INTRODUCTION
The term portal biliopathy (syn. pseudosclerosing cholangitis) was first coined in the 1990s[1], where it was used to describe abnormalities in the intrahepatic and extrahepatic biliary tract, gallbladder and cystic duct secondary to portal hypertension. However, jaundice and common bile duct (CBD) compression associated with portal hypertension had been, in fact, described by Fraser et al[2] in 1944 and by Gibson et al[3] in 1965. Hunt[4], also in 1965, described the treatment of CBD obstruction secondary to distended venous collaterals.
Portal biliopathy is predominantly associated with extrahepatic portal venous obstruction (EHPVO). Studies in these patients using endoscopic retrograde cholangiopancreatography (ERCP) have shown that changes in the bile ducts occur in 81%-100% of them, although only 5%-30% have symptoms of biliary obstruction[5-10]. The condition has also been described in patients who have non-cirrhotic portal fibrosis (NCPF) and cirrhosis, albeit in smaller numbers.
Portosystemic shunting as a decompressive treatment for portal biliopathy was first described in 1989 by Choudhuri et al[11]. However, subsequent studies have shown that in a subset of patients the biliary obstruction is not relieved by portosystemic shunts alone and requires an additional biliary drainage procedure. The characteristics of patients in whom the biliary obstruction is not reversed after a portosystemic shunt and the role of endoscopic management of the condition is still not clear.
In this article, we review the current data on portal biliopathy and outline the various controversies associated with its effective treatment.
The anatomical basis for the condition was suggested by the works of Petren[12] and Saint[13] who described the venous anatomy of the bile ducts in 1932 and 1971 respectively. An epicholedochal plexus (of Saint) forms a reticular network of veins (maximum size 1 mm) on the outer surface of the bile ducts. The paracholedochal network of Petren courses parallel to the CBD and is connected to the gastric, pancreaticoduodenal and portal veins below, and to the liver above.
PATHOGENESIS
There are three main theories for the pathogenesis of portal biliopathy; that it is the result of compression of the bile ducts, ischemia, or infection.
Compression theory
The first cholangiographic evidence of CBD varices compressing the bile duct was published by Williams et al[14] in 1982. In EHPVO, long standing obstruction of the portal vein leads to replacement of the portal vein by large collaterals along the CBD - the so-called cavernomatous transformation of the portal vein. These large collaterals compress the pliable CBD, leading to the changes seen on ERCP[5-7]. Also, with increased duration of portal thrombosis, there is vascular neogenesis and formation of tumor-like connective tissue, which can encase the CBD or cause angulation of the bile ducts[9]. In a study by Dilawari et al[5], 18 out of 20 patients had indentations suggestive of external compressions on ERCP. The reversibility of biliary tract changes after portal decompressive surgery[11,15-17] or transjugular intrahepatic portosystemic shunts (TIPS)[18,19], shown in various studies, further corroborates this mechanism.
Ischaemic theory
According to this theory, longstanding portal thrombosis leads to sclerosis of the veins draining the bile ducts, which in turn can lead to damage to the capillaries and arterioles. This interruption of the vascular supply can, in turn, lead to the development of ischaemic strictures in the bile duct which are not reversed after a portosystemic shunt or TIPS. In a study by Khuroo et al[7], strictures in the CBD, both short segment and long confluent, were the most common findings seen on ERCP suggestive of an ischemic pathology. Dhiman et al[20] studied bile duct changes after shunt surgery in 5 patients by performing ERCP pre- and post-surgery and reported complete reversal in one patient, partial reversal in three and no reversal in one patient, postulating that ischemia or scarring may be the etiology behind persistence of bile duct changes.
Infective theory
Infection or cholangitis was postulated by some authors in earlier studies to be the cause of jaundice in patients with portal vein thrombosis[15,21,22]. However, later cholangiographic studies have shown that changes in the biliary tract are seen even in asymptomatic patients, and cholangitis occurs late in its natural history. Cholangitis, once present, may lead to inflammation, neogenesis and deposition of fibrous tissue, along with persistence of strictures following shunt surgeries.
All the above mentioned mechanisms may be present simultaneously, resulting in the characteristic changes of portal biliopathy.
EPIDEMIOLOGY
Portal biliopathy is an uncommon presentation in patients with portal hypertension. In 1992, Dilawari et al[5] published a series of 20 patients with EHPVO in which ERCP was done prospectively, and found that changes in the biliary tract were seen in all of them. The left hepatic ducts were always involved, the right ductal system was involved in 56% and there were changes in the common bile duct in 90%. Sarin et al[6] found the incidence on ERCP to be 80%. Studies published by various authors utilising ERCP/magnetic resonance cholangiopancreatography (MRCP) found the frequency to be between 81%-100%[5-10]. Portal biliopathy is also seen in patients with cirrhosis of the liver (0%-30%)[1,23,24] and NCPF (9%-40%)[1,24]. The natural history of portal biliopathy is not known. The majority of patients (70%-95%) do not manifest with any symptoms of biliary obstruction. However, patients with symptomatic portal biliopathy are normally older than patients presenting with EHPVO[7,10], which is suggestive of long term obstruction. Patients with long term obstruction, or inadequate endoscopic or surgical management, may develop secondary biliary cirrhosis (2%-4%)[1,9]. No evidence of malignant potential on long term follow-up exists in the literature.
CLINICAL FEATURES
In patients with EHPVO, 5%-38% develop symptomatic portal biliopathy[5-10,25]. These symptoms may be secondary to bile duct obstruction like jaundice and pruritus, or to ductal stones like fever with chills and biliary colic. Dilawari et al[5] reported a 5% incidence of symptoms. Khuroo et al[7] reported a 38% incidence of symptoms ranging from jaundice, recurrent cholangitis and biliary colic. All symptomatic patients in his study were adults and almost a decade older than the patients presenting with variceal bleeding. Sezgin et al[10] studied 10 patients with portal biliopathy who presented with jaundice, cholangitis, pruritus and abdominal pain and their mean age at presentation was 36 years, whereas other patients with EHPVO generally presented with bleeding or splenomegaly during childhood. Their studies also suggest that portal biliopathy is a progressive condition which develops late in the course of portal hypertension and may progress to secondary biliary cirrhosis characterised by decreased serum albumin levels, ascites and a deranged coagulation time[1,9].
INVESTIGATIONS
Most patients with EHPVO with biliary changes detected on ERCP are asymptomatic. However, because ERCP is an invasive procedure, its routine use in all patients with either EHPVO or NCPF is not justified. Liver function tests are the best initial investigations to identify patients who might benefit from imaging studies. A raised serum bilirubin level with a predominant increase in its direct component and an elevated serum alkaline phosphatase is an indication for performing biliary imaging. Serum albumin levels and prothrombin time abnormalities become abnormal only after prolonged biliary obstruction when secondary biliary cirrhosis develops.
Abdomen ultrasound with Doppler
Ultrasonography (USG) has a poor sensitivity in identifying the CBD in EHPVO as it is usually obscured by the portal vein collaterals. However, there is a role for USG in outlining the splenoportal axis before surgery and to identify gallbladder varices, which are seen in about 35% patients, if a cholecystectomy is planned[26,27].
Endoscopic retrograde cholangiopancreatography
ERCP has been used by various authors to define portal biliopathy. The changes seen in the bile ducts include single or multiple smooth strictures of varying length and degree, saccular dilatations, indentations, dilated intrahepatic bile duct radicles, displacement of bile ducts, clustering and pruning of intrahepatic ducts, and filling defects in the CBD which may be due to stones or varices[5-10,25]. These changes occur most commonly in the CBD and the left hepatic ducts. Although seen predominantly in EHPVO, patients with NCPF and cirrhosis also show these changes in 40% and 25% respectively[1,23,24].
The differential diagnoses on cholangiography include sclerosing cholangitis, recurrent pyogenic cholangitis, CBD stones with stricture, and biliary ascariasis. Patients’ history, clinical examination and ultrasound findings showing normal liver, portal cavernoma and echogenic shadowing suggestive of ascariasis may help in reaching a diagnosis[1].
ERCP also has a therapeutic role in portal biliopathy. This includes removal of CBD stones, relief of cholangitis, and dilatation of dominant strictures with stenting. The latter is indicated only in patients not fit for surgery, or in whom shunt surgery is not feasible or has not reversed the biliopathy.
Presently, ERCP is indicated only if a therapeutic intervention is required and not for diagnosis.
Magnetic resonance cholangiopancreatography
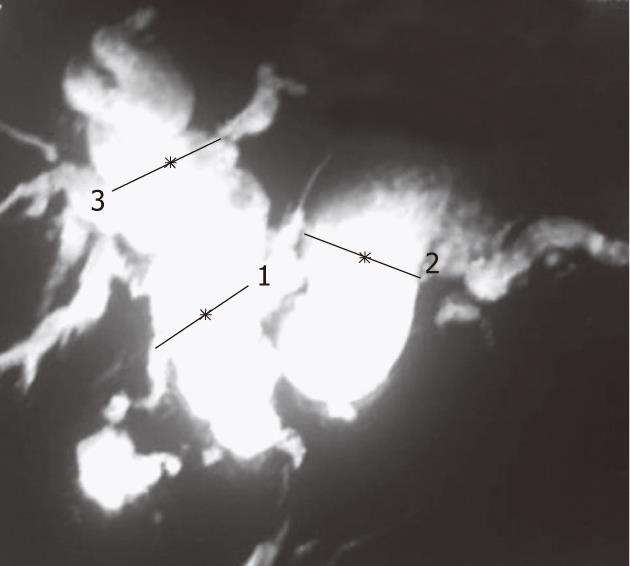
Due to the invasive nature of ERCP and its attendant risks, magnetic resonance cholangiopancreatography (MRCP) with or without magnetic resonance (MR) portography has become the investigation of choice for portal biliopathy. The sensitivity of MRCP has been found to be similar to ERCP by various authors[9,28]. Additional benefits of MR portography are that it differentiates choledochal varices from stones, images the splenoportal axis in surgical planning, and identifies portal collaterals (Figure 1).
Figure 1 Magnetic resonance cholangiopancreatography showing common bile duct stricture with upstream dilatation of intra- and extrahepatic bile ducts.
1: Distance: 1.85 cm; 2: Distance: 2.57 cm; 3: Distance: 2.44 cm.
Endoscopic ultrasonography
The additional information obtained from endoscopic ultrasonography (EUS) pertains to the differentiation between CBD varices[29,30], stones, and tumours when other imaging modalities are not clear. EUS is not routinely recommended in the workup of a patient with portal biliopathy.
MANAGEMENT
Asymptomatic patients do not require any treatment if their liver function tests are within normal limits. Patients with persistently raised serum bilirubin and alkaline phosphatase levels need to be investigated by imaging (MRCP/ERCP/USG) to look for biliary tract changes[1].
There is no consensus on the optimal treatment for symptomatic portal biliopathy. Endoscopic treatment is preferred in patients with CBD stones, cholangitis or if shunt surgery is not feasible[1,17,31-34]. This includes use of a balloon catheter or Dormia basket to clear CBD stones. Mechanical lithotripsy may be required for large stones. Endoscopic papillotomy with stenting or nasobiliary tube drainage may be necessary in patients with cholangitis[1,34-37]. Balloon dilatation of dominant CBD strictures with stone extraction has also been described by various authors[1,10,34].
The problems with endoscopic management are: (1) Filling defects seen on imaging may be due to varices and may lead to bleeding during attempted clearance. Stones usually move with a balloon catheter and varices appear as longitudinal defects on MRCP. Some authors prefer a balloon catheter over a Dormia basket for stone clearance as it is less traumatic[34]; (2) Venous collaterals in the region of the ampulla of Vater may lead to bleeding during papillotomy, so this procedure should only be attempted in experienced centres which have a good surgical backup[38,39]; (3) Balloon dilatation with stenting of dominant strictures may help to relieve biliary obstruction. However these stents become blocked frequently requiring multiple changes with their inherent risk of bleeding[40]. Sezgin et al[10] reported long-term relief of portal biliopathy in only 3 out of 10 patients with endoscopic dilatation with stenting or nasobiliary drainage, whereas 7 out of 10 patients required repeated stent changes every 6 mo or earlier if cholangitis developed. Vibert et al[41] also reported long-term relief of biliary obstruction with percutaneous transhepatic biliary drainage with endobiliary stenting in only 3 out of 19 patients with symptomatic portal biliopathy. Furthermore, recurrent stent blockade with cholangitis may further decrease the chances of reversibility of the CBD obstruction following portosystemic shunt surgery; and (4) Multiple sessions require the patients to be compliant and have ready access to endoscopic expertise.
Therefore, although endoscopic extraction remains the preferred treatment in patients with CBD stones, most centres consider shunt surgery to be the first line of management for biliary obstruction secondary to bile duct strictures, unless complications like cholangitis or absence of a shuntable vein exist.
The role of ursodeoxycholic acid (UDCA) has not been evaluated for treatment or prophylaxis in portal biliopathy in any of the large studies. In a study by Condat et al[9], UDCA was effective in 3 out of 4 patients with portal biliopathy in relieving biliary symptoms and preventing recurrence. Most centres, however, rely on endoscopic extraction of biliary stones and experience in use of UDCA is limited.
Surgical management
The first published report of surgical treatment of portal biliopathy was by Hunt[4] in 1965. He described separating the collaterals from the bile duct wall by dividing the fibrous adhesions between them and reported a relief from jaundice in the early postoperative period. However this technique has a high risk of intraoperative hemorrhage as the collaterals are closely related to the wall of the CBD and attempting to separate them may cause torrential bleeding.
Choudhuri et al[11] in 1988 published the first case report of relief of CBD obstruction secondary to portal cavernoma after a proximal lienorenal shunt, in a patient with symptomatic portal biliopathy with EHPVO. Chaudhary et al[15] then published a series of 9 patients, out of whom 7 underwent proximal lienorenal shunts for symptomatic portal biliopathy. Five patients experienced reversal of their portal biliopathy with two patients requiring a biliary bypass (Roux-en-Y hepaticojejunostomy) for refractory bile duct strictures. Other authors have published similar results[16,41].
In patients with non cirrhotic portal hypertension (EHPVO and NCPF) with portal biliopathy, portosystemic shunt surgery is the treatment of choice. The advantages of this approach are: (1) A successful portosystemic shunt not only prevents variceal bleeding but in a large number of patients may be the only treatment required for portal biliopathy[11,15,16,41-43]; (2) Primary biliary bypass for portal biliopathy is associated with a risk of severe intraoperative bleeding due to the presence of large collaterals in the bile duct wall[7,15,16,41,44,45]; and (3) Even if a portosystemic shunt fails to completely revert the bile duct obstruction, a patent shunt decompresses the collaterals present in the region of the bile duct enough to render a later biliary bypass possible[15,16].
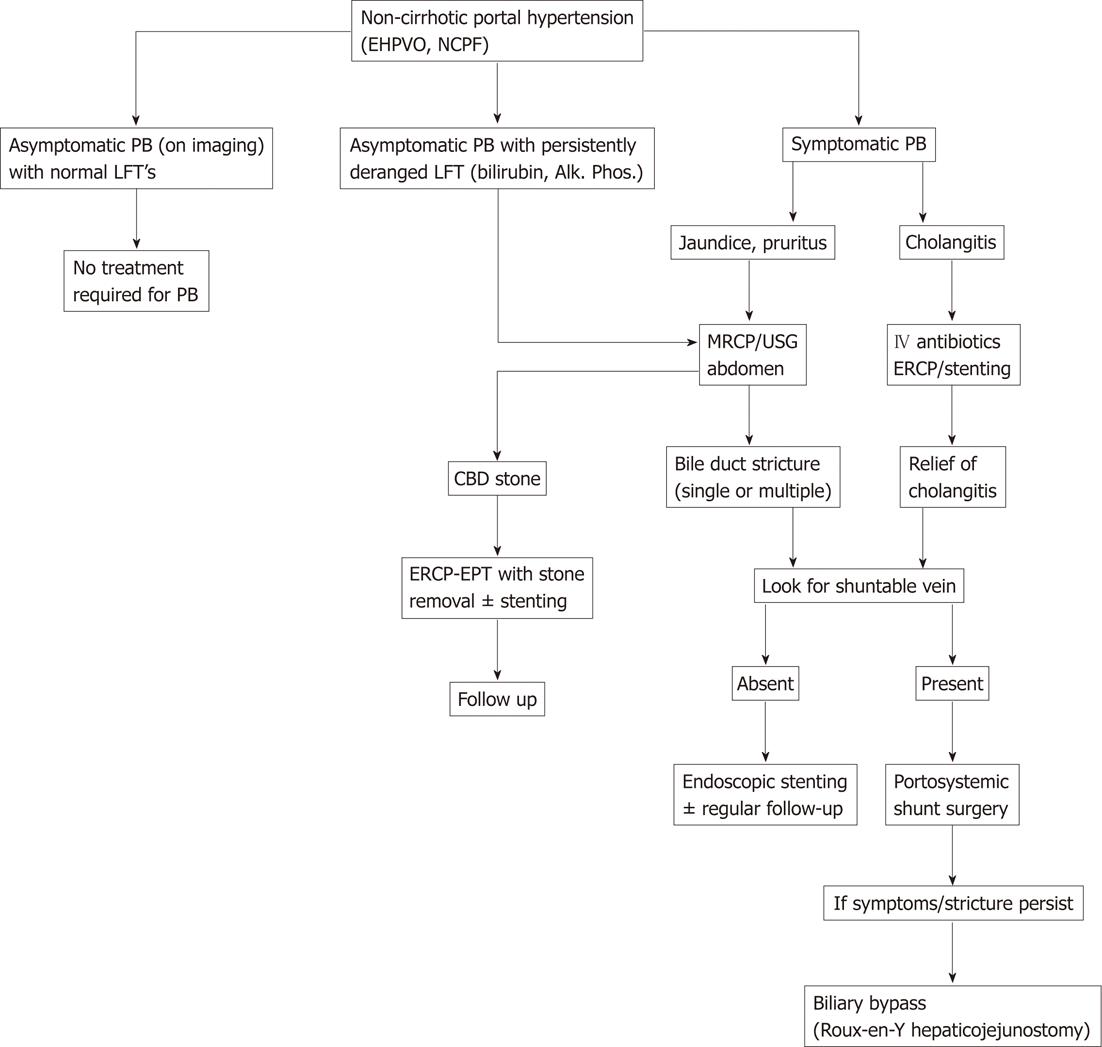
Portosystemic shunt surgery is also the procedure of choice in patients with imaging features of portal biliopathy who are otherwise asymptomatic and are being operated on for other complications of portal hypertension like variceal bleeding or symptomatic hypersplenism, as these patients may develop significant bile duct obstruction later, even after splenectomy and devascularisation[7,46]. A suggested algorithm for the management of portal biliopathy is shown in Figure 2.
Figure 2 Algorithmic approach to management of portal biliopathy in patients with non-cirrhotic portal hypertension.
EHPVO: Extrahepatic portal venous obstruction; NCPF: Non-cirrhotic portal fibrosis; LFT: Liver function test; PB: Portal biliopathy; ERCP: Endoscopic retrograde cholangiopancreatography; EPT: Endoscopic papillotomy; CBD: Common bile duct; MRCP: Magnetic resonance cholangiopancreatography; USG: Ultrasonography; Alk.: Alkaline; Phos.: Phosphatase.
In conclusion, although abnormalities in the biliary tract on imaging are seen in the majority of patients with EHPVO, symptomatic portal biliopathy is a late and uncommon presentation in the natural history of the condition. The exact pathogenesis of portal biliopathy is not clear, but compression by dilated collaterals, ischemia resulting from venous thrombosis, and infection may all have a role to play in its development. The diagnostic investigation of choice is an MRCP which is done based on clinical suspicion and biochemical abnormalities. Endoscopic management is indicated in the presence of CBD stones, cholangitis and dominant strictures without a shuntable vein. Portosystemic shunt surgery, if feasible, is indicated in most patients, as it causes reversal of portal biliopathy and also renders a subsequent biliary bypass easier if the biliary obstruction persists. Thus, in patients with portal biliopathy, both endoscopic and surgical management are complementary and should be used appropriately according to individual situations.