Published online Nov 21, 2012. doi: 10.3748/wjg.v18.i43.6324
Revised: August 22, 2012
Accepted: August 25, 2012
Published online: November 21, 2012
Granular cell tumor (GCT) of the biliary system is rare. It is reported that it occurs more commonly in young black women. We report here our seldom experience of a Japanese case in whom icterus was found as a first symptom just after a caesarean operation. A 36-year-old Japanese woman developed icterus after delivery by the Caesarean operation. A surgical operation was performed without can deny that there was a tumor-related change in a bile duct as a result of examination for various images. As a result of pathological evaluation, GCT was diagnosed. By the preoperative organization biomicroscopy result, it was not able to be attachd a right diagnosis. It was thought that this tumor, although rare, should be considered as one of the causes of biliary stenosis in the younger population.
- Citation: Saito J, Kitagawa M, Kusanagi H, Kano N, Ishii E, Nakaji S, Hirata N, Hoshi K. Granular cell tumor of the common bile duct: A Japanese case. World J Gastroenterol 2012; 18(43): 6324-6327
- URL: https://www.wjgnet.com/1007-9327/full/v18/i43/6324.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i43.6324
Granular cell tumor was initially described by Abrikossoff in 1926; it was found in the skeletal muscle of the tongue[1], and first reported in the biliary system in 1952 by Coggins[2]. A lot of researchers favor the theory that such tumors are generated from Schwann cells. The theory of Schwann cell origin is based on histological, electron microscopic, and immunohistochemical findings[3,4]. The tumors generally arise in the oral cavity, skin, and subcutaneous tissues, and less than 1% of granular cell tumor (GCT) occurs in the biliary tract[5,6]. To date, there have been no reports on malignant GCT in bile duct. Most patients suffering from GCT in the bile duct are young, female, and black. Benign tumors in bile duct are rare (4%) and GCT in bile duct is very rare[5].
This is a Japanese case report of bile duct GCT. This case was first suspected of being adenocarcinoma by the result of tumor biopsy on endoscopic retrograde cholangiopancreatography (ERCP) before the operation, as this was the result in other previous cases.
A 36-year-old Japanese woman was hospitalized for toxemia during pregnancy. She had hypertension and renal disorder at gestational age 30 wk and 2 d. Her blood pressure was 160-170/100 mmHg with albuminuria. Because her condition was resistant to treatment, she gave birth to twins by preparative Caesarean section at gestational age 32 wk and 2 d. During surgical procedures, her and her babies’ general conditions were good. The Caesarean section was completed without noticeable complications.
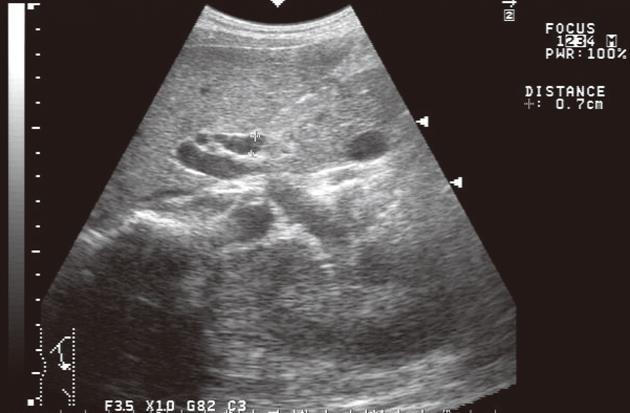
After delivery, her systolic arterial pressure decreased to 120 mmHg and the proteinuria disappeared in several weeks. Three days after the delivery, liver enzymes and bile tract enzymes suddenly increased. In addition, six days after the delivery, icterus was recognized (Table 1). Serum bilirubin increased to 5.0 mg/dL (direct bilirubin 4.0 mg/dL) in 3 d. At first, the liver damage was regarded as a side effect of the various medicines. For example, Cephalosporin antibiotic was administered on the day and the day after the Caesarean operation and Ca blocker was prescribed for the purpose of lowering the blood pressure. Abdominal ultrasonography showed a swollen gallbladder, as well as debris associated with it. However, for the common bile duct (CBD), no expansion was observed given its diameter of 0.7 cm in the measurement (Figure 1). Tumor shadows or stenosis of CBD were also recognized. Contrast-enhanced abdominal computed tomography and magnetic resonance cholangiopancreatography also showed about 10 mm long stenosis and wall thickening in the middle part of the bile duct. Tumor marker Carcinoembryonic antigen was 2.0 ng/mL (normal 0-5 ng/dL) and carbohydrate antigen 19-9 was 32.0 U/mL (0-37 U/mL).
| Complete blood count | Blood chemistry |
| WBC 6000 /μL (3500-9800/μL) | AST 88 IU/L (6-40 IU/L) |
| Hb 12.2 g/dL (11.0-15.3 g/dL) | ALT 132 IU/L (0-35 IU/L) |
| PLT 31.8 × 104/μL (13.0-37.0 × 104/μL) | LDH 580 IU/L (160-420 IU/L) |
| Blood coagulation test | ALP 3016 IU/L (115-360 IU/L) |
| PT-INR 0.93 (0.8-1.3) | γGTP 189 IU/L (5-45 IU/L) |
| Tumor markers | T-bil 5.0 mg/dL (0.2-1.0 mg/dL) |
| CEA 2.0 ng/mL (0-5 ng/mL) | D-bil 4.0 mg/dL (0.0-0.4 mg/dL) |
| CA19-9 32.0 U/mL (0-37 U/mL) | Amy 81 IU/L (50-220 IU/L) |
| CRP 1.09 mg/dL (0.01-0.4 mg/dL) |
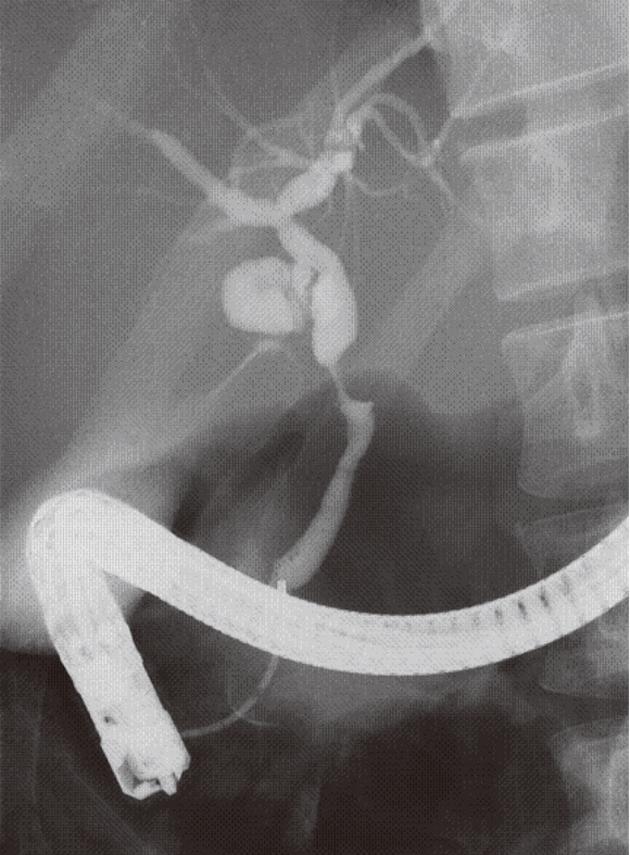
ERCP suggested there was a 6.1 mm-long stenosis at the middle part of the bile duct. The shape of the tumor was nodular (Figure 2). Mucosal layer (m) and fibromucosal layer (fm) thickening was identified by intraductal ultrasonography. Endoscopic brush cytology of the bile duct resulted in a status of “quantity not sufficient” and findings of forceps biopsy were atypical but inconclusive. In spite of these investigations, bile duct carcinoma could not be ruled out, so pancreaticoduodenectomy was performed 37 d after the Caesarean section.
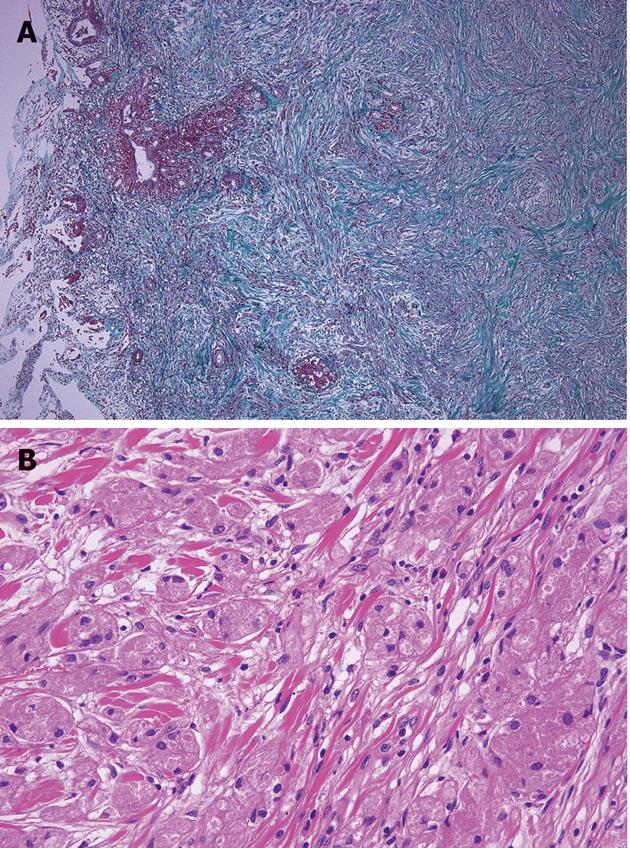
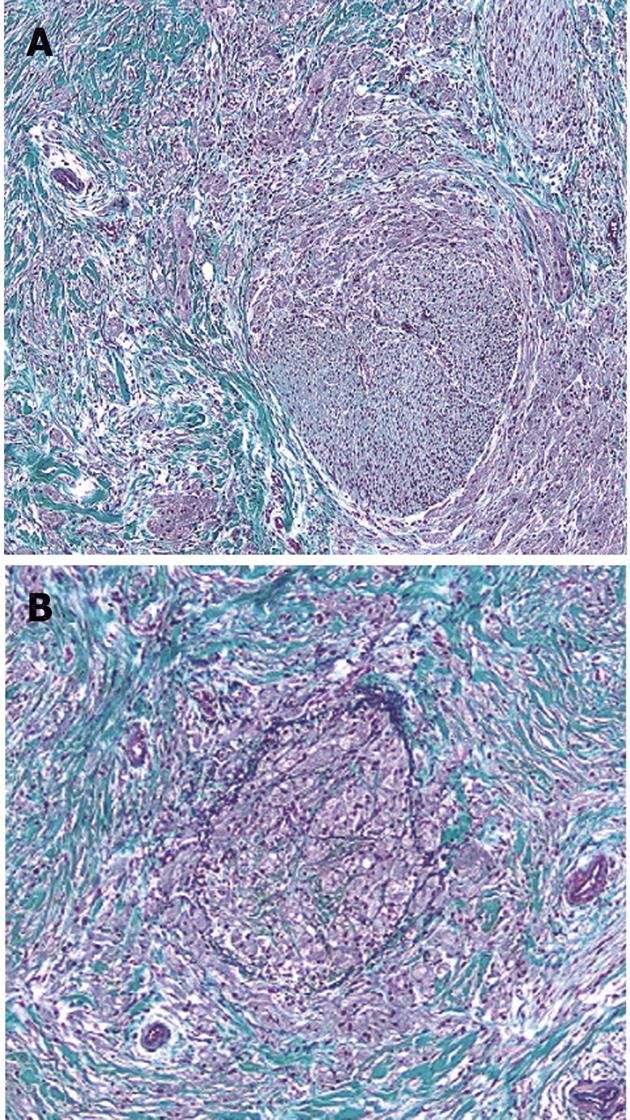
At the operation, the tumor felt hard to touch in liver-duodenum ligament. Lymph nodes around the aorta and the lymph node on the posterior surface of the pancreatic head were slightly swollen, but they were free of malignancy on frozen section. The tumor was a poorly circumscribed nodule, measuring 9.0 mm× 5.5 mm, located at the lower portion of the CBD at the level of the upper margin of the pancreas and involved all layers of the bile duct wall (Figure 3A). Microscopically, tumors were rounded or polygonal with eosinophilic granular cytoplasm, arranged in nests and cords separated by fibrous tissue septa (Figure 3B). The GCT cells partly infiltrated a peripheral nerve fibrous tissue bunch in the bile duct wall. In addition, they infiltrated the vein in the bile duct wall and caused some vein lumina to occlude (Figure 4). Tumor cells are diastase-resistant periodic acid-Schiff stain positive, and immunoreactive for S-100 protein and neuron-specific enolase. The cellular atypia and mitosis were inconspicuous. These findings were compatible with benign GCT. In addition, regional lymph nodes were free of tumor. Five years have passed since the surgery and the patient is well.
Almost all bile duct tumors or stenosis are suspected as being malignant because benign tumors are comparatively rare in the bile duct. In 2003, Principe et al[5] reviewed 179 (90 men and 89 women) patients who were diagnosed with malignant stricture of bile duct and judged to be operable from 1982 to 2001. In 153 cases, an operation was carried out. Of these 147 (96.1%) cases were found to have a malignant cause as gall bladder carcinoma or cholangiocarcinoma. Cases in which radical operation was possible constituted only 24.8% of these. It was judged not to be possible to remove the tumor surgically in the other cases, and only an operation for reducing jaundice was carried out. The remaining 6 cases (4%) were benign strictures and only one of them was GCT (0.7% of the total). Benign tumor in bile duct is rare so almost all cases of stenosis in it tend to be considered as malignant stricture. We searched for “granular cell tumor” and “bile duct” in the Pub-Med database and obtained reports on 47 previous cases of GCT of the bile duct. We verified 48 cases including our case.
Most of the patients were young. The average age at diagnosis was 33.9 years old (range 11 years to 56 years). Almost all of the patients (81.0%) were female and many were black. In terms of ethnic origin, 60.5% of them were black (including Jamaican and Ethiopian), 31.6% were Caucasoid (including Algerian), and 7.9% of them were Mongoloid (including Japanese and Thai). The chief complaints were jaundice, pain, and a pruritus feeling, which are symptoms caused by obstructive jaundice. There is no specific symptom of this tumor, as forecast. The range of tumor size was 0.5 cm[7] to 4.0 cm[8] and the average was 1.6 cm. As for the site of the tumors, 58.1% were in CBD, 23.3% in common hepatic duct (CHD), 14.0% in cystic duct (CD), and 2.3% in gallbladder and ampulla of Vater. They might tend to be located at confluences with 41.9% of them near the confluence of CBD (CHD and CD and CBD), and 11.6% of them near the confluence of CHD (right and left hepatic ducts). However, the divergence position of the CD was not mentioned in all reports, and some contained vague descriptions, for example, “CBD” or “CD”.
It is generally difficult to make diagnoses as GCT histologically before an operation. A correct diagnosis might be made from several repeated biopsies, as in our case. Because almost all tumors of bile duct are malignant, the diagnosis of “suggested adenocarcinoma” is difficult to the default setting doubt.
Histologically, GCT involves large granular-like eosinophilic cells. It generally occurs in skin and the oral cavity, and two-thirds of cases occur in cutis and submucosa[9]. It is rare in infants, and occurs in adults of 30-40 years old. As for the sex ratio, its prevalence is four times higher in women than in men. Many cases of GCT occurring in bile duct in women, the young, and in black people have been reported. In the literature, GCT is described as being not often diagnosed with histology before an operation. Even if it is diagnosed as benign tumor, surgical operation may be chosen for various reasons. One is that it is not easy to rule out malignant tumor completely because of the frequency of malignant tumor in bile duct. Another reason is that some symptoms like jaundice or pain can be caused, no matter how small it is. The average size was 1.6 cm, which is small. The smallest size was 0.5 cm. Generally, it is difficult to discover small tumors without symptoms. These tumors tend to cause small symptoms, at an early stage.
It occurs at a rate under 2%, but there is malignant GCT[9-11]. There are some cases that relapsed after surgical excision and spread, finally resulting in death. These malignant GCTs are usually larger than 5 cm[12]. However, no one has reported malignant GCT in bile duct, for example, a case of it spreading to other organs, recurring several years later, or invading surrounding tissue. Although only one case resulted in mortality[13], this was not due to primary disease but to postoperative severe adult respiratory distress syndrome and disseminated intravascular coagulopathy with fulminant sepsis, 3 wk after Roux-en-Y hepaticojejunostomy.
Benign tumors in the biliary system are so rare that it is difficult to rules out malignant tumor. If intraoperative frozen section could establish an exact diagnosis, we would be able to select reduction operation depending on the location of GCT in bile duct.
GCT is a rare disease among Asian population. According to past case reports, it is a tumor that tends to occur in the bile duct in young black women. A benign tumor developing in the bile duct is very rare and it is unusual in Asians.
In conclusion, we experienced a case of GCT that occurred in a young Japanese woman. It was thought that this tumor, although rare, should be considered as one of the causes of biliary stenosis in the younger population.
| 1. | Abrikosoff A. Ueber Myome ausgehened von der quergesrteifeten willkuerlichen Muskulatur. Virchows Arch Path Anat. 1926;260:215-233. |
| 2. | Coggins RP. Granular-cell myoblastoma of common bile duct; report of a case with autopsy findings. AMA Arch Pathol. 1952;54:398-402. [PubMed] |
| 3. | Fisher ER, Wechsler H. Granular cell myoblastoma--a misnomer. Electron microscopic and histochemical evidence concerning its Schwann cell derivation and nature (granular cell schwannoma). Cancer. 1962;15:936-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Mori K, Chano T, Yamamoto K, Matsusue Y, Okabe H. Expression of macrophage inflammatory protein-1alpha in Schwann cell tumors. Neuropathology. 2004;24:131-135. [PubMed] |
| 5. | Principe A, Ercolani G, Bassi F, Paolucci U, Raspadori A, Turi P, Beltempo P, Grazi GL, Cavallari A. Diagnostic dilemmas in biliary strictures mimicking cholangiocarcinoma. Hepatogastroenterology. 1962;50:1246-1249. [PubMed] |
| 6. | Bilanović D, Boricić I, Zdravković D, Randjelović T, Stanisavljević N, Toković B. Granular cell tumor of the common hepatic duct presenting as cholangiocarcinoma and acute acalculous cholecystitis. Acta Chir Iugosl. 2008;55:99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Altavilla G, Brotto M, Busatto G, Boccù C, Ragni L. Granular cell tumor of the intrapancreatic common bile duct: one case report and review of the literature. Ultrastruct Pathol. 2004;28:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Balart LA, Hines C, Mitchell W. Granular cell schwannoma of the extrahepatic biliary system. Am J Gastroenterol. 1983;78:297-300. [PubMed] |
| 9. | Weiss SW, Goldblum JR. Benign tumors of peripheral nerves. Soft tissue tumors, Philadelphia, PA: Mosby Elsevier 2008; 878-887. |
| 10. | Tonsi A, Standish R, Desai C, Davidson BR. Granular cell tumour of the bile duct mimicking distal cholangiocarcinoma: one case report and review of the literature. Minerva Chir. 2006;61:247-255. [PubMed] |
| 11. | Simsir A, Osborne BM, Greenebaum E. Malignant granular cell tumor: a case report and review of the recent literature. Hum Pathol. 1996;27:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998;22:779-794. [PubMed] |
Peer reviewers: Sharon DeMorrow, Assistant Professor, Division of Research and Education, Scott and White Hospital and The Texas A and M University System, Health Science Center College of Medicine, Temple, TX 76504, United States; Pietro Invernizzi, MD, PhD, Division of Internal Medicine and Hepatobiliary Immunopathology Unit, IRCCS Istituto Clinico Humanitas, Via A Manzoni 113, 20089 Milan, Italy
S- Editor Gou SX L- Editor A E- Editor Xiong L