Published online Nov 21, 2012. doi: 10.3748/wjg.v18.i43.6235
Revised: July 26, 2012
Accepted: July 29, 2012
Published online: November 21, 2012
AIM: To define which segments of the gastrointestinal tract are most likely to yield angioectasias for ablative therapy.
METHODS: A retrospective chart review was performed for patients treated in the Louisiana State University Health Sciences Center Gastroenterology clinics between the dates of July 1, 2007 and October 1, 2010. The selection of cases for review was initiated by use of our electronic medical record to identify all patients with a diagnosis of angioectasia, angiodysplasia, or arteriovenous malformation. Of these cases, chart reviews identified patients who had a complete evaluation of their gastrointestinal tract as defined by at least one upper endoscopy, colonoscopy and small bowel capsule endoscopy within the past three years. Patients without evidence of overt gastrointestinal bleeding or iron deficiency anemia associated with intestinal angioectasias were classified as asymptomatic and excluded from this analysis. Thirty-five patients with confirmed, bleeding intestinal angioectasias who had undergone complete endoscopic evaluation of the gastrointestinal tract were included in the final analysis.
RESULTS: A total of 127 cases were reviewed. Sixty-six were excluded during subsequent screening due to lack of complete small bowel evaluation and/or lack of documentation of overt bleeding or iron deficiency anemia. The 61 remaining cases were carefully examined with independent review of endoscopic images as well as complete capsule endoscopy videos. This analysis excluded 26 additional cases due to insufficient records/images for review, incomplete capsule examination, poor capsule visualization or lack of confirmation of typical angioectasias by the principal investigator on independent review. Thirty-five cases met criteria for final analysis. All study patients were age 50 years or older and 13 patients (37.1%) had chronic kidney disease stage 3 or higher. Twenty of 35 patients were taking aspirin (81 mg or 325 mg), clopidogrel, and/or warfarin, with 8/20 on combination therapy. The number and location of angioectasis was documented for each case. Lesions were then classified into the following segments of the gastrointestinal tract: esophagus, stomach, duodenum, jejunum, ileum, right colon and left colon. The location of lesions within the small bowel observed by capsule endoscopy was generally defined by percentage of total small bowel transit time with times of 0%-9%, 10%-39%, and 40%-100% corresponding to the duodenum, jejunum and ileum, respectively. Independent review of complete capsule studies allowed for deviation from this guideline if capsule passage was delayed in one or more segments. In addition, the location and number of angioectasias observed in the small bowel was further modified or confirmed by subsequent device-assisted enteroscopy (DAE) performed in the 83% of cases. In our study population, angioectasias were most commonly found in the jejunum (80%) followed by the duodenum (51%), stomach (22.8%), and right colon (11.4%). Only two patients were found to have angioectasias in the ileum (5.7%). Twenty-one patients (60%) had angioectasias in more than one location.
CONCLUSION: Patients being considered for endoscopic ablation of symptomatic angioectasias should undergo push enteroscopy or anterograde DAE and re-inspection of the right colon.
- Citation: Bollinger E, Raines D, Saitta P. Distribution of bleeding gastrointestinal angioectasias in a Western population. World J Gastroenterol 2012; 18(43): 6235-6239
- URL: https://www.wjgnet.com/1007-9327/full/v18/i43/6235.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i43.6235
Intestinal angioectasias are culprit lesions in up to 5%-6% of gastrointestinal bleeding cases and are the most common source of bleeding from the small intestine in patients older than 50-60 years[1]. In patients who experience symptomatic blood loss due to these lesions, approximately 40%-50% will experience recurrent bleeding[2]. Endoscopic ablation of these lesions using bipolar cautery or argon plasma coagulation is a standard therapy to prevent bleeding recurrence[1,3]. However, localization of these lesions can be challenging as they are commonly small, evanescent and/or located deep in the small intestine. These lesions are also multiple in many cases[4]. Understanding the natural distribution of these lesions in the gastrointestinal tract is useful to guide endoscopic evaluation and therapy.
Endoscopic visualization of the entire gastrointestinal tract has not been readily available until the advent of wireless capsule endoscopy and double balloon enteroscopy in 2001[5,6]. Prior to the introduction of these technologies, complete examinations of the intestinal lumen were limited to cases in which intraoperative enteroscopy was performed. Recent studies of the distribution of angioectasias using capsule endoscopy and balloon enteroscopy demonstrate that these lesions are common causes of bleeding from the small intestine. However, the most frequent location of lesions within the small intestine has varied between populations. In Eastern populations, angioectasias have been found to be distributed almost equally between the proximal and distal small intestine (44%-69% and 31%-56%, respectively)[7-9]. These findings are in contrast to a recent study in the United States which found that the vast majority of lesions occur in the jejunum (93%) rather than the ileum (7%)[10]. Anecdotal reports from our center or other centers performing balloon enteroscopy in the United States are similar to the findings of Gerson et al[10].
In order to better define the distribution of angioectasias in the gastrointestinal tract, we studied the location of specific lesions in patients with overt or occult gastrointestinal blood loss attributed to symptomatic angioectasias.
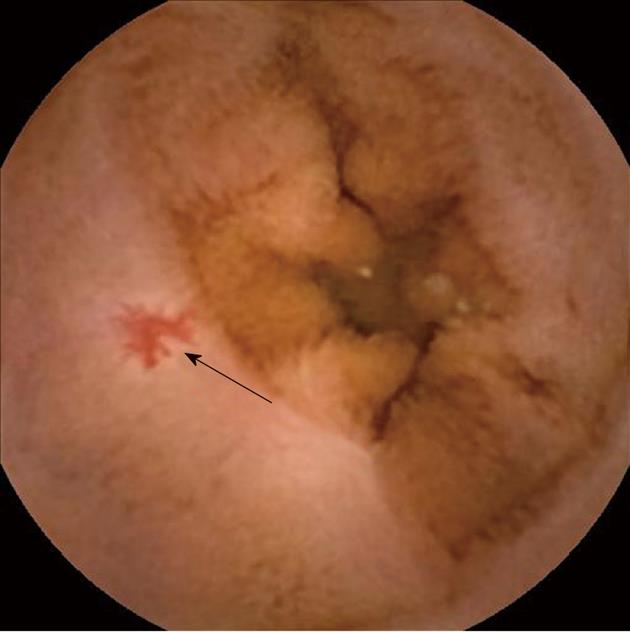
This study was designed as a single-center retrospective chart review. Institutional review board approval was obtained during final protocol development. Prior to study initiation, the terminology regarding intestinal vascular lesions was delineated. Angioectasias were defined pathologically as dilated submucosal veins with overlying ectasia of mucosal venules and capillaries. The term angiodysplasia was used interchangeably with angioectasia although the equivalence of these terms has been debated. Due to the presence of arteriovenous communications in some angioectasias, lesions identified as arteriovenous malformations were also included in this study provided that the observed lesion was not protruding or obviously deep to the submucosa. Using these pathologic definitions as a reference, all superficial lesions 1mm or greater in size which are cherry-red in color with a fern-like pattern were included for analysis (Figure 1). Lesions characterized by pulsatile bleeding but no mucosal defect were generally classified as Dieulafoy lesions and were excluded[1,11].
Selection of cases for review was initiated by use of our electronic medical record to identify cases in which a diagnosis of angioectasia, angiodysplasia, or arteriovenous malformation was made between the dates of July 1, 2007 and October 1, 2010. Of these cases, chart reviews identified patients who had a complete evaluation of their gastrointestinal tract as defined by at least one upper endoscopy, colonoscopy and small bowel capsule endoscopy within the past three years. Patients without evidence of overt gastrointestinal bleeding or iron deficiency anemia associated with intestinal angioectasias were classified as asymptomatic and excluded from this analysis. Patients with a diagnosis of hereditary hemorrhagic telangiectasia or gastric antral vascular ectasia were also excluded.
A total 127 cases were initially reviewed. A large number (66) of these were excluded during subsequent screening due to lack of complete small bowel evaluation (primarily lack of capsule endoscopy) and/or lack of documentation of overt bleeding or iron deficiency anemia. The 61 remaining cases were carefully examined with independent review of endoscopic images as well as complete capsule endoscopy videos. This analysis excluded 26 additional cases due to insufficient records/images for review, incomplete capsule examination, poor capsule visualization or lack of confirmation of typical angioectasias by the principal investigator on independent review. A total of 35 cases met criteria for final analysis. The number and location of angioectasias were documented for each case. Lesions were then classified into the following segments of the gastrointestinal tract: esophagus, stomach, duodenum, jejunum, ileum, right colon and left colon. The location of lesions within the small bowel observed by capsule endoscopy was generally defined by percentage of total small bowel transit time with times of 0%-9%, 10%-39%, and 40%-100% corresponding to the duodenum, jejunum and ileum, respectively. Independent review of complete capsule studies allowed for deviation from this guideline in cases in which capsule passage was delayed in one or more segments. In addition, the location and number of angioectasias observed in the small bowel was further modified or confirmed by subsequent device-assisted enteroscopy (DAE) performed in the 83% of cases.
In the final analysis, all study patients were age 50 years or older (age range 50-91 years) and 13 patients (37.1%) had chronic kidney disease stage 3 or higher (defined by Kidney disease outcomes quality initiative staging: stage 3 = glomerular filtration rate (GFR) 30-59, stage 4 = GFR 15-29, stage 5 = GFR < 15 or on dialysis). Twenty of 35 patients were taking aspirin (81 mg or 325 mg), clopidogrel, and/or warfarin. Of these 20 patients, 8 were on a combination of these medical therapies.
In our study population, the jejunum was the most common location of symptomatic angioectasias in the gastrointestinal tract. Out of 150 total angioectasias observed in 35 patients, 78 were localized to the jejunum (52%), 34 to the duodenum (23%), 22 to the stomach (15%), 9 to the ileum (6%), and 7 to the right colon (4.7%). Twenty-eight patients had lesions in the jejunum (80%), 18 had lesions in the duodenum (51%), 8 had lesions in the stomach (22.8%), 4 had lesions in the right colon (11.4%) and 2 had lesions in the ileum (5.7%). There were no angioectasias localized to the esophagus or left colon. Twenty-five patients had more than one lesion (71.4%). Twenty-one patients had lesions in more than one location (60%).
Spatial clustering of lesions was also observed. In patients with lesions in multiple segments, the most common sites were the duodenum and jejunum (14 patients or 40%). Other clustering occurred in the stomach and jejunum (6 patients or 17%), stomach and duodenum (5 patients or 14.3%), jejunum and right colon (5 patients or 14.3%), duodenum and right colon (3 patients or 8.6%), stomach and right colon (1 patient or 2.9%), and duodenum and ileum (1 patient or 2.9%).
Eight patients had angioectasias isolated to the jejunum (22.8%), while two patients had angioectasias isolated to the duodenum (5.7%). Other angioectasias isolated to a single segment were found in the stomach and ileum in one patient each (2.9%). None of our patients had angioectasias located in the esophagus or left colon and no patients had isolated angioectasias in the right colon.
Recent studies of obscure bleeding in Eastern populations indicate that angioectasias are not the most common cause of small bowel hemorrhage and that these lesions are distributed fairly equally throughout the small intestine[7-9,12-14]. However, studies of Western populations report that angioectasias account for 60%-70% of bleeding sources in the small bowel and are generally found in the proximal small bowel[10,15,16]. One study by May et al[17] described a high incidence of symptomatic small bowel angioectasias in a United States population, with lesions seen in 17 of 52 push enteroscopies, 21 of 52 oral push-and-pull enteroscopies, and 2 of 52 anal push-and-pull enteroscopies. In our study population, angioectasias were most commonly found in the proximal small bowel, namely the jejunum and duodenum. Spatial clustering of these lesions also occurred most frequently in these segments.
Treatment of bleeding angioectasias is difficult. Data regarding the use of hormones, thalidomide or octreotide is limited with pharmacologic therapy reported as effective in some studies but not others. Treatment with estrogen and progesterone is the most common pharmacologic therapy, although recent studies have shown failed to confirm the efficacy observed in smaller, earlier trials[1,18-20]. Somatostatin and octreotide have reportedly shown some reduction of blood loss from angioectasias, although only in case reports and one small study. It is theorized that somatostatin may prevent recurrent bleeding from angioectasias by reduction in mesenteric blood flow and inhibition of vasodilator peptides[19]. Although this rationale seems plausible, there are currently no randomized, double-blinded studies supporting these hypotheses[1]. Endoscopic ablation is a commonly utilized therapy in patients with overt bleeding or iron deficiency anemia attributed to intestinal angioectasias. Although the effectiveness of this therapy has not been proven in a randomized, controlled trial, the practice of endoscopic ablation is supported by the lack of compelling data for medical therapy. Small studies of rebleeding rates after ablative therapy support this practice. In one study conducted in the United Kingdom, heater probe ablation of angioectasias was performed in 23 patients during push enteroscopy. The authors found this therapy to be effective although repeated ablation was required in 30% of cases[21]. Another early study of endoscopic therapy in London found that argon lasers, although seemingly promising, did not completely ablate the angioectasia. The first of their 18 studied cases ultimately required an emergency gastrectomy, so the investigators observed the argon laser’s effercts on the resected specimen. Despite the laser’s small ulcer created during endoscopy, the arteriovenous malformation remained intact under normal mucosa, increasing the likelihood of rebleeding. In a separate case, investigators had better results with the neodymium Yag (Nd YAG) laser, which created fibrotic zones three to four times thicker than the argon laser[22]. This finding was validated by two separate studies which showed a reduction in blood transfusion requirements after treating patients with angioectasias with endoscopic Nd YAG laser. However, the investigators of these studies performed only upper gastrointestinal endoscopy, and admitted that the Nd YAG laser would need to be used with caution in the thinner bowel walls of the lower gastrointestinal tract[23,24]. In Japan, Ohmiya et al[25] used a combination of double balloon endoscopy with enteroscopic electrocoagulation to locate and treat 19 patients with small bowel angioectasias. Of the 19 patients, 7 rebled after treatment. Nakase et al[26] also DBE to localize bleeding angioectasias in the small bowel of 8 patients, and reported successful hemostasis in all cases using combination therapy of local injection of hypertonic-saline epinephrine and heat coagulation. However, since bleeding from angioectasias often occurs intermittently, it is difficult to evaluate the effects of any therapy on rebleeding rates. For this reason, both endoscopic therapy and medical therapy are still used in clinical practice with medical therapy often reserved for patients with comorbidities preventing endoscopic ablation, patients with multiple lesions or in cases in which bleeding recurs despite ablation[1].
Weaknesses of our study include retrospective design and relatively small number of patients included in the final analysis. Also, patients with angioectasias located within reach of standard endoscopy may not have been included if additional evaluation by capsule endoscopy was not pursued thus leading to overstatement of the percentage of patients with small bowel lesions. The lack of angioectasias found in the ileum is still a compelling finding despite these considerations. When applying this finding towards clinical practice, examination of the ileum by retrograde DAE or attempted total enteroscopy by antrograde DAE is unlikely to yield angioectasias candidate for ablation unless lesions are observed in this segment by capsule study. Close inspection of the duodenum and jejunum using push enteroscopy or anterograde DAE combined with re-inspection of the right colon and terminal ileum may be the most effective approach in these patients.
Intestinal angioectasias are the most common source of bleeding from the small intestine in patients older than 50-60 years. Nearly half of patients who experience symptomatic blood loss due to these lesions will experience recurrent bleeding. Additionally, many patients have multiple angioectasias that may bleed. Localization and endoscopic treatment of these lesions can be challenging as they are commonly small, evanescent and/or located deep in the small intestine. In order to better define the distribution of angioectasias in the gastrointestinal tract, we studied the location of specific lesions in patients with overt or occult gastrointestinal blood loss attributed to symptomatic angioectasias.
Endoscopic visualization of the entire gastrointestinal tract has not been readily available until the advent of wireless capsule endoscopy and double balloon enteroscopy in 2001. Since that time, localization and endoscopic treatment of angioectasias have become areas of interest for clinicians and researchers alike.
Recent studies of the distribution of angioectasias using capsule endoscopy and balloon enteroscopy demonstrate that these lesions are common causes of bleeding from the small intestine. However, the most frequent location of lesions within the small intestine has varied between populations. In Eastern populations, angioectasias are almost equally distributed between the proximal and distal small intestine. In Western populations, however, the vast majority of lesions occur in the proximal small bowel.
The majority of small bowel angioectasias in the study were located in the proximal small bowel. When applying this finding towards clinical practice, examination of the ileum by retrograde device-assisted enteroscopy (DAE) or attempted total enteroscopy by antrograde DAE is unlikely to yield angioectasias candidate for ablation unless lesions are observed in this segment by capsule study. Close inspection of the duodenum and jejunum using push enteroscopy or anterograde DAE combined with re-inspection of the right colon and terminal ileum may be the most effective approach in these patients.
It is a retrospective study of 35 patients with bleeding intestinal angioectasias who had undergone complete endoscopic evaluation of the gastrointestinal tract. They showed that angioectasias were most commonly found in the jejunum (80%) followed by the duodenum (51%), stomach (22.8%), and right colon (11.4%). It has an interesting report but the main limitation its small sample size.
| 1. | Regula J, Wronska E, Pachlewski J. Vascular lesions of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2008;22:313-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Junquera F, Feu F, Papo M, Videla S, Armengol JR, Bordas JM, Saperas E, Piqué JM, Malagelada JR. A multicenter, randomized, clinical trial of hormonal therapy in the prevention of rebleeding from gastrointestinal angiodysplasia. Gastroenterology. 2001;121:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 3. | Kwan V, Bourke MJ, Williams SJ, Gillespie PE, Murray MA, Kaffes AJ, Henriquez MS, Chan RO. Argon plasma coagulation in the management of symptomatic gastrointestinal vascular lesions: experience in 100 consecutive patients with long-term follow-up. Am J Gastroenterol. 2006;101:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Gerson LB. Outcomes associated with deep enteroscopy. Gastrointest Endosc Clin N Am. 2009;19:481-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1994] [Cited by in RCA: 1410] [Article Influence: 54.2] [Reference Citation Analysis (2)] |
| 6. | Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 867] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 7. | Chen LH, Chen WG, Cao HJ, Zhang H, Shan GD, Li L, Zhang BL, Xu CF, Ding KL, Fang Y. Double-balloon enteroscopy for obscure gastrointestinal bleeding: a single center experience in China. World J Gastroenterol. 2010;16:1655-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Sun B, Rajan E, Cheng S, Shen R, Zhang C, Zhang S, Wu Y, Zhong J. Diagnostic yield and therapeutic impact of double-balloon enteroscopy in a large cohort of patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2006;101:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 9. | Kameda N, Higuchi K, Shiba M, Machida H, Okazaki H, Yamagami H, Tanigawa T, Watanabe K, Watanabe T, Tominaga K. A prospective, single-blind trial comparing wireless capsule endoscopy and double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. J Gastroenterol. 2008;43:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Gerson LB, Batenic MA, Newsom SL, Ross A, Semrad CE. Long-term outcomes after double-balloon enteroscopy for obscure gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2009;7:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Selby W. Vascular Abnormalities of the Small Bowel. Capsule Endoscopy. Philadelphia: Saunders 2007; 165-181. |
| 12. | Okazaki H, Fujiwara Y, Sugimori S, Nagami Y, Kameda N, Machida H, Yamagami H, Tanigawa T, Shiba M, Watanabe K. Prevalence of mid-gastrointestinal bleeding in patients with acute overt gastrointestinal bleeding: multi-center experience with 1,044 consecutive patients. J Gastroenterol. 2009;44:550-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Shinozaki S, Yamamoto H, Yano T, Sunada K, Miyata T, Hayashi Y, Arashiro M, Sugano K. Long-term outcome of patients with obscure gastrointestinal bleeding investigated by double-balloon endoscopy. Clin Gastroenterol Hepatol. 2010;8:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, Yano T, Iwamoto M, Sekine Y, Miyata T, Kuno A. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2:1010-1016. [PubMed] |
| 15. | Carey EJ, Leighton JA, Heigh RI, Shiff AD, Sharma VK, Post JK, Fleischer DE. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Cellier C. Obscure gastrointestinal bleeding: role of videocapsule and double-balloon enteroscopy. Best Pract Res Clin Gastroenterol. 2008;22:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | May A, Nachbar L, Schneider M, Ell C. Prospective comparison of push enteroscopy and push-and-pull enteroscopy in patients with suspected small-bowel bleeding. Am J Gastroenterol. 2006;101:2016-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 18. | Szilagyi A, Ghali MP. Pharmacological therapy of vascular malformations of the gastrointestinal tract. Can J Gastroenterol. 2006;20:171-178. [PubMed] |
| 19. | Barkin JS, Ross BS. Medical therapy for chronic gastrointestinal bleeding of obscure origin. Am J Gastroenterol. 1998;93:1250-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (3)] |
| 20. | van Cutsem E, Rutgeerts P, Vantrappen G. Treatment of bleeding gastrointestinal vascular malformations with oestrogen-progesterone. Lancet. 1990;335:953-955. [PubMed] |
| 21. | Hayat M, Axon AT, O’Mahony S. Diagnostic yield and effect on clinical outcomes of push enteroscopy in suspected small-bowel bleeding. Endoscopy. 2000;32:369-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Bown SG, Swain CP, Storey DW, Collins C, Matthewson K, Salmon PR, Clark CG. Endoscopic laser treatment of vascular anomalies of the upper gastrointestinal tract. Gut. 1985;26:1338-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Sargeant IR, Loizou LA, Rampton D, Tulloch M, Bown SG. Laser ablation of upper gastrointestinal vascular ectasias: long term results. Gut. 1993;34:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Rutgeerts P, Van Gompel F, Geboes K, Vantrappen G, Broeckaert L, Coremans G. Long term results of treatment of vascular malformations of the gastrointestinal tract by neodymium Yag laser photocoagulation. Gut. 1985;26:586-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Ohmiya N, Yano T, Yamamoto H, Arakawa D, Nakamura M, Honda W, Itoh A, Hirooka Y, Niwa Y, Maeda O. Diagnosis and treatment of obscure GI bleeding at double balloon endoscopy. Gastrointest Endosc. 2007;66:S72-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Nakase H, Matsuura M, Mikami S, Chiba T. Diagnosis and treatment of obscure GI bleeding with double balloon endoscopy. Gastrointest Endosc. 2007;66:S78-S81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Peer reviewers: Rafiq A Sheikh, MBBS, MD, MRCP, FACP, FACG, Department of Gastroenterology, Kaiser Permanente Medical Center, 6600 Bruceville Road, Sacramento, CA 95823, United States; Orhan Sezgin, Professor, Department of Gastroenterology, Mersin University School of Medicine, 33190 Mersin, Turkey
S- Editor Lv S L- Editor A E- Editor Xiong L