Published online Feb 7, 2012. doi: 10.3748/wjg.v18.i5.393
Revised: August 11, 2011
Accepted: August 15, 2011
Published online: February 7, 2012
Esophageal adenocarcinoma is a cancer with poor prognosis, and its incidence has risen sharply over recent decades. Obesity is a major risk factor for developing this cancer and there is a clear male gender bias in the incidence that cannot be fully explained by known risk factors. It is possible that a difference in the expression of estrogen, or its signaling axes, may contribute to this gender bias. We undertook a comprehensive literature search and analyzed the available data regarding estrogen and estrogen receptor expression, and the possible sex-specific links with esophageal adenocarcinoma development. Potentially relevant associations between visceral vs subcutaneous fat deposition and estrogen expression, and the effect of crosstalk between estrogen and leptin signaling were identified. We also found limited studies suggesting a role for estrogen receptor β expression in esophageal adenocarcinoma development. The current literature supports speculation on an etiological role for estrogen in the male gender bias in esophageal adenocarcinoma, but further studies are required.
- Citation: Yang H, Sukocheva OA, Hussey DJ, Watson DI. Estrogen, male dominance and esophageal adenocarcinoma: Is there a link? World J Gastroenterol 2012; 18(5): 393-400
- URL: https://www.wjgnet.com/1007-9327/full/v18/i5/393.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i5.393
Esophageal carcinoma is the eighth most common cancer worldwide, and over the last three decades its incidence has risen significantly in all Western countries[1-4]. This change is entirely due to an increase in the adenocarcinoma subtype, and this has predominantly occurred in males[2,5-9]. Recent Western experiences report that the male:female ratio for patients undergoing esophagectomy for adenocarcinoma now exceeds 8:1. However, the identified risk factors for esophageal adenocarcinoma, including gastroesophageal reflux disease, Barrett’s esophagus, obesity, alcohol, and tobacco consumption cannot adequately explain this profound gender difference. The dramatic gender difference for esophageal adenocarcinoma suggests there should be a gender-related mechanism underpinning this phenomenon. Estrogens, the primary female sex hormones, are mechanistically linked to aspects of cancer risk and cancer development. Therefore it seems reasonable to consider that estrogens might contribute towards the gender difference for esophageal adenocarcinoma.
A link between estrogen-activated signaling and carcinogenesis in many organs, including mammary glands[10], ovaries and colon[11] has been clearly defined, although it is unclear whether a similar connection exists for the esophagus, and esophageal adenocarcinoma in particular. Additionally, estrogen is actively involved in the regulation of metabolism in adipose tissues[12], and it can be synthesized locally by activated aromatase in adipocytes in both men and women[13-15]. Involvement of estrogen signaling in regulation of adipose tissue metabolism indicates a possible connection between the effects of estrogen and male obesity - one of the main risk factors for esophageal adenocarcinoma. Given the established regulatory role for estrogen in carcinogenesis and metabolic homeostasis for other cancers, and the strong gender differences for the incidence of esophageal adenocarcinoma, it is plausible to suggest that the estrogen signaling network is involved in the progression of this cancer, and an understanding of estrogen and estrogen receptor (ER) roles in the regulation of carcinogenesis, and how this might be relevant in the esophagus, could provide a basis for developing either preventive measures or new treatments.
In this paper we review potential links between estrogen signaling and esophageal adenocarcinoma, to determine whether this might contribute to the dominance of esophageal adenocarcinoma in males. Literature pertinent to gender specific differences in estrogen synthesis, estrogen-regulated carcinogenesis, specific differences between ERs and signaling in cancer cells, and available information about estrogen signaling in esophageal adenocarcinoma is reviewed.
In premenopausal women the ovaries are the principal source of estrogen[16]. Serum estradiol concentration is much higher in premenopausal women, compared to men, but decreases substantially after the menopause, and ultimately becomes lower than in elderly men[17]. Mass spectrometry has shown that average levels of serum estradiol in elderly men are approximately 73 pmol/L, whereas levels in postmenopausal women are markedly lower (about 15 pmol/L)[17].
When the ovaries cease to produce estrogens in postmenopausal women the main characteristics of estradiol function change, and it is produced in extragonadal sites, and acts locally at these sites as a paracrine or intracrine factor[16-20]. These sites are similar in men and postmenopausal women and include the mesenchymal cells of adipose tissue[13], osteoblasts and chondrocytes of bone[21], the vascular endothelium and aortic smooth muscle cells[22], and numerous sites in the brain[16,17,23].
Importantly, in men and postmenopausal women, circulating estrogens are not the main drivers of estrogen action, but locally produced estrogens originating in extragonadal sites are responsible for the majority of paracrine and intracrine effects of these hormones[20]. The total amount of estrogen synthesized by these extragonadal sites may be small, but the local tissue concentrations achieved are probably high and exert biological influence locally. This might impact on tumor biology. For example, it has been determined that the concentration of estradiol present in breast tumors in postmenopausal women is at least 20-fold higher than in the plasma. Aromatase inhibitor therapy is associated with a major decrease in intratumoral concentrations of estradiol and estrone and loss of intratumoral aromatase activity, which is followed by downregulation of cancer cell growth[10,24]. Local estrogen biosynthesis has also been demonstrated in men, where aromatase expression in adipose tissue is greatly increased by this process[25,26]. However, with respect to esophageal adenocarcinoma, no studies have evaluated whether the amount of estrogen synthesized in abdominal adipose tissue is sufficient to exert any paracrine effect in the esophagus.
The effects of estrogens are mediated by their ligation to ERα and ERβ. ERα and ERβ both belong to the nuclear steroid/thyroid hormone receptor family and they are encoded by two distinct genes [encoding estrogen receptor 1 (ESR1) and ESR2] which are located on two different chromosomes 6q25.1 and 14q22-24[27,28]. ERα and ERβ have distinct cellular distributions and regulate separate sets of genes. ERα is predominantly expressed in female sex organs such as the breast, uterus and ovaries especially during the reproductive years. ERβ is widely expressed in many other tissues in both genders, but to a lesser degree in males compared to females[15,29]. Although the role of ERs in male physiology has long been neglected, there is growing evidence for estrogen involvement in multiple areas of male physiology[15-17].
The mechanism for ER signaling has been widely investigated. ERα and ERβ share common functional domains, with a conserved central DNA-binding domain which is often involved in receptor dimerization[30,31]. ERs possess two activation function domains; activation function-1 and activation function-2, with the former interacting with non-ER transcription factors, and the latter containing the ligand binding domain[31,32]. One of the most important differences between ERα and ERβ is that activation function-1 in ERβ lacks functional activity[30]. Also, it has been suggested that the main function of ERβ is to bind ERα and suppress its activation, so that ERα and ERβ as a dimer might exert inverse biological effects. Another difference between ERα and ERβ signaling is their interaction with the activator protein-1[32-35]. The activator protein-1 complex of Jun/Fos hetero- or homo-dimers is a key regulator of cell proliferation, with one of its target genes identified as cyclin D1[33]. Depending on whether ERα or ERβ is activated, the activator protein-1 complex acts in a reciprocal fashion to stimulate or inhibit cell proliferation[35].
After binding estrogen, the receptor ligand-binding domain undergoes a conformational and surface-charge change that results in receptor dimerization. Ligand-binding is accompanied by the dissociation of intracellular ER from chaperone proteins, subsequently releasing the hormone/ER complex for attachment to estrogen response elements in the promoter region of target genes. The dimer then binds DNA to regulate gene expression at specific regions of the DNA named hormone response elements[31,35]. As a consequence, transcription of 17β-estradiol-responsive genes increases, and proliferation or differentiation of steroid-sensitive tissue is augmented. Although most steroid hormone receptors primarily localize to the nuclei, additional ERs have been identified in the cytoplasm and on the plasma membrane. Activation of cytoplasm signaling cascades has been detected after estrogen binding to its plasma membrane receptors[36].
Several isoforms of ERβ able to mediate estrogen signaling have also been found. The isoforms can exert diverse functions, and significantly complicate understanding of cellular responses to estrogens. ERβ isoforms can inhibit ERα transcriptional activity at the estrogen response elements and potentially reverse estrogen signaling[34]. A splice variant of ERβ, termed ERβcx, has been characterized[37]. ERβcx is expressed in the breast[38], the prostate and testis[37], the esophagus[39], and in gastric tissue[40]. Interestingly, ERβcx does not bind estrogen[41]. Instead it inhibits ERα from binding DNA, whilst it does not influence ERβ. The role and mechanism of ERβcx downstream signaling in esophageal tissue is largely unclear and needs to be further investigated.
The biological significance of ERs in breast tumorigenesis has been studied extensively. In breast tumors, ER signaling promotes malignancy due to oncogenic mutations, sustained exposure of ERα with endogenous or exogenous estrogen, and abnormal coupling of estrogen-activated cytoplasmic machinery to growth and anti-apoptosis, all well established causative triggers of cancer in postmenopausal women[41]. Several large prospective studies have confirmed the role of estrogen in stimulation of breast tumor growth, and have demonstrated that the risk of breast cancer is increased in women taking estradiol after the menopause[42-44].
In females with breast cancer, ERα is instrumental in promoting cell proliferation and cancer progression, whereas ERβ exerts anti-proliferative effects by induction of cell cycle and growth arrest[34]. For instance, the downregulation of the cyclin D1 gene by ERβ prevents cellular progression from the G1 to S-phase of the cell cycle[45]. Loss of ERβ expression is considered to be a common feature in estrogen-dependent breast tumor progression[34,35] supporting the hypothesis that ERβ acts as a protector against the mitogenic activity of estrogen in breast pre-malignant tissues.
Estrogen is also critical for the progression of ovarian cancer[46-48]. A strong association between long-term estrogen replacement therapy and increased risk of ovarian cancer has been detected in several studies[45-47]. Similar to breast cancer, the imbalance between ERα and ERβ, along with decreasing expression of ERβ in the ovaries can also lead to uncontrolled cellular proliferation, subsequent malignancy and metastasis[49,50]. Thus, ERβ appears to be pro-apoptotic, facilitating the destruction of malignant cells, whereas ERα has anti-apoptotic activity, indicating its growth stimulatory role[34,45,49]. Confirming the role of ERβ as a tumor-suppressor, deletion of chromosome 14q, where ERβ co-localizes with some other tumor suppressors, is often detected in breast, colon, ovarian and prostate malignant tissue[51-54].
In contrast to the cancer-promoting role of estrogen in breast and ovarian cancers, it has been shown that estrogen works as a cancer suppressor for several gastrointestinal malignancies[41,42,44-56]. The Women’s Health Initiative study, which included a cohort of 16 608 women randomized to hormone replacement therapy (HRT) vs no HRT, showed that the risk of colorectal cancer was almost halved in women using HRT[55]. A similar study in the United Kingdom of patients with esophageal and gastric cancer concluded that HRT was associated with a 50% reduction in the risk of gastric and colon adenocarcinoma, but had no significant benefit for esophageal adenocarcinoma[56]. However, due to the relatively small number of females with esophageal adenocarcinoma in this study (n = 299), the power of the study was limited and the question remains, thus, unresolved[41,42].
The male predominance of approximately 2:1 in gastric cancer incidence across the world cannot be explained on the basis of gender differences for the pre-valence of known risk factors[57]. It has been hypothesized that estrogens play a protective role against gastric cancer. This statement has gained further support from a clinical study of a male cohort of patients with prostate cancer. In this study the risk of developing gastric cancer was lower amongst those who had been treated with estrogen than in those without such treatment (standardized incidence ratio, 0.87; 95% confidence interval, 0.78-0.98)[58]. Further supporting this argument are studies which have shown decreased ERβ expression in other gastrointestinal cancers, such as colon cancer, compared to benign tumors and normal tissues[59]. Tamoxifen exposure has also been shown to be a risk factor for gastric cancer[60,61], adding support to the idea that estrogen signaling has a protective role against gastrointestinal cancer.
There is a growing appreciation that estrogens are not only directly involved in the reproductive process and in regulation of carcinogenesis, but also have general metabolic roles in both sexes[15-17]. Estrogen signaling has a complex relationship with obesity that differs for premenopausal and postmenopausal women[12]. Importantly, obesity is a risk factor for esophageal adenocarcinoma in both women[62] and men[63]. In a recent study of 23 women with esophageal adenocarcinoma[63], 21 (91.3%) were in the top half of the distribution of the studied cohort with regard to waist-to-hip ratio, waist circumference, and body mass index. Multiple studies of male cohorts have demonstrated a strong association between increased abdominal diameter and esophageal adenocarcinoma, after controlling for body mass index and gastroesophageal reflux[63-68]. It is possible that associations between obesity and esophageal cancer are similar for both sexes, even though the regulation of adiposity in men and women differs significantly. For instance, distribution of body fat in men is characterized by the accumulation of visceral fat, but in women by subcutaneous fat.
Subcutaneous and visceral fat tissues express variable levels of both types of ER[69-71]. However, only ERα has a significant influence on energy homeostasis. The role of ERα in estradiol regulation of body weight and obesity is supported by the following observations: (1) both male and female mice that have been genetically altered to reduce the ability to produce estrogen by knocking out aromatase (an enzyme that catalyzes the conversion of androgen to estrogen) became obese when fed the same amounts as normal mice[72]; and (2) increased white adipose tissue and body fat were seen in both sexually mature male and female ERα-knockout mice[73,74]. Further supporting a role for estrogen signaling through ERα in the regulation of body weight are the findings that abnormal adiposity has been associated with the XbaI polymorphism of the human ERα gene[75,76].
The role of ERβ in estradiol regulation of body weight and obesity is less clear and somewhat controversial suggesting that ERβ functions more as a modulator of estrogen actions[71].
Estrogen has also been shown to contribute to the regulation of body adiposity and fat distribution through ERs in the brain[77], and by interacting with leptin signaling pathways[78]. 17β-estradiol increases leptin mRNA levels in adipose tissue[79]. Consistently, estrogen deficiency impairs central leptin sensitivity[77,78]. In women, leptin fluctuations during the menstrual cycle correlate directly with secretion of estrogen[79,80]. Estrogen has also been found to influence leptin receptor expression and hypothalamic sensitivity to leptin driving subcutaneous body fat accrual over visceral fat during the estrous cycle in rats[81]. Hence, visceral fat varies inversely with estrogen levels. Visceral fat accumulates in females when circulating estrogen levels become sufficiently low, as in postmenopausal women[76,78,82]. The accumulation of visceral fat is associated with an increased risk of various gastrointestinal malignancies, including esophageal adenocarcinoma[83]. Thus, estrogen regulation of leptin levels in women may play a protective role, directing accumulation of subcutaneous in preference to visceral fat.
The situation for men, however, is less clear, although a high level of leptin is considered to be a risk factor for males to develop esophageal adenocarcinoma[63,83]. Speculatively, the production of, and sensitivity to, leptin in men may be increased in visceral fat, and locally in tissues located in close proximity to adipose tissue where estrogen synthesis may be increased. However, mechanisms of ER and leptin signaling in males remain obscure, mostly because the majority of laboratory findings and clinical investigations of leptin and estrogen signaling have used tissues from females. To address this issue, studies are needed that specifically address the role of estrogen signaling in male adipose tissue.
In 1998, Lagergren et al[83] hypothesized that high estrogen and/or progesterone levels, low testosterone, or a combination of both, might contribute to the lower incidence of esophageal carcinoma in women. Epidemiological data for esophageal adenocarcinoma demonstrates a profound gender difference, with the male:female ratio exceeding 8:1, strongly supporting this hypothesis[1-4]. There are no detailed studies that compare the expression of ERs in esophageal tissues between males and females, but a limited number of studies have provided some preliminary data comparing ER expression in esophageal adenocarcinoma and its precursor lesion, Barrett’s esophagus. These studies are summarized in Table 1.
| No. of patients with EAC | ERα | ERβ | Conclusion | |
| Akgun et al[84] | 31 | Not expressed | Increased expression as esophageal lesions progressed | ERβ is suggested as a EAC therapy target |
| Tiffin et al[85] | 20 (8) | Type of ER was not specified; ER were detected in EAC patients | ER may be important for further investigation | |
| Liu et al[39] | 33 | Not expressed | Expressed in EAC, but not in Barrett’s esophagus | Anti-estrogen treatment could be a promising therapeutic target for EAC |
| Kalayaransan et al[86] | 45 (15) | Not expressed | Detected in all 45 patients; Expressed higher in EAC, compared to normal esophageal mucosa | ERβ suggested as marker and/or prognostic factor |
In contrast to the anti-tumor role of ERβ in other cancers, some studies have identified a positive association between ERβ expression and esophageal adenocarcinoma development. Akgun et al[84] determined ERβ expression in the esophageal mucosa from patients with Barrett’s metaplasia negative for dysplasia, Barrett’s metaplasia with low grade dysplasia and Barrett’s metaplasia with high grade dysplasia. The results of this study showed significant expression of ERβ (more than 50% of cells positive) in all patients with esophageal adenocarcinoma, and there was a trend towards increased expression of ERβ as the esophageal lesions progressed[85]. These results raise the possibility of ERβ as a target of therapy for esophageal adenocarcinoma. Similarly, another investigation showed a moderate increase in ER expression in tissue samples from men and women with Barrett’s esophagus and esophageal adenocarcinoma. However, the subtype of ER was not determined in this study[39].
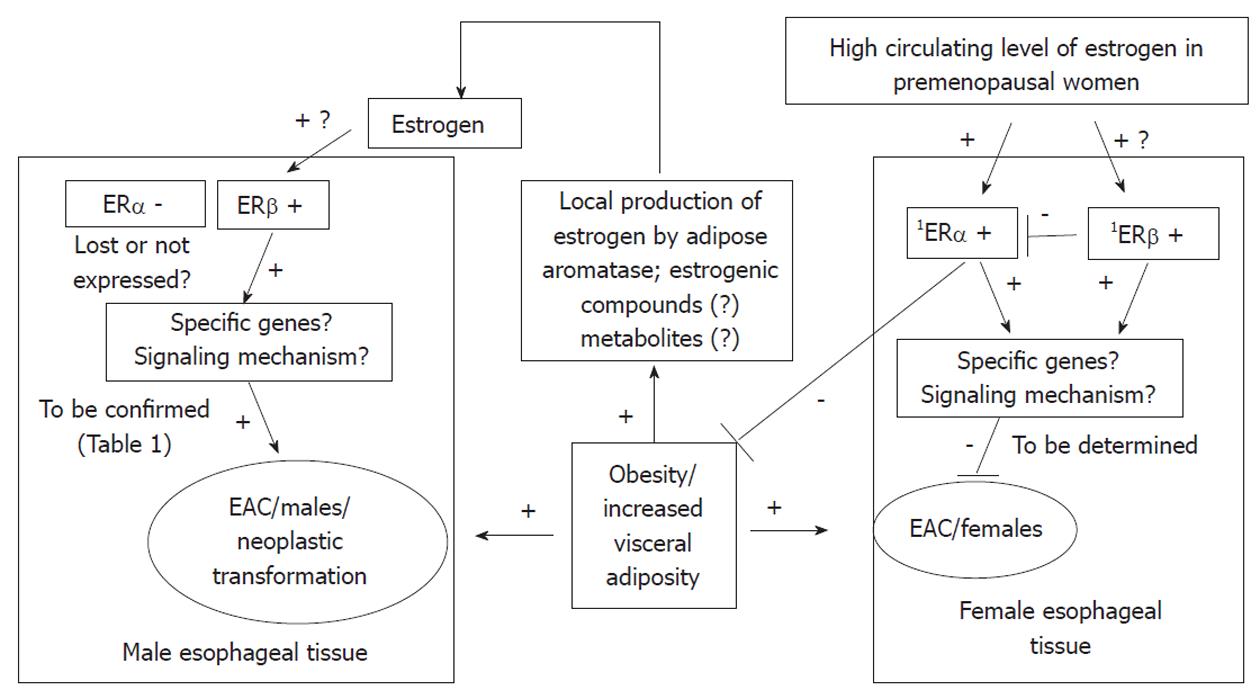
As ERβ has several isoforms, and these isoforms have different functions, Liu et al[39] identified which isoforms of ERβ were expressed in esophageal adenocarcinoma but not in Barrett’s esophagus. All isoforms of ERβ showed much higher expression in esophageal adenocarcinoma, than in its precursor lesion, Barrett’s esophagus. Thus, a possible role for ERβ isoforms in the maintenance and evolution of esophageal adenocarcinoma was suggested. Although the study did not find a correlation between immunoreactivity and cancer proliferative activity, it showed that ERβ1 tended to have higher expression in invasive tumors which had penetrated the full thickness of the esophageal wall, compared to tumors limited to the esophageal wall (P = 0.05), and ERβ1 immunostaining tended to be most prominent in invasive esophageal adenocarcinoma. Conclusively, the study detected the presence of ERβ isoforms in esophageal adenocarcinoma and suggested the potential use of anti-estrogen treatment as a therapeutic target for esophageal adenocarcinoma. Manipulation of ERβ signaling may be considered as a potential prevention strategy to delay or block progression from dysplasia to esophageal adenocarcinoma. Figure 1 summarizes a potential mechanism for interaction between estrogen, ERs and esophageal adenocarcinoma.
Another study by Kalayaransan et al[86] determined the expression of ERα and ERβ in esophageal adenocarcinoma across various classifications of tumor stage, and compared expression with adjacent normal esophageal mucosa. No significant expression levels of ERα were found in esophageal adenocarcinoma, suggesting ERα is unlikely to mediate the growth of esophageal adenocarcinoma. However, immunostaining with ERβ antibodies yielded significantly higher results in esophageal adenocarcinoma, compared to normal esophageal mucosa[87]. In each group with the same degree of tumor differentiation, tumor samples had significantly higher staining scores compared to normal esophageal mucosa. Tumors with good or moderate differentiation had lower staining scores than those which were poorly differentiated, indicating that the potential effect of estrogen on esophageal adenocarcinoma could be mediated by ERβ[84,86,87]. Overall, most studies that have evaluated esophageal adenocarcinoma are consistent in suggesting a detrimental effect and prognostic value for ERβ.
Unfortunately, these clinical findings have not yet been supported by in vitro experiments using esophageal adenocarcinoma cells. The few in vitro studies that have addressed the role of estrogen in the regulation of esophageal cell growth were conducted using squamous cancer cells[87,88]. It has been shown that the growth of an ER-positive esophageal squamous carcinoma cell line (ES-25C) is significantly inhibited by 17β-estradiol, whereas this effect is not observed in an ER-negative squamous carcinoma cell line (ES-8C)[87]. A similar finding was seen in another study, in which the proliferation of the ER-positive KSE-1 esophageal squamous carcinoma cell line was inhibited by 17β-estradiol[88]. In addition, in vivo growth of this cell line in both female and male mice was suppressed by the administration of 17β-estradiol, raising the possibility of manipulating the growth of esophageal carcinoma by manipulating the estrogen-ER system[88]. However, esophageal squamous cell carcinoma and esophageal adenocarcinoma are two biologically distinct diseases, so estrogen responsiveness in squamous cell carcinoma lines does not automatically mean that esophageal adenocarcinoma cell lines will also respond. Similar experiments need to be performed on esophageal adenocarcinoma cell lines in order to explore this possibility further.
Current literature provides only limited evidence for a link between estrogen and the development of esophageal adenocarcinoma. Hence, a series of questions can be proposed, and further studies will be needed to determine whether there is any link. It is unclear whether there is a gender difference for the expression of ERβ, or correlation between tumor stage and the expression of ERβ. Most previous studies have not compared estrogen effects in both genders, and have only addressed men and women separately. Detailed comparisons have not been done for various esophageal pathologies vs normal esophageal mucosa within both gender groups. Another limitation of previous studies is the small number of patients studied, and for this reason reported data is yet to be verified. A systematic study which includes a sufficiently large number of men and women is needed to determine whether, within each gender group, ERβ expression is associated with the development and progression of esophageal adenocarcinoma. A confirmed link might provide support for ERβ to be used as a target for therapy, or as a prognostic marker.
| 1. | Armstrong RW, Borman B. Trends in incidence rates of adenocarcinoma of the oesophagus and gastric cardia in New Zealand, 1978-1992. Int J Epidemiol. 1996;25:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Hansson LE, Sparén P, Nyrén O. Increasing incidence of both major histological types of esophageal carcinomas among men in Sweden. Int J Cancer. 1993;54:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Lepage C, Rachet B, Jooste V, Faivre J, Coleman MP. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol. 2008;103:2694-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 194] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 4. | Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 528] [Cited by in RCA: 536] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 5. | Powell J, McConkey CC, Gillison EW, Spychal RT. Continuing rising trend in oesophageal adenocarcinoma. Int J Cancer. 2002;102:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Hansen S, Wiig JN, Giercksky KE, Tretli S. Esophageal and gastric carcinoma in Norway 1958-1992: incidence time trend variability according to morphological subtypes and organ subsites. Int J Cancer. 1997;71:340-344. [PubMed] |
| 7. | McKinney A, Sharp L, Macfarlane GJ, Muir CS. Oesophageal and gastric cancer in Scotland 1960-90. Br J Cancer. 1995;71:411-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13561] [Article Influence: 645.8] [Reference Citation Analysis (3)] |
| 9. | Nordenstedt H, El-Serag H. The influence of age, sex, and race on the incidence of esophageal cancer in the United States (1992-2006). Scand J Gastroenterol. 2011;46:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Russo J, Russo IH. Breast development, hormones and cancer. Adv Exp Med Biol. 2008;630:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Chen JQ, Brown TR, Yager JD. Mechanisms of hormone carcinogenesis: evolution of views, role of mitochondria. Adv Exp Med Biol. 2008;630:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Rose DP, Vona-Davis L. Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas. 2010;66:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Zhao Y, Nichols JE, Bulun SE, Mendelson CR, Simpson ER. Aromatase P450 gene expression in human adipose tissue. Role of a Jak/STAT pathway in regulation of the adipose-specific promoter. J Biol Chem. 1995;270:16449-16457. [PubMed] |
| 14. | Zhou C, Zhou D, Esteban J, Murai J, Siiteri PK, Wilczynski S, Chen S. Aromatase gene expression and its exon I usage in human breast tumors. Detection of aromatase messenger RNA by reverse transcription-polymerase chain reaction. J Steroid Biochem Mol Biol. 1996;59:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Sharpe RM. The roles of oestrogen in the male. Trends Endocrinol Metab. 1998;9:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 140] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Simpson E, Rubin G, Clyne C, Robertson K, O'Donnell L, Jones M, Davis S. The role of local estrogen biosynthesis in males and females. Trends Endocrinol Metab. 2000;11:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Simpson ER, Clyne C, Speed C, Rubin G, Bulun S. Tissue-specific estrogen biosynthesis and metabolism. Ann N Y Acad Sci. 2001;949:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Labrie F, Cusan L, Gomez JL, Martel C, Bérubé R, Bélanger P, Bélanger A, Vandenput L, Mellström D, Ohlsson C. Comparable amounts of sex steroids are made outside the gonads in men and women: strong lesson for hormone therapy of prostate and breast cancer. J Steroid Biochem Mol Biol. 2009;113:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Labrie F, Bélanger A, Cusan L, Candas B. Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: intracrinology. J Clin Endocrinol Metab. 1997;82:2403-2409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 131] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Vandenput L, Ohlsson C. Estrogens as regulators of bone health in men. Nat Rev Endocrinol. 2009;5:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Cho JJ, Cadet P, Salamon E, Mantione K, Stefano GB. The nongenomic protective effects of estrogen on the male cardiovascular system: clinical and therapeutic implications in aging men. Med Sci Monit. 2003;9:RA63-RA68. [PubMed] |
| 23. | Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126:2-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Huiart L, Dell'Aniello S, Suissa S. Use of tamoxifen and aromatase inhibitors in a large population-based cohort of women with breast cancer. Br J Cancer. 2011;104:1558-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Dieudonné MN, Sammari A, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Sex steroids and leptin regulate 11beta-hydroxysteroid dehydrogenase I and P450 aromatase expressions in human preadipocytes: Sex specificities. J Steroid Biochem Mol Biol. 2006;99:189-196. [PubMed] |
| 26. | Wilson JD, Aiman J, MacDonald PC. The pathogenesis of gynecomastia. Adv Intern Med. 1980;25:1-32. [PubMed] |
| 27. | McInerney EM, Tsai MJ, O'Malley BW, Katzenellenbogen BS. Analysis of estrogen receptor transcriptional enhancement by a nuclear hormone receptor coactivator. Proc Natl Acad Sci USA. 1996;93:10069-10073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 170] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258-4265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 281] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Rochira V, Granata AR, Madeo B, Zirilli L, Rossi G, Carani C. Estrogens in males: what have we learned in the last 10 years? Asian J Androl. 2005;7:3-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Delaunay F, Pettersson K, Tujague M, Gustafsson JA. Functional differences between the amino-terminal domains of estrogen receptors alpha and beta. Mol Pharmacol. 2000;58:584-590. [PubMed] |
| 31. | Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535-1565. [PubMed] |
| 32. | Hayashi SI, Eguchi H, Tanimoto K, Yoshida T, Omoto Y, Inoue A, Yoshida N, Yamaguchi Y. The expression and function of estrogen receptor alpha and beta in human breast cancer and its clinical application. Endocr Relat Cancer. 2003;10:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, Price RH, Pestell RG, Kushner PJ. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem. 2002;277:24353-24360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 335] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 34. | Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11:537-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 319] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 35. | Chen GG, Zeng Q, Tse GM. Estrogen and its receptors in cancer. Med Res Rev. 2008;28:954-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 36. | Levin ER, Pietras RJ. Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res Treat. 2008;108:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Ogawa S, Inoue S, Watanabe T, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor ofestrogen action in human. Nucleic Acids Res. 1998;26:3505-3512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 332] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 38. | Palmieri C, Lam EW, Mansi J, MacDonald C, Shousha S, Madden P, Omoto Y, Sunters A, Warner M, Gustafsson JA. The expression of ER beta cx in human breast cancer and the relationship to endocrine therapy and survival. Clin Cancer Res. 2004;10:2421-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Liu L, Chirala M, Younes M. Expression of estrogen receptor-beta isoforms in Barrett's metaplasia, dysplasia and esophageal adenocarcinoma. Anticancer Res. 2004;24:2919-2924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Hankinson SE, Colditz GA, Willett WC. Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res. 2004;6:213-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 180] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 41. | Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872-881. [PubMed] |
| 42. | Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 664] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 43. | Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11239] [Cited by in RCA: 10563] [Article Influence: 440.1] [Reference Citation Analysis (1)] |
| 44. | Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 922] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 45. | Lacey JV, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, Schatzkin A, Schairer C. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 456] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 46. | Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, Liu J, McNeeley SG, Lopez AM. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial. JAMA. 2003;290:1739-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 374] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 47. | Folsom AR, Anderson JP, Ross JA. Estrogen replacement therapy and ovarian cancer. Epidemiology. 2004;15:100-104. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Pujol P, Rey JM, Nirde P, Roger P, Gastaldi M, Laffargue F, Rochefort H, Maudelonde T. Differential expression of estrogen receptor-alpha and -beta messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Res. 1998;58:5367-5373. [PubMed] |
| 49. | Rutherford T, Brown WD, Sapi E, Aschkenazi S, Muñoz A, Mor G. Absence of estrogen receptor-beta expression in metastatic ovarian cancer. Obstet Gynecol. 2000;96:417-421. [PubMed] |
| 50. | Bandera CA, Takahashi H, Behbakht K, Liu PC, LiVolsi VA, Benjamin I, Morgan MA, King SA, Rubin SC, Boyd J. Deletion mapping of two potential chromosome 14 tumor suppressor gene loci in ovarian carcinoma. Cancer Res. 1997;57:513-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Young J, Leggett B, Gustafson C, Ward M, Searle J, Thomas L, Buttenshaw R, Chenevix-Trench G. Genomic instability occurs in colorectal carcinomas but not in adenomas. Hum Mutat. 1993;2:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Loveday RL, Greenman J, Simcox DL, Speirs V, Drew PJ, Monson JR, Kerin MJ. Genetic changes in breast cancer detected by comparative genomic hybridisation. Int J Cancer. 2000;86:494-500. [PubMed] |
| 53. | Kasahara K, Taguchi T, Yamasaki I, Kamada M, Yuri K, Shuin T. Detection of genetic alterations in advanced prostate cancer by comparative genomic hybridization. Cancer Genet Cytogenet. 2002;137:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, Rosenberg CA, Taylor VM, Harris R, Chen C. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 474] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 55. | Lindblad M, García Rodríguez LA, Chandanos E, Lagergren J. Hormone replacement therapy and risks of oesophageal and gastric adenocarcinomas. Br J Cancer. 2006;94:136-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002;5:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 57. | Lindblad M, Ye W, Rubio C, Lagergren J. Estrogen and risk of gastric cancer: a protective effect in a nationwide cohort study of patients with prostate cancer in Sweden. Cancer Epidemiol Biomarkers Prev. 2004;13:2203-2207. [PubMed] |
| 58. | Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G, Papavassiliou AG. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. Eur J Cancer. 2003;39:1251-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 251] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 59. | Rutqvist LE, Johansson H, Signomklao T, Johansson U, Fornander T, Wilking N. Adjuvant tamoxifen therapy for early stage breast cancer and second primary malignancies. Stockholm Breast Cancer Study Group. J Natl Cancer Inst. 1995;87:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 334] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 60. | Curtis RE, Boice JD, Shriner DA, Hankey BF, Fraumeni JF. Second cancers after adjuvant tamoxifen therapy for breast cancer. J Natl Cancer Inst. 1996;88:832-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Bodelon C, Anderson GL, Rossing MA, Chlebowski RT, Ochs-Balcom HM, Vaughan TL. Hormonal factors and risks of esophageal squamous cell carcinoma and adenocarcinoma in postmenopausal women. Cancer Prev Res (Phila). 2011;4:840-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Whiteman DC, Sadeghi S, Pandeya N, Smithers BM, Gotley DC, Bain CJ, Webb PM, Green AC. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 63. | Edelstein ZR, Farrow DC, Bronner MP, Rosen SN, Vaughan TL. Central adiposity and risk of Barrett's esophagus. Gastroenterology. 2007;133:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 64. | MacInnis RJ, English DR, Hopper JL, Giles GG. Body size and composition and the risk of gastric and oesophageal adenocarcinoma. Int J Cancer. 2006;118:2628-2631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Corley DA, Kubo A, Levin TR, Block G, Habel L, Zhao W, Leighton P, Quesenberry C, Rumore GJ, Buffler PA. Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology. 2007;133:34-41; quiz 311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 263] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 66. | Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:352-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 67. | Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4204] [Cited by in RCA: 3770] [Article Influence: 209.4] [Reference Citation Analysis (1)] |
| 68. | Mizutani T, Nishikawa Y, Adachi H, Enomoto T, Ikegami H, Kurachi H, Nomura T, Miyake A. Identification of estrogen receptor in human adipose tissue and adipocytes. J Clin Endocrinol Metab. 1994;78:950-954. [PubMed] [DOI] [Full Text] |
| 69. | Price TM, O'Brien SN. Determination of estrogen receptor messenger ribonucleic acid (mRNA) and cytochrome P450 aromatase mRNA levels in adipocytes and adipose stromal cells by competitive polymerase chain reaction amplification. J Clin Endocrinol Metab. 1993;77:1041-1045. [PubMed] [DOI] [Full Text] |
| 70. | Schomberg DW, Couse JF, Mukherjee A, Lubahn DB, Sar M, Mayo KE, Korach KS. Targeted disruption of the estrogen receptor-alpha gene in female mice: characterization of ovarian responses and phenotype in the adult. Endocrinology. 1999;140:2733-2744. [PubMed] [DOI] [Full Text] |
| 71. | Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735-12740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 563] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 72. | Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97:12729-12734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1015] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 73. | Cooke PS, Heine PA, Taylor JA, Lubahn DB. The role of estrogen and estrogen receptor-alpha in male adipose tissue. Mol Cell Endocrinol. 2001;178:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 74. | Speer G, Cseh K, Winkler G, Vargha P, Braun E, Takács I, Lakatos P. Vitamin D and estrogen receptor gene polymorphisms in type 2 diabetes mellitus and in android type obesity. Eur J Endocrinol. 2001;144:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 75. | Bjørntorp P. Hormonal effects on fat distribution and its relationship to health risk factors. Acta Paediatr Suppl. 1992;383:59-60; discussion 61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 430] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 76. | Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25:1680-1688. [PubMed] |
| 77. | Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978-987. [PubMed] |
| 78. | Quinton ND, Laird SM, Okon MA, Li TC, Smith RF, Ross RJ, Blakemore AI. Serum leptin levels during the menstrual cycle of healthy fertile women. Br J Biomed Sci. 1999;56:16-19. [PubMed] |
| 79. | Quinton ND, Smith RF, Clayton PE, Gill MS, Shalet S, Justice SK, Simon SA, Walters S, Postel-Vinay MC, Blakemore AI. Leptin binding activity changes with age: the link between leptin and puberty. J Clin Endocrinol Metab. 1999;84:2336-2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 246] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 81. | Morita Y, Iwamoto I, Mizuma N, Kuwahata T, Matsuo T, Yoshinaga M, Douchi T. Precedence of the shift of body-fat distribution over the change in body composition after menopause. J Obstet Gynaecol Res. 2006;32:513-516. [PubMed] |
| 82. | Howard JM, Beddy P, Ennis D, Keogan M, Pidgeon GP, Reynolds JV. Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br J Surg. 2010;97:1020-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 83. | Lagergren J, Nyrén O. Do sex hormones play a role in the etiology of esophageal adenocarcinoma? A new hypothesis tested in a population-based cohort of prostate cancer patients. Cancer Epidemiol Biomarkers Prev. 1998;7:913-915. [PubMed] |
| 84. | Akgun H, Lechago J, Younes M. Estrogen receptor-beta is expressed in Barrett's metaplasia and associated adenocarcinoma of the esophagus. Anticancer Res. 2002;22:1459-1461. [PubMed] |
| 85. | Tiffin N, Suvarna SK, Trudgill NJ, Riley SA. Sex hormone receptor immunohistochemistry staining in Barrett's oesophagus and adenocarcinoma. Histopathology. 2003;42:95-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Kalayarasan R, Ananthakrishnan N, Kate V, Basu D. Estrogen and progesterone receptors in esophageal carcinoma. Dis Esophagus. 2008;21:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Utsumi Y, Nakamura T, Nagasue N, Kubota H, Harada T, Morikawa S. Effect of 17 beta-estradiol on the growth of an estrogen receptor-positive human esophageal carcinoma cell line. Cancer. 1991;67:2284-2289. [PubMed] |
| 88. | Ueo H, Matsuoka H, Sugimachi K, Kuwano H, Mori M, Akiyoshi T. Inhibitory effects of estrogen on the growth of a human esophageal carcinoma cell line. Cancer Res. 1990;50:7212-7215. [PubMed] |
Peer reviewer: Guy D Eslick, PhD, MmedSc (Clin Epi), MmedStat, Department of Medicine, The University of Sydney Nepean Hospital, Level 5, South Block, PO Box 63, Penrith, Sydney NSW 2751, Australia
S- Editor Tian L L- Editor Cant MR E- Editor Li JY