP, Heo NJ, Joo KW, Yi NJ, Suh KS, Moon KC, Kim SG, Kim YS. Risk factors for consequent kidney impairment and differential impact of liver transplantation on renal function. Nephrol Dial Transplant. 2010;25:2772-2785. [PubMed] |
| 13. | Shenoy S, Hardinger KL, Crippin J, Desai N, Korenblat K, Lisker-Melman M, Lowell JA, Chapman W. Sirolimus conversion in liver transplant recipients with renal dysfunction: a prospective, randomized, single-center trial. Transplantation. 2007;83:1389-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Neau-Cransac M, Morel D, Bernard PH, Merville P, Revel P, Potaux L, Saric J. Renal failure after liver transplantation: outcome after calcineurin inhibitor withdrawal. Clin Transplant. 2002;16:368-373. [PubMed] |
| 15. | Cantarovich M, Tzimas GN, Barkun J, Deschênes M, Alpert E, Tchervenkov J. Efficacy of mycophenolate mofetil combined with very low-dose cyclosporine microemulsion in long-term liver-transplant patients with renal dysfunction. Transplantation. 2003;76:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. [PubMed] |
| 17. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [PubMed] |
| 18. | Committee for Guidelines on Prevention and Treatment of Blood Lipid Abnormality in Chinese Adults. Guidelines on Prevention and Treatment of Blood Lipid Abnormality in Chinese Adults. Chin J Cardio. 2007;35:390-413. |
| 19. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266. [PubMed] |
| 20. | Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, Balk E, Lau J, Levin A, Kausz AT. National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. 2003;111:1416-1421. [PubMed] [DOI] [Full Text] |
| 21. | Morard I, Mentha G, Spahr L, Majno P, Hadengue A, Huber O, Morel P, Giostra E. Long-term renal function after liver transplantation is related to calcineurin inhibitors blood levels. Clin Transplant. 2006;20:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Pawarode A, Fine DM, Thuluvath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl. 2003;9:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 224] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Velidedeoglu E, Bloom RD, Crawford MD, Desai NM, Campos L, Abt PL, Markmann JW, Mange KC, Olthoff KM, Shaked A. Early kidney dysfunction post liver transplantation predicts late chronic kidney disease. Transplantation. 2004;77:553-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Tchervenkov JI, Tzimas GN, Cantarovich M, Barkun JS, Metrakos P. The impact of thymoglobulin on renal function and calcineurin inhibitor initiation in recipients of orthotopic liver transplant: a retrospective analysis of 298 consecutive patients. Transplant Proc. 2004;36:1747-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 25. | Bajjoka I, Hsaiky L, Brown K, Abouljoud M. Preserving renal function in liver transplant recipients with rabbit anti-thymocyte globulin and delayed initiation of calcineurin inhibitors. Liver Transpl. 2008;14:66-72. [PubMed] |
| 26. | Soliman T, Hetz H, Burghuber C, Györi G, Silberhumer G, Steininger R, Mühlbacher F, Berlakovich GA. Short-term induction therapy with anti-thymocyte globulin and delayed use of calcineurin inhibitors in orthotopic liver transplantation. Liver Transpl. 2007;13:1039-1044. [PubMed] |
| 27. | Stratta P, Canavese C, Quaglia M, Balzola F, Bobbio M, Busca A, Franchello A, Libertucci D, Mazzucco G. Posttransplantation chronic renal damage in nonrenal transplant recipients. Kidney Int. 2005;68:1453-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Campbell MS, Rai J, Kozin E, Bloom RD, Markmann JF, Olthoff KM, Shaked A, Rajender Reddy K. Effects of sirolimus vs. calcineurin inhibitors on renal dysfunction after orthotopic liver transplantation. Clin Transplant. 2007;21:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Neugarten J. Gender and the progression of renal disease. J Am Soc Nephrol. 2002;13:2807-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Tsui JI, Vittinghoff E, Shlipak MG, Bertenthal D, Inadomi J, Rodriguez RA, O'Hare AM. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med. 2007;167:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Jiang L, Yan L, Li B, Wen T, Zhao J, Jiang L, Cheng N, Wei Y, Yang J, Xu M. Prophylaxis against hepatitis B recurrence posttransplantation using lamivudine and individualized low-dose hepatitis B immunoglobulin. Am J Transplant. 2010;10:1861-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Zhao J, Yan L, Li B, Zeng Y, Wen T, Zhao J, Wang W, Xu M, Yang J, Ma Y. Diabetes mellitus after living donor liver transplantation: data from mainland China. Transplant Proc. 2009;41:1756-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Filler G, Webb NJ, Milford DV, Watson AR, Gellermann J, Tyden G, Grenda R, Vondrak K, Hughes D, Offner G. Four-year data after pediatric renal transplantation: a randomized trial of tacrolimus vs. cyclosporin microemulsion. Pediatr Transplant. 2005;9:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Lucey MR, Abdelmalek MF, Gagliardi R, Granger D, Holt C, Kam I, Klintmalm G, Langnas A, Shetty K, Tzakis A. A comparison of tacrolimus and cyclosporine in liver transplantation: effects on renal function and cardiovascular risk status. Am J Transplant. 2005;5:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Peer reviewer: Rubén Ciria, MD, PhD, Hepatobiliary Surgery and Liver Transplantation Unit. Hospital Universitario Reina Sofía, Avenida Menendez Pidal s/n. Servicio de Cirugia General, Cordoba 14004, Spain
S- Editor Tian L L- Editor Ma JY E- Editor Xiong L