Published online Mar 7, 2012. doi: 10.3748/wjg.v18.i9.889
Revised: October 16, 2011
Accepted: January 18, 2012
Published online: March 7, 2012
AIM: To assess quantitative endoscopic ultrasound (EUS)-guided elastography in the nodal staging of oesophago-gastric cancers.
METHODS: This was a single tertiary centre study assessing 50 patients with established oesophago-gastric cancer undergoing EUS-guided fine needle aspiration biopsy (FNAB) of lymph nodes between July 2007 and July 2009. EUS-guided elastography of lymph nodes was performed before EUS-FNAB. Standard EUS characteristics were also described. Cytological determination of whether a lymph node was malignant or benign was used as the gold standard for this study. Comparisons of elastography and standard EUS characteristics were made between the cytologically benign and malignant nodes. The main outcome measure was the accuracy of elastography in differentiating between benign and malignant lymph nodes in oesophageal cancers.
RESULTS: EUS elastography and FNAB were performed on 53 lymph nodes. Cytological malignancy was found in 23 nodes, one was indeterminate, one was found to be a gastrointestinal stromal tumor and 25 of the nodes were negative for malignancy. On 3 occasions insufficient material was obtained for analysis. The area under the curve for the receiver operating characteristic curve for elastography strain ratio was 0.87 (P < 0.0001). Elastography strain ratio had a sensitivity 83%, specificity 96%, positive predictive value 95%, and negative predictive value 86% for distinguishing between malignant and benign nodes. The overall accuracy of elastography strain ratio was 90%. Elastography was more sensitive and specific in determining malignant nodal disease than standard EUS criteria.
CONCLUSION: EUS elastography is a promising modality that may complement standard EUS and help guide EUS-FNAB during staging of upper gastrointestinal tract cancer.
- Citation: Paterson S, Duthie F, Stanley AJ. Endoscopic ultrasound-guided elastography in the nodal staging of oesophageal cancer. World J Gastroenterol 2012; 18(9): 889-895
- URL: https://www.wjgnet.com/1007-9327/full/v18/i9/889.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i9.889
Endoscopic ultrasound (EUS) is an integral investigation in the staging of oesophageal and oesophago-gastric junctional tumours[1,2]. It provides both an accurate assessment of the tumour (T) stage of a cancer and associated lymphadenopathy (N)[3-5].
EUS lymph node assessment can be challenging. EUS-guided fine needle aspiration biopsy (FNAB) of lymph nodes remains the pre-operative gold standard for determining nodal involvement, with diagnostic accuracy of up to 95% reported in previous studies[6,7]. However, EUS-FNAB cannot be undertaken if the needle passes through the primary tumour, and there can be technical difficulties in obtaining lymph node material for analysis.
Using conventional grey-scale B-mode EUS, lymph nodes are characterised by size, shape, density and distinction of the border in an attempt to distinguish between benign and malignant nodes[8]. However, the sensitivity and specificity of these features in distinguishing malignant involvement only exceeds 80% when all four of these features are present[8].
Elastography measures the stiffness of a structure and it is known that pathophysiological processes such as malignancy lead to stiffer tissue that deforms less. Over the past 15 years, elastography during ultrasound has been applied to measure tissue elasticity in breast, thyroid and liver disease[9-13]. Initial studies assessing nodes with elastography used processing to produce a colour elastography image[14-16]. More recent software technology allows quantification of the stiffness in the form of strain. Comparing two different areas of tissue allows calculation of a strain ratio between the two.
There have been several recent reports using EUS elastography to assess pancreatic lesions[17,18] as well as lymph nodes[13,18-20]. The results from these studies, using first wave software to produce descriptive colour elastograms, are encouraging, suggesting a high sensitivity and specificity for detecting malignant involvement. However, there is limited data on oesophageal cancer nodal staging. This study aimed to compare conventional EUS with quantitative EUS-guided elastography in the assessment of nodal staging in oesophageal and junctional cancers and to assess whether quantitative EUS could accurately distinguish malignant from benign lymph nodes.
This was a prospective single centre study. Glasgow Royal Infirmary is a West of Scotland tertiary referral centre for EUS staging of upper gastrointestinal tract cancers. All patients who, as part of routine clinical care, were undergoing EUS-FNA lymph node biopsy for staging of upper gastrointestinal tract cancer, during the period July 2007 to July 2009, had elastography of the node prior to sampling.
Initial staging EUS was undertaken by one of two endoscopists (SP/AJS) using a Pentax radial echoendoscope, attached to a Hitachi EUB-8500 ultrasound processor. Standard EUS grey-scale images of suspicious lymph nodes were obtained and conventional characteristics of size, shape, distinction of border and density were recorded. After the decision was made to undertake a FNA biopsy, a Pentax linear array echoendoscope was then used with the same ultrasound processor.
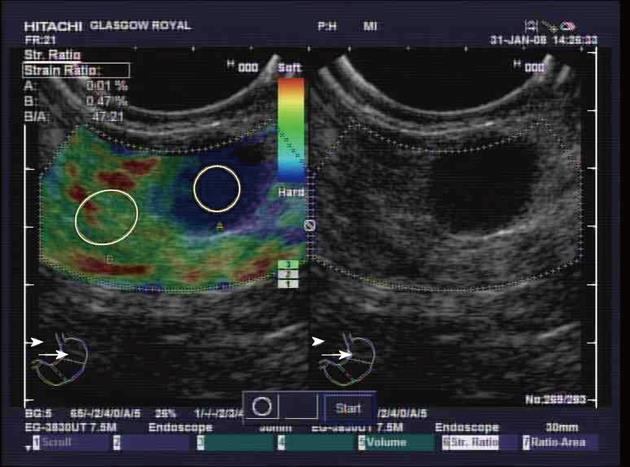
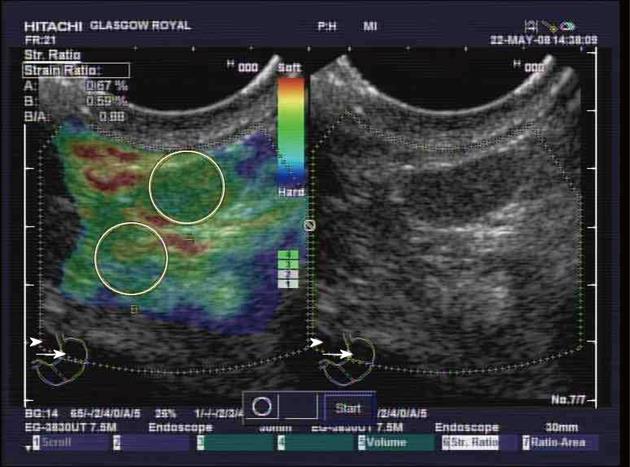
Elastography is a standard function of the Hitachi EUB-8500 ultrasound processor. A conventional grey-scale image was displayed on the right-hand side of the monitor, while the superimposed elastography image was displayed on the left-hand side (Figures 1 and 2). The elastography image and measurements rely on compressions from vascular pulsation and respiratory movement. Measurements were only taken when there was good contact and appropriate compression of the transducer, as indicated on the elastography image on the ultrasound processor. Using the superimposed elastography image, the largest area possible of the node was outlined, as was a similar sized area of surrounding apparently normal tissue. The ultrasound processor measured the strain of each area as a quantitative figure and calculated a strain ratio between the two areas. The strain ratio was recorded a minimum of three times prior to EUS-FNAB. The mean of these recordings was calculated.
EUS-FNAB was performed using a Wilson Cook 22 gauge needle. Three passes were obtained, the samples stored in formalin and sent to the laboratory for later cytological analysis by specialist pathologists who were blinded to the elastography values.
Cytological determination of whether a lymph node was benign or malignant was used as the gold standard for the purposes of this study, as surgical resection specimens and follow up imaging were not available. Comparison of the demographic variables between cytologically proven benign and malignant nodes was carried out using either contingency table (χ2) analysis or Mann-Whitney test, as appropriate. In order to assess the intra-observer variation, and hence the reproducibility, for strain ratio, 8 patients had 8 strain ratio values determined and the coefficient of variation was determined. In order to compare the relative sensitivity and specificity of the EUS elastography and 4 conventional EUS criteria (both individually and in combination) with EUS-FNAB for detecting malignant lymph nodes, the area under the receiver operator curve (ROC) was analysed. The area under an ROC curve (AUC) for a measurement to discriminate between two disease conditions may be viewed as the probability that the measurement would correctly discriminate between two patients, one with and one without disease, each selected randomly from their group (those with and without disease). Note that the P-value cited with a single ROC curve is for the rejection of the null hypothesis that the expected value of the strain ratio AUC = 0.50, i.e., non-informative, equivalent to flipping a fair coin; whereas, the other P-values for AUCs other than the strain ratio ROC are for rejecting the null hypotheses that those expected values of AUCs are no different than that of the strain ratio AUC. Comparison between ROC curves AUC was undertaken by the statistics software package which uses the DeLong, DeLong, Clarke-Pearson methodology[21]. Because of the number of statistical comparisons, a P value of < 0.01 was considered to be significant. Analysis was performed with the use of Analyse-It Statistical Package (Analyse-it Software, Ltd. http://www.analyse-it.com/; 2009).
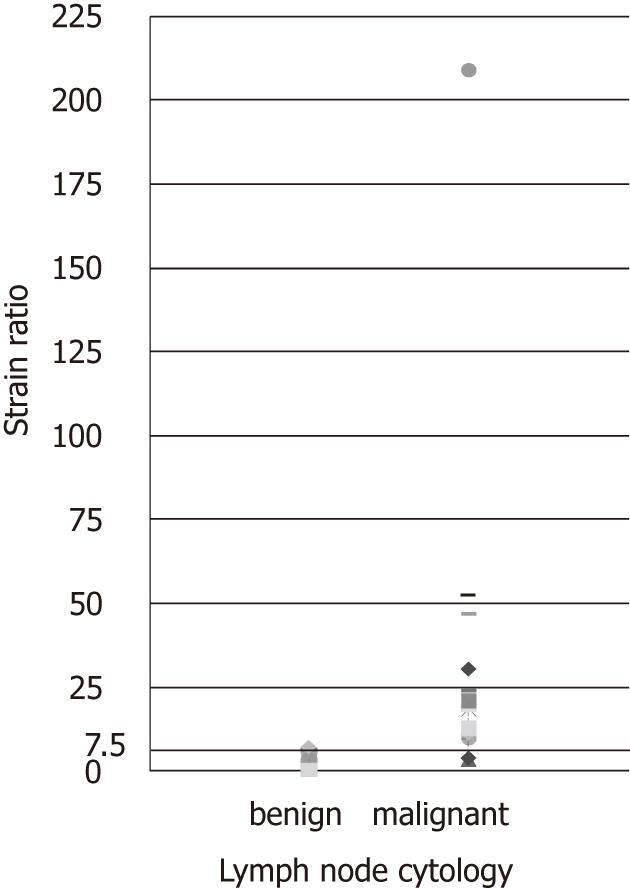
EUS elastography, prior to lymph node EUS-FNAB, was performed in 53 patients undergoing EUS for oesophago-gastric cancer staging. Cytological evidence of malignant disease was found in 23 nodes, while 25 nodes were considered benign (Figure 3). On 3 occasions insufficient node material was sampled to allow cytological analysis and on one occasion the cytological analysis was indeterminate. The pathological analysis of one lesion in a patient with confirmed oesophageal adenocarcinoma revealed a gastrointestinal stromal tumor rather than a lymph node.
Patient demographics are shown in Table 1. There were no statistically significant differences between the cytologically benign and malignant node groups.
| Benign node | Malignant node | |
| Gender | M 20:F 5 | M 18:F 5 |
| Age (mean, yr) | 66.8 | 67.9 |
| Oesophageal adenocarcinoma | 17 (68) | 16 (70) |
| Oesophageal squamous cancer | 5 (20) | 7 (30) |
| Gastric adenocarcinoma | 2 (8) | 0 (0) |
| Barrett’s oesophagus with HGD | 1 (4) | 0 (0) |
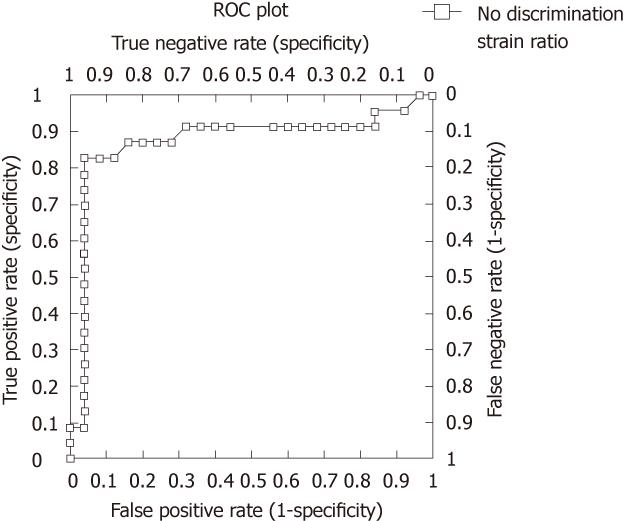
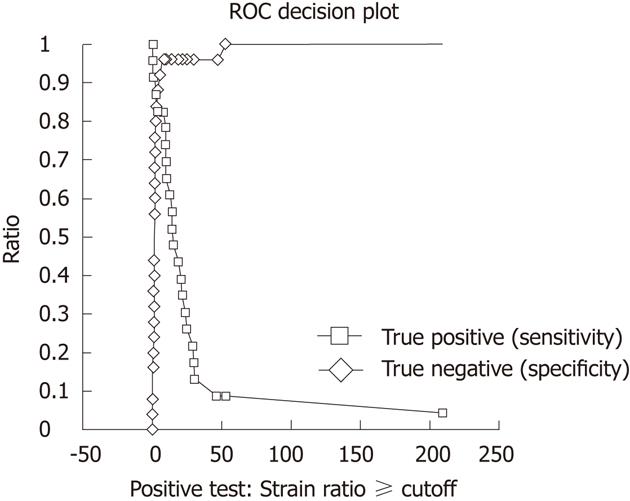
The ROC area under the curve, using elastography strain ratio, was 0.87 (95% CI: 0.75-1.00, P < 0.0001) (Figure 4). The strain ratio which was the optimal cut-off point for distinguishing malignant from benign nodes was ≥ 7.5 as determined by a ROC sensitivity specificity decision plot (Figure 5), with a strain ratio above this indicating malignant involvement. The likelihood ratio was 20.65 for a strain ratio of 7.5. This gave sensitivity 83%, specificity 96%, positive predictive value (PPV) 95%, and negative predictive value (NPV) 86% (Table 2). The accuracy of elastography with a strain ratio ≥ 7.5 was 90%.
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| Strain ratio | 83 (61-95) | 96 (80-100) | 95 (75-100) | 86 (67-96) | 90 (77-97) |
| 4/4 conventional criteria present | 22 (07-43) | 96 (80-100) | 83 (36-99) | 57 (41-72) | 60 (45-72) |
| 3/4 conventional criteria present | 48 (27-69) | 84 (64-96) | 75 (45-92) | 64 (45-80) | 67 (52-80) |
| Size > 1 cm | 61 (39-80) | 64 (43-82) | 61 (38-80) | 64 (43-82) | 62 (47-76) |
| Shape round | 65 (43-84) | 84 (64-96) | 79 (54-94) | 72 (53-87) | 75 (60-86) |
| Clear border | 56 (34-77) | 68 (47-85) | 62 (38-82) | 63 (42-81) | 62 (47-76) |
| Hypodense | 70 (47-87) | 68 (46-85) | 67 (45-84) | 71 (49-87) | 69 (53-81) |
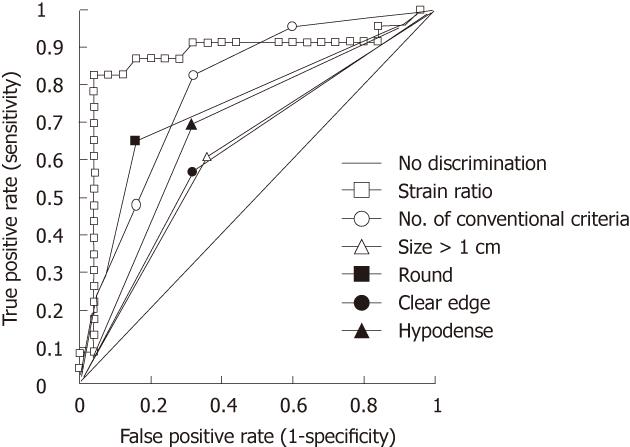
ROC area under the curve comparisons showed that there was a trend for elastography strain ratio to be the best diagnostic test for distinguishing benign from malignant nodes when compared to standard EUS nodal assessment, i.e., size, shape, density, distinction of border and any combination of these four conventional characteristics, although statistical significance was reached for only individual comparisons of size and clear edge (Figure 6 and Table 3).
| ROC AUC | P value1 | |
| Strain ratio | 0.87 (0.75-1.00) | NA |
| Number of conventional criteria | 0.79 (0.66-0.92) | 0.2846 |
| Size > 1cm | 0.62 (0.48-0.76) | 0.0078 |
| Shape round | 0.75 (0.62-0.87) | 0.1329 |
| Clear edge | 0.62 (0.48-0.76) | 0.0035 |
| Hypodense | 0.69 (0.55-0.82) | 0.0100 |
Elastography strain ratio assessment was more accurate than all other assessed EUS characteristics of lymph nodes, with an accuracy of 90% (Figure 6, Tables 2 and 3).
Elastography strain ratio was favourable compared to other nodal assessments with regard to sensitivity, specificity, PPV and NPV (Table 2).
The mean coefficient of variation (CV) for all recorded node strain ratio recordings was 44.8%. The mean CV for cytologically malignant nodes was 51.7%, while the mean CV for cytologically benign nodes was 34%.
This study has shown EUS-guided elastography to be effective for distinguishing between malignant and benign lymph nodes in patients with oesophago-gastric cancer and that it is more accurate than conventional EUS nodal characteristics.
Elastography of tissue is a new technology that has been successfully applied to several clinical fields. The specific modality of EUS-guided elastography has been developed over the past few years and first generation technology using elastographic colour imaging has shown promising results in the assessment of pancreatic disease[17,18]. There has also been encouraging, though limited, data on lymph node assessment[13,18-20].
Conventional EUS lymph node assessment using size, shape, density and distinction of node border does not give adequate sensitivity and specificity to confidently distinguish between malignant and benign nodes. In this study, accuracy of these characteristics is poor (Table 3). This is consistent with previously published data[8,19,22]. However, using EUS elastography with a strain ratio cut off of ≥ 7.5 for malignancy, we found a sensitivity and specificity of 83% and 96%, respectively. The ROC AUC was highest for strain ratio compared to all of the conventional EUS criteria, either individually or in combination. The strain ratio cut-off point of ≥ 7.5 to distinguish malignant nodal involvement was derived from a ROC sensitivity and specificity decision plot. The specificity and the sensitivity using this strain ratio cut-off point were 96% and 83%, respectively.
Out of the 23 cytologically determined malignant cases, there were 4 cases where elastography strain ratio gave a false negative result. This may be explained by vascular or necrotic lymph nodes giving the elastographic appearance of soft tissue[19,23]. There were no surgical resection specimens in this study to confirm or refute this hypothesis.
The gold standard for determination of malignant cells within a lymph node at the time of pre-operative staging remains FNAB. However, there are challenges encountered during endosonography such as large peri-tumoural reactive lymph nodal assessment, targeting which suspicious nodes on which to perform FNAB, and avoiding passing the needle through interposed major neurovascular structures. Although EUS-FNAB is considered the gold standard for the purposes of this study, there are previous reports suggesting EUS-FNAB has a false negative rate of 5%-10%[24,25]. EUS-FNAB was used as the gold standard due to the lack of availability of routine imaging follow up or surgical resection specimens. This is a limitation of our study. In this study, there was only one patient who had an apparently striking falsely positive strain ratio value of 47.0. Despite radical chemotherapy this patient has not progressed well with rapid deterioration. It is possible that the EUS-FNA was cytologically falsely negative, rather than there being a falsely positive elastography strain ratio value.
Technical challenges were encountered in performing elastography during EUS. The technique relies on respiratory movement and vascular pulsation to generate appropriate compression displacement of the digitalised radiofrequency echo lines. Obtaining consistent compression recordings was not always possible, leading to some variability in the elastogram produced, as has previously been described by other groups[26]. This will have led, in part, to the moderately large intra-observer variation calculated which is a limitation of this technique. It would have been desirable to have a coefficient of variance not exceeding 30%. It is, however, worth noting that the intra-observer variation was only significant when strain ratio values were high and none of the range of values obtained changed the likelihood of a node from being malignant to benign.
The method described relies on comparison of the strain within a node and surrounding tissue. This comparison assumes that the surrounding tissues are normal. It is recognised that the surrounding tissues may have different physical characteristics, which may influence the results. It is also worth noting that, as central necrosis and vascular invasion of a lymph node occur, the strain within the node may start to reduce as these “softer” components of the node are assessed. This is the basis of a fifth colour pattern analysis described by other authors[18,23]. However, the use of a strain ratio is an objective measure that is not limited by the inter-observer variability which is inherent in elastogram colour pattern analysis.
A limitation of this study is that it was undertaken within a single centre. However, there are supporting data from a recently reported large European multi-centre trial. Giovannini et al[23] report on 38 cases of oesophago-gastric cancer staging EUS-guided elastography lymph node assessments in a larger cohort of lymph node analysis. The elastography sensitivity and specificity of 91.8% and 82.5% recorded from this multicentre study are similar to those presented in this paper. The sensitivity and specificity recorded from the data presented in our paper are also similar to those found when cervical lymph nodes were assessed by sonographic elastography[19].
It is also recognised that there is the potential for selection bias within this study, in that all patients were having EUS-FNAB of nodes which were considered suspicious for malignancy. However, it is proposed that this modality is used in those very situations where EUS-FNAB will have been contemplated but may not be practical.
Patients undergoing staging of oesophago-gastric cancers are often subjected to several staging investigations. EUS-guided elastography offers an additional assessment of any suspicious lymph nodes that can be undertaken at the time of the standard EUS evaluation and is no more invasive. The recent change in the staging system for oesophago-gastric cancers means that the absolute number of involved malignant nodes becomes critical in determining the tumour stage[27-30]. Therefore, any modality that improves nodal staging is of critical importance. We believe EUS elastography is complementary to FNA in distinguishing between benign and malignant lymphadenopathy.
In conclusion, EUS elastography has been shown in this study to be superior to standard EUS assessment in characterising benign from malignant lymph nodes in oesophago-gastric cancer. It appears complementary to current staging investigations and has the potential to improve the staging and management of this disease.
We would like to acknowledge Professor Donald McMillan of the University of Glasgow for his comments and guidance on writing the statistical analysis section of this paper.
Endoscopic ultrasound (EUS) is an integral investigation in the staging of oesophageal and oesophago-gastric junctional tumours. Determination of lymph node involvement in particular is of critical prognostic importance. At present, staging relies largely on EUS-fine needle aspiration biopsy (FNAB) of suspicious nodes after initial determination of standard EUS characteristics. However, the invasive process of FNAB is not always possible.
Elastography has been shown to be useful in distinguishing benign from malignant tissue in the pancreas. However, there are limited data assessing the accuracy of EUS elastography for nodal assessment in upper gastrointestinal malignancy.
There have been recent reports of the use of elastography to distinguish between benign and malignant tissue, including lymph nodes. Earlier studies used qualitative descriptions of elastogram patterns. More recently, there have been technological advances allowing quantitative assessment of elastography strain ratio which measures, and compares, the stiffness of tissues.
This study suggests that quantitative EUS elastography is accurate at distinguishing between malignant and benign lymph nodes in the staging of oesophago-gastric malignancy. Compared to standard EUS characterisation of lymph nodes, elastography is superior in this study. EUS elastography appears complementary to current staging investigations and has the potential to improve the staging and management of this disease.
Elastography is a measure of the stiffness, or strain, of tissue. This can be useful in many disease processes including malignancy where tissue is often “harder” or “stiffer” where there is cancerous tissue present. Measuring elastography relies on applying a mechanical compression and determining how much the tissue deforms. Harder tissue deforms less than soft tissue.
Overall, a very interesting article looking at something that remains a problem without tissue diagnosis. The paper is well written and reads well. The methodology is appropriate, the results presented well and the discussion is appropriate. This paper will add to our understanding and serve as a platform for further studies looking at the characteristics of lymph nodes, not just for the esophago-gastric lymph nodes but other malignancies as well.
| 1. | Management of oesophageal and gastric cancer: A national and clinical guideline. Available from URL: http: www.sign.ac.uk/pdf/sign.87.pdf. . |
| 2. | Staging of oesophago-gastric carcinoma by endoscopic ultrasonography: Guidance and minimum standards. Available from URL: http: www.bsg.org.uk/pdf_word_docs/eus_staging.doc. . |
| 3. | Smith BR, Chang KJ, Lee JG, Nguyen NT. Staging accuracy of endoscopic ultrasound based on pathologic analysis after minimally invasive esophagectomy. Am Surg. 2010;76:1228-1231. [PubMed] |
| 4. | Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 243] [Cited by in RCA: 254] [Article Influence: 14.1] [Reference Citation Analysis (2)] |
| 5. | van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98:547-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 351] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 6. | Fritscher-Ravens A, Sriram PV, Bobrowski C, Pforte A, Topalidis T, Krause C, Jaeckle S, Thonke F, Soehendra N. Mediastinal lymphadenopathy in patients with or without previous malignancy: EUS-FNA-based differential cytodiagnosis in 153 patients. Am J Gastroenterol. 2000;95:2278-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Larsen SS, Krasnik M, Vilmann P, Jacobsen GK, Pedersen JH, Faurschou P, Folke K. Endoscopic ultrasound guided biopsy of mediastinal lesions has a major impact on patient management. Thorax. 2002;57:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Catalano MF, Sivak MV, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 311] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 9. | Moon WK, Chang RF, Chen CJ, Chen DR, Chen WL. Solid breast masses: classification with computer-aided analysis of continuous US images obtained with probe compression. Radiology. 2005;236:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Lyshchik A, Higashi T, Asato R, Tanaka S, Ito J, Mai JJ, Pellot-Barakat C, Insana MF, Brill AB, Saga T. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237:202-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 442] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 11. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 966] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 12. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1862] [Article Influence: 88.7] [Reference Citation Analysis (1)] |
| 13. | Gómez-Domínguez E, Mendoza J, Rubio S, Moreno-Monteagudo JA, García-Buey L, Moreno-Otero R. Transient elastography: a valid alternative to biopsy in patients with chronic liver disease. Aliment Pharmacol Ther. 2006;24:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Saftoiu A, Vilman P. Endoscopic ultrasound elastography-- a new imaging technique for the visualization of tissue elasticity distribution. J Gastrointestin Liver Dis. 2006;15:161-165. [PubMed] |
| 15. | Janssen J, Dietrich CF, Will U, Greiner L. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy. 2007;39:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Faige DO. EUS in patients with benign and malignant lymphadenopathy. Gastrointest Endosc. 2001;53:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Alam F, Naito K, Horiguchi J, Fukuda H, Tachikake T, Ito K. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: comparison with conventional B-mode sonography. AJR Am J Roentgenol. 2008;191:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Zhang Y, Lv Q, Yin Y, Xie M, Xiang F, Lu C, Yan T, Li W, Xu H, Huang Y. The value of ultrasound elastography in differential diagnosis of superficial lymph nodes. Front Med. 2009;3:368-372. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13220] [Cited by in RCA: 13268] [Article Influence: 349.2] [Reference Citation Analysis (0)] |
| 22. | Săftoiu A, Vilmann P, Hassan H, Gorunescu F. Analysis of endoscopic ultrasound elastography used for characterisation and differentiation of benign and malignant lymph nodes. Ultraschall Med. 2006;27:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine needle aspiration is superior to lymph node echofeatures: a prospective evaluation of mediastinal and peri-intestinal lymphadenopathy. Am J Gastroenterol. 2004;99:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Zhang S, Defrias DV, Alasadi R, Nayar R. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA): experience of an academic centre in the USA. Cytopathology. 2010;21:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 26. | Fritscher-Ravens A. Blue clouds and green clouds: virtual biopsy via EUS elastography? Endoscopy. 2006;38:416-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6606] [Article Influence: 412.9] [Reference Citation Analysis (0)] |
| 28. | Rice TW, Rusch VW, Ishwaran H, Blackstone EH. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763-3773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 360] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 29. | Akutsu Y, Matsubara H. The significance of lymph node status as a prognostic factor for esophageal cancer. Surg Today. 2011;41:1190-1195. [PubMed] |
Peer reviewer: Dr. Vui Heng Chong, Gastroenterology and Hepatology Unit, Department of Medicine, Raja Isteri Pengiran Anak Saleha Hospital, Bandar Seri Begawan BA 1710, Brunei Darussalam
S- Editor Shi ZF L- Editor Logan S E- Editor Xiong L