Published online Feb 7, 2012. doi: 10.3748/wjg.v18.i5.458
Revised: April 26, 2011
Accepted: May 3, 2011
Published online: February 7, 2012
AIM: To investigate the clinical features and survival of patients treated for cholangiocarcinoma in our institution and to analyze the factors affecting their survival.
METHODS: This retrospective cohort study assessed patients diagnosed with cholangiocarcinoma between January 1997 and December 2007 at the University Malaya Medical Centre in Malaysia. The clinical data and associated outcomes were collected using a structured proforma.
RESULTS: Of the 69 patients diagnosed with cholangiocarcinoma, 38 (55%) were male; mean patient age was 61 years. Twelve patients (17%) had intrahepatic, 38 (55%) had perihilar and 19 (28%) had distal tumors. Only 12 patients underwent curative surgery, including seven R0 resections. Only one patient died within 30 d after surgery. The overall median survival was 4 mo, whereas the median survival of R0 resected patients was 16 mo. The overall 1-, 2- and 3-year cumulative survival rates were 67%, 17% and 17%, respectively. Survival rates were significantly associated with curative resection (P = 0.002), intrahepatic tumor (P = 0.003), negative margin status (P = 0.013), early tumor stage (P = 0.016), higher tumor differentiation (P = 0.032) and absence of jaundice (P = 0.038). Multivariate analysis showed that tumor location was a significant independent predictor of patient survival.
CONCLUSION: Curative, margin-negative resection of early stage, well-differentiated intrahepatic tumors is associated with improved patient survival.
- Citation: Yusoff AR, Abdul Razak MM, Yoong BK, Vijeyasingam R, Siti ZM. Survival analysis of cholangiocarcinoma: A 10-year experience in Malaysia. World J Gastroenterol 2012; 18(5): 458-465
- URL: https://www.wjgnet.com/1007-9327/full/v18/i5/458.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i5.458
Cholangiocarcinoma is a rare malignant neoplasm of bili-ary tract epithelium, accounting for less than 2% of all human malignancies[1]. Cholangiocarcinoma is the second most common primary hepatic malignancy after hepatocellular carcinoma (HCC)[2] and is associated with poor patient outcomes. Over the past three decades, however, the worldwide incidence of and mortality from cholangiocarcinoma have steadily increased[3].
Cholangiocarcinoma is difficult to diagnose and is usually fatal, due to its late clinical presentation and the absence of effective non-surgical therapeutic modalities[4]. The incidence of cholangiocarcinoma peaks in patients aged 50-70 years, and there is a slight male predominance[5]. Most patients have unresectable disease at the time of diagnosis and usually die within 6-12 mo from the cancer cachexia, liver failure and biliary sepsis[6]. The 5-year survival rate is low (about 5%), and has remained virtually unchanged over the past 20 years[5,7]. Surgical resection remains the only hope for cure, and radical resection has improved outcomes, although it is also associated with high perioperative morbidity and mortality rates[8]. Since most patients with cholangiocarcinoma present with advanced disease, palliative stenting and chemoradiation are reserved for non-resectable patients, those with recurrence, and those who refuse surgical treatment[3].
Little is known about the survival of cholangiocarcinoma patients in Malaysia. We therefore analyzed the factors affecting survival of patients with cholangiocarcinoma treated at the University Malaya Medical Centre, Malaysia.
This retrospective cohort study assessed patients diagnosed with cholangiocarcinoma and treated between January 1997 and December 2007 at our center, a tertiary referral center in Malaysia with a specialized hepatobiliary surgery unit and a gastroenterology unit. Patients with a diagnosis of cholangiocarcinoma confirmed histologically by tissue biopsy, and patients without a histologically confirmed diagnosis but with a strong provisional diagnosis by clinical examination, biochemical results and positive endoscopic or imaging [i.e., endoscopic retrograde cholangiopancreatography (ERCP)/magnetic resonance cholangiopancreatography (MRCP) or computed tomography (CT) of the abdomen] results[7] were included. Patients with HCC, tumor at the head of the pancreas and gallbladder carcinoma were excluded.
Combinations of ultrasonography, CT, MRCP, ERCP and percutaneous cholangiography (PTC) were used for tumor diagnosis and staging, and for assessment of resectability. Metastatic disease was evaluated by CT of the thorax, abdomen and pelvis and/or chest radiography (CXR).
Patients were classified into three groups based on the anatomic location of the primary lesion, specifically intrahepatic, perihilar and distal types as proposed by the guidelines for the diagnosis and treatment of cholangiocarcinoma[5]. Intrahepatic tumors were defined as those confined to the liver and not involving the extrahepatic biliary tree. Perihilar tumors were defined as those involving or requiring resection of the hepatic duct bifurcation and were typically located in the extrahepatic biliary tree proximal to the origin of the cystic duct. Distal tumors were defined as extrahepatic lesions located in the peripancreatic region[9].
Patients were staged according to the tumor-node-metastasis (TNM) system[6,10] and assessed for resectability. Variables assessed included therapeutic options (surgical or palliative treatment), operative data, and 30-d postoperative morbidity and mortality. Survival was measured from the date of first presentation to the date of death or last follow-up visit.
Statistical calculations were performed using SPSS version 13.0. Categorical variables were compared using χ2 tests for association. One-way analysis of variance was used to compare continuous variables among the three groups of patients classified by tumor location. Results were presented as means ± SD, unless otherwise specified. Survival curves were calculated using the Kaplan-Meier method and compared using log-rank tests (Mantel-Cox). Cox proportional hazard models were used to calculate adjusted hazard ratios. A P value < 0.05 was considered significant.
Of the 69 patients included in this study, 12 (17%) had intrahepatic, 38 (55%) had perihilar and 19 (28%) had distal tumors. Thirty-eight patients were male (55%) and 31 (45%) were female (a male to female ratio, 1.2:1). Mean ± SD patient age was 61 ± 14.2 years (range, 18-91 years), although patients with intrahepatic tumors were younger than those with perihilar and distal tumors (P < 0.05). When subdivided ethnically, 42 patients (61%) were Chinese, 20 (29%) were Malay and 7 (10%) were Indian.
Of the 69 patients, 22 (29%) had a history of chronic cigarette smoking, 13 (19%) each had diabetes mellitus and regular alcohol intake, and 5 (7%) had chronic hepatitis B, with only 1 each (1.4%) having hepatolithiasis and choledochal cyst. None of our patients had other strong risk factors, such as inflammatory bowel disease, primary sclerosing cholangitis or liver fluke infestation.
The most common symptoms among our patients were jaundice (78%), anorexia (57%), weight loss (52%), abdominal pain (44%), abdominal mass (44%), itchiness (25%), vomiting (9%) and fever (7%). Ninety-eight percent of the patients with extrahepatic cholangiocarcinoma were jaundiced (63% of perihilar tumor and 35% of distal tumor patients), whereas only 2% of intrahepatic patients was jaundiced (P < 0.001). No specific symptom was significantly related to any of the three types of cholangiocarcinoma. The median duration of complaints prior to medical consultation was 30 d (range 1-365 d). However, patients with intrahepatic lesions present somewhat later (P < 0.05) than those with perihilar or distal lesions.
When we assessed laboratory variables in these patients, we found that total bilirubin concentration was significantly higher in patients with perihilar than in those with distal or intrahepatic lesions (P = 0.003) (Table 1). The international normalized ratio (INR) value was also predictive of lesion site, as it was higher in patients with distal than with perihilar or intrahepatic lesions (P = 0.005).
| Total | Intrahepatic | Perihilar | Distal | P | |
| (n = 69) | (n = 12) | (n = 38) | (n = 19) | value1 | |
| Total bilirubin (mmol/L) | 178 (158) | 45 (109) | 216 (163) | 188 (131) | 0.003 |
| Conjugated bilirubin (mmol/L) | 140 (122) | 31 (87) | 170 (123) | 151 (102) | 0.002 |
| INR | 1.2 (0.7) | 1.1 (0.1) | 1.0 (0.1) | 1.6 (1.1) | 0.005 |
The tumor markers carcinoembryonic antigen, alpha-fetoprotein and carbohydrate antigen 19-9 were measured in about 50% of these patients, but none of them significantly correlated with tumor location.
Ultrasound was the first line non-invasive imaging me-thod used in 45 (65%) patients, detecting 50%-76% of obstructed biliary systems. Almost 91% of these patients underwent CT scans to further assess the extent of pathology, including liver masses, lymph nodes and the involvement of major vessels. We found that about 20% of the abdominal lesions were not detected by ultrasound alone, but were detected on CT scan. Only four patients underwent MRCP.
In 58 (84%) patients, ERCP was the first invasive method used to assess the obstructed biliary system. ERCP showed that 51 (74%) patients had strictures at various levels of their biliary trees. Plastic stents were inserted successfully into 44 (76%) of these patients to relieve their biliary obstructions, whereas the other seven (14%) failed in stent insertion; five later underwent PTC drainage. Cytology brushing of suspicious strictures was performed in 22 (38%) patients, with 10 (45%) being positive for cancer.
Preoperative staging for resectability was predicted based on ultrasound, CT scan, MRCP and CXR. None of these patients underwent staging laparoscopy because it was not a routine practice in our center.Based on the American Joint Committee on Cancer TNM staging system, 23 (33%) patients had stage 1 tumors, 7 (10%) had stage 2, 17 (25%) had stage 3 and 22 (32%) had stage 4. Stages 3 and 4 were considered unresectable.
Although preoperative staging indicated that 30 (43%) patients were candidates for surgical resection, only 22 (32%) patients underwent laparotomy with curative intent. Three patients (and their relatives) refused surgery, citing age as their primary concern (mean age 78 years), whereas five were excluded from surgery due to age (mean 71 years) and/or associated comorbidity. At laparotomy, 10 patients had extensive local disease and/or hepatic metastasis that precluded resection. The remaining 12 patients underwent potentially curative resection (resectability rate, 55%). All except one with evidence of biliary obstruction underwent preoperative biliary drainage either by endoscopic stenting or percutaneous transhepatic biliary drainage (PTBD). The type of surgery depended on the location of the tumor (Table 2). In general, intrahepatic tumors were treated by hepatic resection; perihilar lesions by excision of the extrahepatic biliary tree and lymph node dissection, with or without hepatic resection; and distal tumors by Whipple pancreaticoduodenectomy. Biliary reconstructions were mostly by Roux-ex-Y hepaticojejunostomy. All operations were performed by trained hepatobiliary surgeons.
| Types of surgery | Patients (n = 12) |
| Left hemihepatectomy | 2 |
| Right hemihepatectomy | 1 |
| Segmental hepatic resections | 2 |
| Extrahepatic bile duct resection | 5 |
| Whipple’s procedure | 2 |
Complications occurred in patients with all three tumor types, but the differences were not statistically significant. Three patients developed post-operative ileus, which resolved after conservative treatment. Two patients had intra-abdominal hemorrhage. One had undergone a Whipple procedure and was re-explored within 24 h because of portovenous bleeding; unfortunately, this patient died the next day. The second patient did not require intervention and was treated with blood transfusion and correction of coagulopathy. Three patients developed intra-abdominal collection, later complicated by abscesses, including one caused by bile leakage from the anastomosis. None of these patients, however, required surgical or percutaneous intervention. Two patients had surgical site infections, including one with anastomotic stenosis and the other with deep venous thrombosis (DVT) despite DVT prophylaxis. Only one patient died of complications within 30 postoperative days; hence the perioperative mortality rate was 8%.
Of the 10 patients who underwent surgery but were found to have advanced inoperable disease at laparotomy, 3 underwent a palliative bypass procedure, consisting of hepaticojejunostomy and gastrojejunostomy, whereas four underwent gastrojejunostomy alone. Two patients underwent cholecystectomy and biliary stent insertion, whereas one underwent only laparotomy and biopsy.
Of the 12 patients who underwent curative resection, 11 (92%) had adenocarcinoma and one had a papillary adenocarcinoma. The mean ± SD tumor diameter was 9.6 ± 15.2 cm (range, 0.5 cm-55 cm). Intrahepatic tumors were larger than perihilar and distal tumors (P < 0.05). Five (42%) patients had well-differentiated adenocarcinoma and the others had moderately or poorly differentiated adenocarcinoma. Seven (58%) patients had perineural involvement, 5 (42%) had lymphovascular invasion and 4 (33%) had regional lymph node metastases. Seven (58%) patients were resected with negative margins (R0 resection), whereas the other 5 (42%) had microscopically positive margins. Table 3 summarizes the characteristics of these tumors.
| Total | Intrahepatic | Perihilar | Distal | P | |
| (n = 12) | (n = 3) | (n = 6) | (n = 3) | value1 | |
| Tumour histology | |||||
| Adenocarcinoma | 11 (92) | 2 (18%) | 6 (55%) | 3 (27) | |
| Other | 1 (8) | 1 (100%) | 0 | 0 | |
| Degree of differentiation | |||||
| Well | 5 (42) | 2 (40%) | 2 (40%) | 1 (20) | |
| Moderate | 6 (50) | 1 (17%) | 3 (50%) | 2 (34) | |
| Poor | 1 (8) | 0 | 1 (100%) | 0 | |
| Tumour diameter, cm | |||||
| Size, mean ± SD | 9.6 ± 15.2 | 27.1 ± 24.3 | 2.8 ± 2.2 | 5.7 ± 15.2 | 0.0481 |
| Margin | |||||
| Negative | 7 (58) | 3 (43) | 3 (43) | 1 (14) | |
| Positive | 5 (42) | 0 | 3 (60) | 2 (40) | |
| Lymph node involvement | |||||
| Negative | 8 (67) | 2 (25) | 4 (50) | 2 (25) | |
| Positive | 4 (33) | 1 (25) | 2 (50) | 1 (25) | |
| Perineural involvement | |||||
| Negative | 5 (42) | 3 (60) | 2 (40) | 0 | |
| Positive | 7 (58) | 0 | 4 (57) | 3 (43) | |
| Lymphovascular invasion | |||||
| Negative | 7 (58) | 2 (29) | 4 (57) | 1 (14) | |
| Positive | 5 (42) | 1 (20) | 2 (40) | 2 (40) | |
Forty-seven (68%) patients did not undergo surgery but received palliative treatment, including 31 (66%) who underwent palliative biliary drainage by endoscopic stenting, 23 (49%) who underwent PTBD with or without concurrent stenting and 13 who underwent both. Sixteen patients had their stents changed on subsequent follow-up, of whom, nine had self-expanding metal stents. Three (6%) patients received palliative chemotherapy alone, using a variety of chemotherapeutic agents (5-fluorouracil, cisplatin, gemcitabine). Three (6%) patients were too ill and hence received best supportive care.
We have described our experience managing patients with cholangiocarcinoma, a rare type of tumor. The incidence of this tumor is likely increasing in Malaysia. The latest National Cancer Registry 2003-2005 Peninsular Malaysia has classified cholangiocarcinoma into the category of liver and gallbladder cancers rather than as a separate tumor. The incidence of liver cancer in Malaysia has been reported to be 3.6% for males and 1.2% for females, while the rates of gallbladder cancer were 0.8% and 0.7%, respectively. Morphologically, about 2.2% of these liver cancers and 33.9% of these gallbladder cancers were cholangiocarcinomas. The incidence of both liver and gallbladder cancers was higher for Chinese than for Malays and Indians.
Cholangiocarcinoma is best classified according to its anatomical location into intrahepatic, perihilar and distal tumors[9,11,12]. Most (40%-60%) tumors are perihilar or Klatskin tumors, with 20%-30% being distal and 10% being intrahepatic tumors[9,13]. Our findings were similar to these rates, in that 17% of tumors were intrahepatic, 54% were perihilar and 29% were distal.
The demographic characteristics of our patients were comparable to those of patients in other larger series. Mean patient age was 61 years (range, 18-91 years), while a review of 294 patients with cholangiocarcinoma by Nakeeb et al[9] showed a mean age of 62.2 years and a review by DeOliveira et al[13] of 564 patients showed a median age of 65 years. We found, however, that patients with intrahepatic tumors were significantly younger than those with perihilar or distal tumors. We observed a slight male predominance, with a male to female ratio of 1.2:1, similar to the ratios of 1.2:1[9] and 1.38:1 reported previously[13].
Of these patients, 32% presented in 1997-2002, wher-eas 68% presented in 2003-2007. We observed similar increases in the number of patients with primary tumors, suggesting that these higher rates may be due to improvement in tumor detection, ERCP proficiency, or increased awareness of the availability of local surgical expertise.
Only a few patients in our series had strong risk factors such as chronic hepatitis B, hepatolithiasis and choledochal cyst[14-16]. None had a history of primary sclerosing cholangitis[14] or liver fluke infestation[17], the latter of which is not endemic in Malaysia. Chronic cigarette smoking, regular alcohol intake and diabetes mellitus were among the risk factors[18,19] observed in our population, but none of these risk factors was associated with any particular tumor site.
The most common symptom observed in these pati-ents was jaundice. Jaundice was significantly more common in patients with extrahepatic (i.e., perihilar and distal) than with intrahepatic cholangiocarcinoma (P < 0.001). This was confirmed by laboratory results showing that the concentration of bilirubin was significantly higher in patients with perihilar (extrahepatic) than in those with intrahepatic tumors (P = 0.002). Similar results have been reported previously[13,20]. We also found that INR was higher in patients with distal tumors (P = 0.005). Obstructive jaundice may decrease the concentrations of vitamin K-dependent coagulation factors, resulting in aberrant coagulation profiles[5]. However, none of the other symptoms or blood parameters we assayed was significantly related to tumor location.
Many of our patients initially underwent ERCP, not MRCP. ERCP enables cytological brushing and can decompress the obstructed biliary system[6]. Although we found that the success rate of internal stent placement for drainage was high, PTBD may also play a role, especially when the endoscopic approach has failed. PTBD can also be used to visualize proximal biliary tumors and anatomy[5]. Failure of biliary decompression may result in an infected biliary system and further risks of liver failure and sepsis[21]. These risks may be prevented by decompression of an obstructed biliary system in patients with potentially resectable cholangiocarcinoma, although a series by Figueras et al[22] demonstrated no significant differences in morbidity and mortality between patients with and without preoperative biliary drainage. None of our patients who underwent hepatic resection developed postoperative liver failure.
Despite improved diagnostic methods and a relatively early presentation (median duration of symptoms, 30 d), only about one-third of our patients (32%) underwent surgery, and only 12 underwent curative resection. More than half of our patients (58%) were considered to have advanced unresectable disease, whereas the remaining patients were considered unsuitable for surgery because of comorbidity and/or advanced age, findings similar to those observed previously[7]. Our overall resectability, 55% (12/22), was similar to previously reported rates 18%-70%[23]. Postoperative complications were not associated with tumor location. In high volume centers with considerable experience, the operative mortality and morbidity rates varied from 6%-14% and 32%-65%, respectively[24-26]. Our 30-d postoperative mortality and morbidity rates were similar, 8% and 67%, respectively.
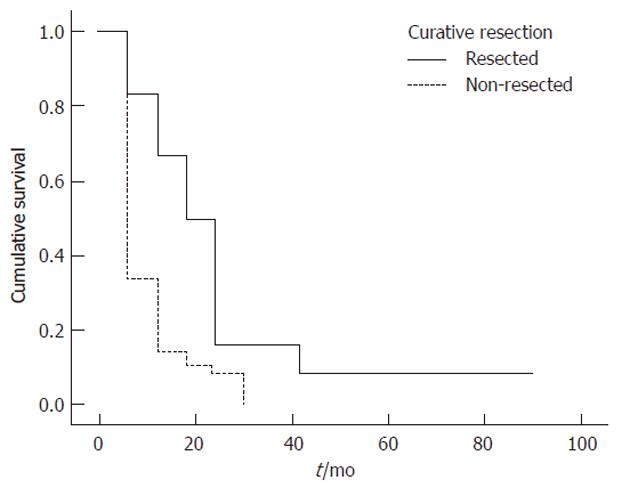
Patients who underwent surgical resection had a definite survival advantage over those who did not, confirming that surgical resection is the best treatment available for patients with cholangiocarcinoma and providing further evidence that potentially resectable patients should be referred early to a specialized surgical team[7,10]. The median survival time for the 12 patients who underwent curative resection was 16 mo, compared with 3 mo for the 57 patients who did not undergo curative resection (P = 0.002). The 1-, 2- and 3-year cumulative survival rates in patients who underwent resection were 67%, 17% and 17%, respectively, significantly higher than the 14%, 8% and 0%, respectively, in those who did not. Furthermore, complete surgical resection with histologically negative margins offers the best chance for cure and long-term survival[27-30].
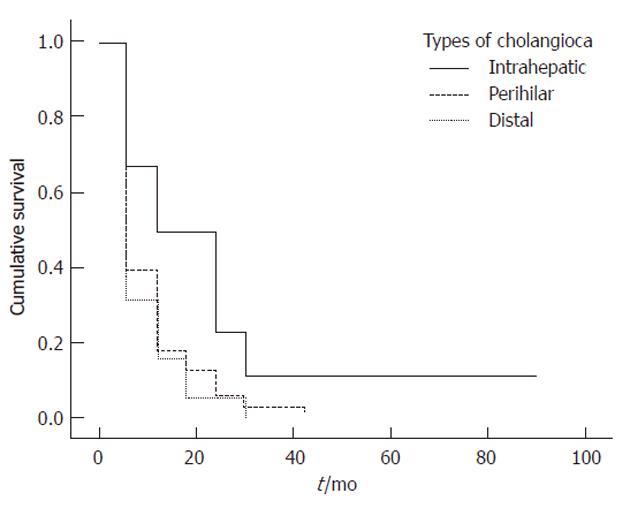
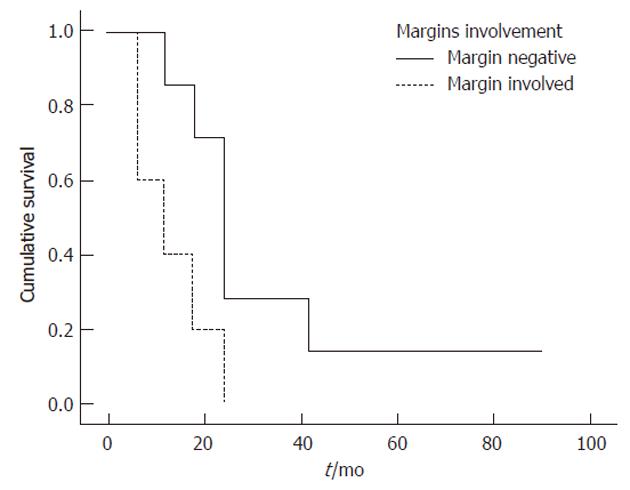
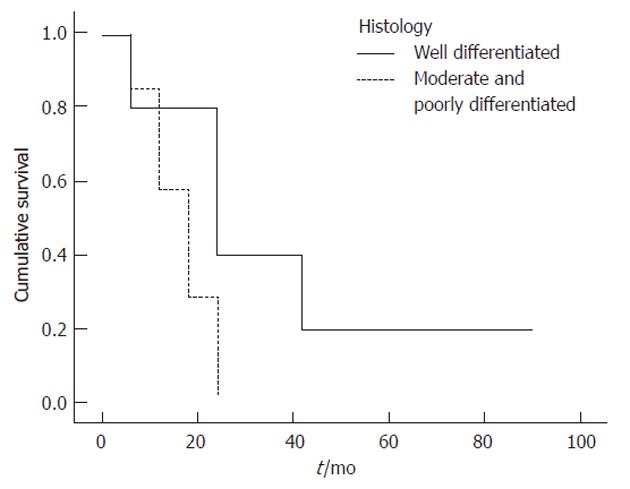
Univariate log-rank analyses of tumor related variables in Table 4 showed that absence of jaundice (P = 0.038), tumor location (P = 0.027) (Figure 1)[17], curative resection (P = 0.002) (Figure 2)[31], early tumor stage (P = 0.016)[32], negative margin status (P = 0.013) (Figure 3)[17,32,33] and higher degree of tumor differentiation (P = 0.032) (Figure 4)[17] were significant predictors of longer survival. In contrast to previous reports, regional lymph node metastasis[28] and perineural invasion[34] were not associated with survival in resected patients. Moreover, we found that age, gender, race and risk factors were not predictors of survival.
| Variables | Survival rates (%) | P value | ||
| 1-year | 2-year | 3-year | ||
| Jaundice | 0.038 | |||
| Absent | 40 | 19 | 9 | |
| Present | 19 | 6 | 2 | |
| Staging | 0.016 | |||
| Stage 1 | 43 | 17 | 9 | |
| Stage 2 | 43 | 14 | 0 | |
| Stage 3 | 12 | 4 | 0 | |
| Stage 4 | 5 | 0 | 0 | |
| Surgery (curative or palliative) | 0.017 | |||
| No | 13 | 10 | 0 | |
| Yes | 45 | 9 | 9 | |
| Curative resection | 0.002 | |||
| No | 14 | 8 | 0 | |
| Yes | 67 | 17 | 17 | |
| Type (among resected patient) | 0.003 | |||
| Intrahepatic | 100 | 33 | 33 | |
| Perihilar | 67 | 17 | 17 | |
| Distal | 33 | 0 | 0 | |
| Type (among all patient) | 0.027 | |||
| Intrahepatic | 50 | 23 | 11 | |
| Perihilar | 18 | 6 | 3 | |
| Distal | 16 | 5 | 0 | |
| Histology | 0.032 | |||
| Well | 80 | 40 | 40 | |
| Moderate and poor | 57 | 0 | 0 | |
| Margin involvement | 0.013 | |||
| Negative | 86 | 29 | 29 | |
| Positive | 40 | 0 | 0 | |
Multivariate analysis showed that tumor location was the only independent predictor of long-term survival. Survival was significantly lower in patients with perihilar than in those with intrahepatic and distal tumors (HR = 0.016, 95% CI: 0.01-0.607; P = 0.026), in agreement with the findings of other series showing that patients with intrahepatic tumors had the best survival[17,2]. This could be explained by the fact that, compared with extrahepatic tumors, intrahepatic tumors are characterized by different epidemiology and tumor biology, younger age (P < 0.05), and lower rate of tumor negative margins[10,17].
For the majority of patients with cholangiocarcinoma who cannot undergo curative resection, palliative treatment to relieve jaundice, pruritus and cholangitis and to avoid liver failure becomes the priority. This can be achieved surgically via biliary-enteric bypass or stent placement via PTBD or ERCP[33]. We found however, that neither palliative surgical bypass nor biliary drainage had significant survival benefits, in agreement with previous findings by Prat et al[34] Both groups had a median survival of 3 mo.
A novel palliative therapy for unresectable cholangiocarcinoma, photodynamic therapy (PDT), has shown promising benefits in terms of patient survival, cholestasis and quality of life[35]. PDT utilizes the intravenous photosensitizer sodium porfimer, which accumulates in tumor tissues. Upon illumination of the tumor bed by a specific endoscopic light, the porfimer becomes activated and forms oxygen free radicals, resulting in tumor necrosis[36].
The major limitation of our study was its involvement of patients at a single center. Therefore, our findings may not be representative of patients with cholangiocarcinoma throughout Malaysia.
The incidence of cholangiocarcinoma is increasing throughout Malaysia, as shown by the increase in the number of patients diagnosed per year throughout our study period. This may be due to an increased detection rate and to increases in referrals to our center. Curative surgical resection with clear histological margins of early stage well-differentiated intrahepatic tumors is associated with improved long-term survival. Further prospective randomized studies involving multiple centers are warranted.
The authors would like to thank Dr. Aqel Mohd Dhaher of Universiti Teknologi MARA and Dr. Azlan Darus of the University of Malaya for their assistance with the data analysis.
Cholangiocarcinoma is a rare malignant cancer of the bile duct with poor prognosis. This cancer is difficult to diagnose and often the patient presented late when curative surgical resection that can provide the chance for cure, is not feasible. Any person in his or her fifth decade with jaundice and significant weight loss should raise a suspicion of this cancer. Despite its rarity, the incidence of this cancer has been steadily increasing worldwide.
Many studies have concurred that early detection together with radical surgery will increase the survival period of these patients. A research article to be published in the World Journal of Gastroenterology has further emphasized the importance of early diagnosis and early referral to a specialized surgical centre with vast experience in the management of this cancer.
The authors have analysed various factors that affect the survival of their cholangiocarcinoma patients treated over a 10-year period. It was found that the survival period will improve if complete tumour excision is performed whereby the margins are tumour-free on histology. The outcome is also favourable if surgery is performed early when the abnormal cells are still at their early grade and have not spread elsewhere. Despite a range of palliative procedures available for inoperable cancer, none could surpass the result of a successful surgical treatment. These have further consolidated similar findings and recommendations of early aggressive surgery from other larger centres in the West and Far East.
The study also shows that cholangiocarcinoma is an important health problem in Malaysia. Although the increase in number of patient being treated may not truly reflect a true increase in incidence, this would mean more need to be done to address such problem. This study provides a framework for future studies in Malaysia and hopefully stimulates other groundbreaking research in this region especially concerning the epidemiology and pathophysiology of this fatal cancer.
This 10-year retrospective review defines treatment modalities and survival statistics for cholangiocarcinoma in a Malaysian Referral Hospital. Not surprisingly, patients who underwent R-O resections had higher cumulative survival rates than patients palliated surgically, endoscopically or by percutaneous transhepatic biliary drainage. This manuscript is worthy of publication.
| 1. | Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. CA Cancer J Clin. 1996;46:5-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1305] [Cited by in RCA: 1242] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 2. | Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 424] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 3. | Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66:167-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 187] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75:171-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Thursz MR, Wasan H. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51 Suppl 6:VI1-VI9. [PubMed] |
| 6. | Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 908] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 7. | Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 345] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 8. | Liu CL, Fan ST, Lo CM, Tso WK, Lam CM, Wong J. Improved operative and survival outcomes of surgical treatment for hilar cholangiocarcinoma. Br J Surg. 2006;93:1488-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 9. | Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463-473; discussion 473-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 872] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 10. | Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87:13-15. [PubMed] |
| 11. | de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 687] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 12. | Singh P, Patel T. Advances in the diagnosis, evaluation and management of cholangiocarcinoma. Curr Opin Gastroenterol. 2006;22:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755-762. [PubMed] |
| 14. | Chapman RW. Risk factors for biliary tract carcinogenesis. Ann Oncol. 1999;10 Suppl 4:308-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Chen MF, Jan YY, Wang CS, Hwang TL, Jeng LB, Chen SC, Chen TJ. A reappraisal of cholangiocarcinoma in patient with hepatolithiasis. Cancer. 1993;71:2461-2465. [PubMed] |
| 16. | Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, Martelli C, Savio A, Trevisi P, Nardi G. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Parkin DM, Srivatanakul P, Khlat M, Chenvidhya D, Chotiwan P, Insiripong S, L'Abbé KA, Wild CP. Liver cancer in Thailand. I. A case-control study of cholangiocarcinoma. Int J Cancer. 1991;48:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Bergquist A, Glaumann H, Persson B, Broomé U. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology. 1998;27:311-316. [PubMed] |
| 19. | Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 390] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | Alexopoulou A, Soultati A, Dourakis SP, Vasilieva L, Archimandritis AJ. Cholangiocarcinoma: a 7-year experience at a single center in Greece. World J Gastroenterol. 2008;14:6213-6217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Anderson CD, Pinson CW, Berlin J, Chari RS. Diagnosis and treatment of cholangiocarcinoma. Oncologist. 2004;9:43-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 251] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 22. | Figueras J, Llado L, Valls C, Serrano T, Ramos E, Fabregat J, Rafecas A, Torras J, Jaurrieta E. Changing strategies in diagnosis and management of hilar cholangiocarcinoma. Liver Transpl. 2000;6:786-794. [PubMed] |
| 23. | Hammill CW, Wong LL. Intrahepatic cholangiocarcinoma: a malignancy of increasing importance. J Am Coll Surg. 2008;207:594-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, Sano T, Yamamoto H, Hayakawa N. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 270] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Lillemoe KD, Cameron JL. Surgery for hilar cholangiocarcinoma: the Johns Hopkins approach. J Hepatobiliary Pancreat Surg. 2000;7:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507-517; discussion 517-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 981] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 27. | Jarnagin WR. Cholangiocarcinoma of the extrahepatic bile ducts. Semin Surg Oncol. 2000;19:156-176. [PubMed] |
| 28. | Su CH, Tsay SH, Wu CC, Shyr YM, King KL, Lee CH, Lui WY, Liu TJ, P'eng FK. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 220] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Silva MA, Tekin K, Aytekin F, Bramhall SR, Buckels JA, Mirza DF. Surgery for hilar cholangiocarcinoma; a 10 year experience of a tertiary referral centre in the UK. Eur J Surg Oncol. 2005;31:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Lai EC, Lau WY. Aggressive surgical resection for hilar cholangiocarcinoma. ANZ J Surg. 2005;75:981-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Bhuiya MR, Nimura Y, Kamiya J, Kondo S, Fukata S, Hayakawa N, Shionoya S. Clinicopathologic studies on perineural invasion of bile duct carcinoma. Ann Surg. 1992;215:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 112] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Madariaga JR, Iwatsuki S, Todo S, Lee RG, Irish W, Starzl TE. Liver resection for hilar and peripheral cholangiocarcinomas: a study of 62 cases. Ann Surg. 1998;227:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 170] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 33. | Abu-Hamda EM, Baron TH. Endoscopic management of cholangiocarcinoma. Semin Liver Dis. 2004;24:165-175. [PubMed] |
| 34. | Prat F, Chapat O, Ducot B, Ponchon T, Fritsch J, Choury AD, Pelletier G, Buffet C. Predictive factors for survival of patients with inoperable malignant distal biliary strictures: a practical management guideline. Gut. 1998;42:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J, Lochs H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 386] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 36. | Berr F. Photodynamic therapy for cholangiocarcinoma. Semin Liver Dis. 2004;24:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Peer reviewer: Richard A Kozarek, MD, Executive Director, Digestive Disease Institute, Virginia Mason Medical Center 1100 Ninth Avenue, PO Box 900, Seattle, WA 98111-0900, United States
S- Editor Sun H L- Editor Ma JY E- Editor Li JY