Published online Nov 7, 2012. doi: 10.3748/wjg.v18.i41.5848
Revised: June 12, 2012
Accepted: June 28, 2012
Published online: November 7, 2012
Cytokines are indispensable signals of the mucosa-associated immune system for maintaining normal gut homeostasis. An imbalance of their profile in favour of inflammation initiation may lead to disease states, such as that is observed in inflammatory bowel diseases (IBD). Although Crohn’s disease (CD) is often described as a prototype of T-helper 1-type diseases, and ulcerative colitis (UC) is traditionally viewed as a T-helper 2-mediated condition, the classic paradigm, which categorises cytokines into pro- and anti-inflammatory groups, has recently been changed. The inflammation regulatory pathways may not be mutually exclusive as individual cytokines can have diverse and even opposing functions in various clinical and immunological settings. None the less there are many common immunological responses in IBD that are mediated by cytokines. Although they regulate and influence the development, course and recurrence of the inflammatory process, the concrete pathogenic role of these small signaling molecules is sometimes not unambiguous in the subtypes of the disease. Our aim is to review the current information about pro- and anti-inflammatory effects of traditionally studied and recently discovered cytokines in the pathogenesis of UC and CD. The better understanding of their production and functional activity may lead to the development of new therapeutic modalities.
- Citation: Műzes G, Molnár B, Tulassay Z, Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol 2012; 18(41): 5848-5861
- URL: https://www.wjgnet.com/1007-9327/full/v18/i41/5848.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i41.5848
The balance of pro- and anti-inflammatory cytokines in the colonic mucosa is essential for normal gut homeostasis. A disturbance of the cytokine profile in favour of pro-inflammatory cytokine overproduction leads to disease states, such as that observed in inflammatory bowel diseases (IBD)[1,2].
The concept that ulcerative colitis (UC) and Crohn’s disease (CD) are two distinct forms of IBD has been changed recently. Instead, they are considered as a spectrum from mildly inflamed mucosa to severely active bowel inflammation with or without extraintestinal manifestations and different clinical behaviour.
CD is often described as a prototype of T-helper (Th) 1-mediated diseases because the primary inflammatory mediators are the Th1 cytokines such as interleukin (IL)-12, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α[3,4]. However, UC is usually viewed as a Th2-type condition because of the increased intestinal expression of the Th2-associated cytokine IL-5 and IL-13, although a clear association with IL-4, another definitive Th2 cytokine, has never been established[3,4]. The role of pro-inflammatory cytokines such as IL-1α, IL-1β, IL-2, -6, -8, -12, -17, -23, IFN, or TNF in IBD is associated with the initiation and progression of UC and CD. Cytokines with anti-inflammatory effects, like IL-4, -10, and partly IL-13 also contribute to the pathogenesis of IBD, decreasing the inflammatory process by down-regulating pro-inflammatory cytokine production.
However, this classic paradigm has recently been changed. These pathways may not be mutually exclusive as individual cytokines can have diverse and even opposing functions in various clinical and immunological settings[4].
Although many common immunological responses in IBD are mediated by cytokines, the concrete pathogenic role of these small peptide molecules is sometime not unambiguous in the subtypes of the disease. Therefore we aimed to review the current information about pro- and anti-inflammatory effects of traditionally studied and recently discovered cytokines in the pathogenesis of UC and CD. Controlling their expression, production and functional activity is an approach that may allow the development of more efficient and less harmful therapeutic strategies.
The cytokines of the IL-1 family play a major role in several autoimmune inflammatory diseases, including IBD[5]. IL-1 represents two structurally distinct forms: IL-1α and IL-1β. For both IL-1α and -β, the most significant and relevant properties are the initiation of cyclooxygenase type 2, inducible nitric oxide synthase and phospholipase A2, which are produced by various cell types[6]. Endogenous IL-1 receptor antagonist (IL-1Ra), a natural occurring antagonist of IL-1, regulates normal immune homeostasis in the gut[1]. The increase of the IL-1/IL-1Ra ratio is parallel with the activity of colitis, while the IL-1/IL-1Ra ratio remains constant in the non-affected part of the colon and in non-IBD inflammatory controls[7]. The main source of IL-1 in IBD is the monocyte/macrophage system as it can activate the IL-1 converting enzyme, therefore active IL-1β is released into the colonic mucosa[8].
IL-33, as known as IL-F11, is the newest identified member of the IL-1 family[9,10]. It has been detected in several different cell types such as myofibroblasts, adipocytes, smooth muscle cells, endothelial cells bronchial and intestinal epithelial cells, macrophages and dendritic cells[10-12]. For the expression of the biological effect of IL-33 the binding to its receptor, IL-1 receptor like 1 (also known as ST2), is required[10]. IL-33 has a pathogenic role in allergy[13], airway inflammation[14] and rheumatic diseases[15]. Based on the results of Schmitz et al[10], IL-33 appears to reconstitute mucosal barrier defences against luminal pathogens, increasing epithelial protection by mucus secretion and augmenting immune response via type 2 helper T cell (Th2)-related cytokines, such as IL-5 and IL-13. In 2010, elevated expression of IL-33 in UC was reported by four independent groups[16-19]. In active UC, the expression of the full-length, biologically active form of IL-33 is markedly increased in epithelial cells and in the infiltrating macrophages and B-cells of the lamina propria, while in the serum only the cleaved form of IL-33 is detectable[18]. This latter possesses reduced biological activity[20] therefore leading to the speculation that the presence of extracellular proteases has the ability to inactivate full-length IL-33 preventing possible harmful effects (i.e., anaphylactic shock) triggered by high levels of circulating IL-33[21].
Similarly to IL-33, the expression of its receptor, ST2, was shown to be increased in both colonic wall and serum of IBD patients[16]. Although the epithelial-derived ST2 expression is decreased and redistributed in IBD[20], a marked infiltration of ST2 expressing antigen presenting cells and Th cells is present in the lamina propria and perivisceral adipose tissue[18]. The same epithelial expression of ST2 was not detected in non-IBD colitis samples, such as diverticulitis or infectious colitis[18]. Regarding the colon, the IL-33/ST2 axis could have a dual and perhaps dichotomous role in the pathogenesis of IBD. Pro-inflammatory cytokine stimuli, such as TNF-α and IL-1β, and signals from pathogen-associated molecular patterns result in an increased IL-33 level in epithelial cells. After epithelial damage the released IL-33 may enhance the immune responses via ST2 expressing immune cells, therefore exacerbating the severity of inflammation[20,22]. Thus, it is tempting to speculate that the blockade of IL-33 during UC may help to reduce the severity of the disease.
In line with the activation of inflammation IL-33, partly come from endothelial cells, may also act on ST2 expressing epithelial cells and myofibroblasts, promoting wound healing and angiogenesis[20,23].
The TNF protein superfamily consists of 18 type 2 proteins that exist in either membrane-bound or soluble forms[24]. Receptors for these ligands are type 1 transmembrane proteins[25]. Binding of TNF-like ligands to their receptors triggers intracellular pathways that are directly involved in cell proliferation, differentiation, and survival[26]. Most members of the TNF/TNF-receptor protein superfamilies are expressed on immune cells and play a critical role in multiple components of the immune response, including defence against microorganisms, inflammation, programmed cell death, and the development of the immune system[24-26].
TNF-α is a master cytokine in the pathogenesis if IBD[27]. It exerts its pleiotropic effects through the expression of adhesion molecules, fibroblast proliferation, procoagulant factors, as well as the initiation of cytotoxic, apoptotic, and acute-phase responses[28]. It also has the ability to increase IL-1β, IL-6, and IL-33 production as well as modulate ST2 expression in epithelial cells[18,29]. The source of TNF-α in IBD is partly the innate immune cells, such as macrophages or monocytes, and also differentiated Th1 cells[30]. The serum levels of TNF-α correlate with the clinical activity of UC and CD[31]. Its orchestrating role in colonic inflammation established the basis of anti-TNF-α antibody therapy in IBD.
Tumor necrosis factor-like factor (TL1A), another newly discovered member of the TNF family, stimulates IFN-γ secretion by binding to death receptor 3 (DR3)[32]. DR3 is expressed by a high percentage of cells from mucosal biopsies of UC and CD, and an increase of IFN-γ level has been observed with disease activity in IBD patients[32]. Although TL1A seems to be involved in intestinal epithelial cell apoptosis in IBD[30], its concrete role in UC pathogenesis still remains unknown.
IL-6, IL-11, IL-31, leukemia inhibitory factor, oncostatin M, cardiotrophin-1, ciliary neurotrophic factor, and cardiotrophin-like cytokine belong to the IL-6 family of cytokines.
IL-6 is an immunoregulatory cytokine that activates a cell surface signaling assembly composed of IL-6, soluble IL-6 receptor (sIL-6R), and the shared signaling receptor gp130[33-35]. The combination of IL-6 and sIL-6R only stimulates gp130 expressing cells, a mechanism that is called trans-signalling. IL-6 signaling via signal transducer and activator of transcription-3 (STAT3) plays an important role in UC pathogenesis, moreover in carcinogenesis of UC-associated colorectal cancers[36].
Mitsuyama et al[37] found that sIL-6R levels were significantly increased in patients with active UC and CD compared with inactive disease. Thereby, serum IL-6 and sIL-6R levels correlated strongly with C-reactive protein levels.
Besides mononuclear cells, intestinal epithelial cells are supposed to contribute to IL-6 production in the lamina propria[38,39]. Recent data have shown interesting new aspects of epithelial function[40]. It was demonstrated in Caco2 cells that IL-6 induces NF-kappaB activation and then enhanced expression of the intercellular adhesion molecule 1, which is important in IBD pathogenesis and most likely in extraintestinal manifestations of the disease[41,42].
Based on these data, the blockade of IL-6/STAT3 signaling and the use of anti-IL-6R antibodies have been suggested as promising therapeutic approaches for the future.
IL-8, a small basic heparin-binding protein, is a member of the cysteine-amino acid-cysteine chemokine family (2 cysteines are separated by a single amino acid in the first 2 of the 4 conserved cysteine residues)[43]. It primarily mediates the activation and migration of neutrophils into tissue from peripheral blood. In a recent study[44], the tissue level of IL-8 was found to be higher in active UC compared to normal colonic tissue, and its serum concentration was also related to endoscopic and histological severity of UC. Based on these results, IL-8 seems to be a reliable biomarker, closely related to disease activity, but its pathogenic role in the initiation and maintain of colitis needs to be further studied.
IL-12, IL-23, IL-27 and IL-35 belong to the IL-12 family of pro-inflammatory heterodimeric cytokines and comprise IL-12p40/IL-12p35, IL-12p40/IL-23p19, Epstein-Barr virus-induced gene 3 (EBI3)/IL27p28(IL-30) and IL12p35/EBI3 subunits[45-48].
IL-12 and IL-23 are mainly produced by antigen presenting cells, dendritic cells and phagocytes[49]. Their receptors are also heterodimeric[49].
IL-12 receptor (IL-12R) is expressed mainly on T cells, natural killer (NK) cells and natural killer T (NKT) cells[49]. IL-12 expression is elevated in the mucosa of UC patients and it correlates with disease activity[50]. Recently, the basic leucine zipper protein, NFIL3, was shown to be a regulator of IL-12p40 in macrophages and mucosal immunity[51]. Interactions of macrophages with the colonic microbiota induce NFIL3 to limit their inflammatory capacity.
IL-23 promotes the differentiation of naïve CD4+ T cells into Th17 cells[52]. The production of IL-23 by the cells of innate immunity is a response to pattern-recognition-receptor (toll-like- and nucleotide oligomerization domain-like receptors) stimulation or endogenous signals, indicating a potential role for T cells in reinforcement of the IL-23 response[53]. The pathogenic role of IL-23 receptor (IL-23R) polymorphisms in UC may result in part from its wide distribution among other immune cells. IL-23R is expressed by NK cells, NKT cells, CD4+ T cells and CD8+ T cells[54]. It is possible that some of the disease-associated polymorphisms observed in the IL-23R gene region may influence IL-12RB2 expression, given their adjacent location on the genome[55]. The regulation of IL-23R and IL-12RB2 expression has a key role in the regulation of T cell differentiation.
Although in most colitis mouse models IL-23 plays a pro-inflammatory role, in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis, which is T cell mediated, IL-23 functions as an anti-inflammatory cytokine because it suppresses IL-12 production[56,57].
There is a strong link between IL-23 and Th17 response in vivo. It appears that IL-23 is not necessary for the initiation of Th17 cell differentiation but it is required at a crucial point in controlling the Th17 response[57]. IL-23 signaling is primarily mediated through the adapter molecule, STAT3. STAT3 was also shown to be essential in the Th17 response, as it binds to the Il-17a gene promoter and mediates IL-23-regulated expression of IL-17A, the main effector cytokine of Th17 cells. In lack of IL-23, a decrease in the accumulation of Th17 cells appears in response to inflammatory stimuli, suggesting a regulatory role of IL-23 in Th17 cell response[57,58]. Besides Th17 cell differentiation, IL-23 also influences the development of regulatory T cells (Tregs) by suppressing Foxp3 expression[59,60]. IL-23 reduces the frequency of Foxp3+ Tregs in the colon and is dispensable for the pathogenesis of mucosal inflammation in the lack of Tregs[61].
The main source of the newly discovered IL-35 is the Tregs[48]. Recently, it has been shown[62] that IL-35 controls the development of T-cell-dependent colitis in mice models, suggesting the potential in targeting IL-35 for patients with chronic intestinal inflammation. The role of IL-35 in the pathogenesis of human IBD needs to be further investigated.
UC has been traditionally considered as a Th2 mediated disease, in which IL-13 was identified as an important effector cytokine[63]. The mRNA expression of IL-13 in UC mucosa is increased[64], and ex vivo cultured lamina propria mononuclear cells from UC patients secrete significantly higher amounts of IL-13 upon stimulation than those from both healthy controls and CD patients[63]. The critical cell population for IL-13 secretion is CD161+ NKT cells, producing IL-13 in response to stimulation by CD1d+ antigen presenting cells in UC[63].
The functional importance of NKT cell-derived IL-13 in UC has been studied in detail. It was shown that both receptors of IL-13, IL-4Rα and IL-13Rα2, were expressed in colonic epithelial cells, which proves the ability for functional IL-13 signaling in UC[65]. The UC-specific CD161+ NKT cells show cytotoxic activity against colon epithelium, which effect is, at least partially, dependent upon functional IL-13[63]. IL-13 was also shown to exert pernicious effects on epithelial barrier function by increasing epithelial cell apoptosis, unmaking tight junction integrity, and decreasing restitution velocity[65]. Based on these results, it was hypothesized by Fuss et al[66] that stimuli from commensal flora-derived microbial products stimulate CD161+ NKT cells to produce IL-13 in the colonic mucosa. Then, the downstream effects, such as the cytotoxic activity of NKT cells, the IL-13 induced epithelial cell apoptosis, and the disruption of tight junctions, culminate to epithelial injury. In active UC, the suppression of IL-13 production by interferon-β1 administration or the inhibition of STAT6, a key adaptor molecule in IL-13 signaling, by small interfering RNA or a histone deacetylase inhibitor result in significant epithelial healing, supporting the aforementioned hypothesis[67-69].
Recently, it was also shown that IL-13 signaling through IL-13Rα2 led to the increase of transforming growth factor (TGF)-β1 production, which favours to the progression of colonic wall fibrosis[70,71].
IL-17, which is mainly produced by Th17 cells, is acting as a key mediator in delayed-type immune reactions by increasing chemokine production and recruiting monocytes and neutrophils to the inflammatory site[72]. After sequencing the human genome 6 structurally related isoforms of the IL-17 family were described: IL-17A (also known as IL-17), IL-17B, IL-17C, IL-17D, IL-17E (also known as IL-25), and IL-17F[73]. IL-17A and IL-17F share 55% homology, which is the highest amongst the family members, but IL-17F has significantly weaker activity than IL-17A[57]. There are more receptors for IL-17 (i.e., IL-17RA, IL-17RC), widely expressed by epithelial cells, endothelium or fibroblasts, and it is supposed that the different receptors show different affinity for IL-17s and different signaling pathways[74].
Although the main source of IL-17A is the Th17 cells, CD8+ T cells are also able to produce this cytokine during chronic inflammation[57]. However, a causative relationship between UC and IL-17A remains controversial.
In most of IL-17A knock out mice dextrane sulfate sodium (DSS) treatment failed to result in a typical acute colitis[75], and after the administration of TNBS, the TNBS-induced colitis was attenuated in the IL-17RA knock out animals[76]. These data support the pro-inflammatory role of IL-17 in colitis models.
On the other hand, O’Connor et al[77] demonstrated IL-17A-mediated protection in the CD45RBhi transfer model of colitis. An accelerated wasting disease elicited by IL-17A-/- CD45RBhi CD4+ T cells correlated with higher expression of genes encoding Th1 type cytokines in colon tissue. Furthermore, IL-17RA-/- T cells elicited an accelerated wasting disease in Rag1-/- recipients. Their findings support the observation that surprisingly IL-17A can mediate protective function rather than pathology in experimental colitis. Additionally, they have also identified T cells as not only the source but also a target of IL-17 in vivo.
In humans, it was recently shown that IL-17 levels were increased in UC compared to healthy colonic mucosa, but in the most reliable studies in which protein rather than messenger RNA was measured this increase was found to be far less than that found in CD[78].
Since different results were obtained from different studies, it will be important to clarify the source and function of IL-17A in the pathogenesis of UC.
IL-25 was shown to inhibit CD14+ cell-derived cytokines, mainly IL-12 production and Th1 cell-driven experimental colitis in mice, suggesting its potential therapeutic role in both UC and CD[79].
IL-5, as known as eosinophil differentiation factor, is a selective eosinophil activating growth hormone and a member of the common β-chain-dependent cytokine family. The source of mucosal IL-5 is the mononuclear cells, which produce a high amount of this cytokine in active UC but not in CD[3,65]. IL-5 together with IL-13 and granulocyte/monocyte colony stimulating factor have been recognized as activators of eosinophil function, including migration to the site of inflammation[80]. Though IL-5 seems to have a regulatory role in eosinophil recruitment in UC mucosa, the role of this cytokine in priming of the blood eosinophils is not as obvious[81,82]. There is no enhanced IL-5 production of circulating lymphocytes in UC, which indicates that in addition to IL-5 other factors may be involved in the priming of blood eosinophils in IBD.
IL-21 is a T cell derived member of the common γ-chain-dependent cytokine family, acting as a maintainer of the Th1 mediated inflammation in the colonic epithelium by inducing IFN-γ production[83]. In IBD, IL-21 is mostly produced by CD4+ lamina proprial T cells coexpressing IFN-γ and follicular T cells[84,85]. The number of these cells is higher in CD than UC[84]. Based on the recent results, IL-21 inhibits Treg differentiation and leads to the resistance of CD4+ T cells to Treg-mediated immune-suppression, therefore enhances the inflammatory process[85].
IL-10 may be considered the most important anti-inflammatory cytokine in humans, secreted by CD4+ Th2 cells[86]. During the last two decades, a range of cytokines related to IL-10 were discovered, making IL-10 the founding member of the type II cytokine family that includes IL-19, IL-20, IL-22, IL-24, IL-26, IL-28 and IL-29[87].
IL-10 inhibits antigen presentation and the release of pro-inflammatory cytokines, hereby attenuates the inflammatory process in the mucosa. It is expressed by many cells of the innate and adaptive immune system. The former triggers IL-10 expression in a toll-like receptor (TLR)-dependent and a TLR-independent way. The major sources of IL-10 are macrophages and dendritic cells[88]. Regarding the adaptive immune system, Th2 cells primarily promote humoral immunity express IL-10[88].
The key role of IL-10 within the colonic mucosal immune system has been extensively studied in IL-10 knockout mice models[89]. In UC, IL-10 mRNA expression was found to be highly increased in mucosal T cells, and the IL-10 production of Tregs is also important in the pathogenesis of IBD[59,90]. A subset of IL-10 and TGF-β1 producing B cells, namely the regulatory B cells (Bregs), are involved in UC pathogenesis as well[91].
The mutations of the IL-10 pathway genes such as Il-10 1q32 or Stat3 17q21 have also been shown to be associated with UC[92,93].
IL-19 is associated with the pathogenesis of both Th1 and Th2 mediated diseases[94]. IL-19 produced by lipopolysaccharide activated macrophages suppresses pro-inflammatory cytokine release, especially the secretion of TNF-α, IL-6 and IL-12 by an IL-10 independent way[95]. IL-19 deficient mice are susceptible to DSS-induced colitis[94]. In a recent study, it was shown that IL-19 polymorphisms (rs2243188 and rs2243193) might have a protective role in the development of UC[96]. Although these results are promising, the exact role of IL-19 in IBD needs to be further studied.
IL-22 has elevated levels in both serum and mucosa of active CD[97], but it has been recently proven that it has a protective role in DSS-colitis murine model of UC by inducing mucin membrane bound production by goblet cells[98]. In humans, the mucosal level of IL-22 was found to be elevated in active UC compared to inactive disease or healthy control samples[99]. It was also recently published, that after Trichuris trichiura therapeutic self-infection, the active UC went into remission, and IL-22-producing Th cells accumulated in the mucosa[100]. It seems that this kind of helminthiasis may reduce symptomatic colitis by promoting goblet cell hyperplasia and mucus production through Th2 cytokines and IL-22.
TGF-β has multiple biological effects on both hematopoietic and nonhematopoietic cells[101]. Binding of TGF-β to its receptor, TGF-βRII, phosphorylates sma- and mothers against decapentaplegic-related protein transcription factors that have primarily immunosuppressive function[101]. Genetic mutations in TGF-βRII are linked to UC and UC-associated cancer in humans[102], and mice lacking TGF-β responsiveness in epithelial cells or T lymphocytes develop severe intestinal inflammation[103,104].
In human UC patients, IL-33 expression is highly upregulated within the colonic mucosa and IL-33-deficient mice are protected from DSS-induced colitis[10,17,19]. Recent data[105] show that CD68TGF-βDNRII mice, lacking normal TGF-β signaling, produce high levels of IgE and IL-33 within the colon following oral DSS administration. One source of IL-33 in these mice was the intestinal macrophages, which demonstrates that TGF-β serves as an anti-inflammatory factor via suppressing the production of IL-33. This may be an important mechanism that could partially explain the reason how mutations in TGF-βRII in humans are associated with increased risk for UC and UC-associated neoplasias[106].
IL-4, an anti-inflammatory cytokine, is a stimulatory factor for B and T cells, and has an immunosuppressive effect in the colon[107,108]. IL-4 and IL-10 are able to down-regulate inflammatory mediators including TNF-α and IL-1 and favour a humoral immune response[109]. In proctitis, the combined effects of IL-4 and IL-10 were shown to shift the Th1/Th2 cell activation in favour of a Th2-type response[109], which eventually ameliorated mucosal healing.
In T-cell receptor-α chain-deficient (TCR-α -/-) mice, anti-IL-4 monoclonal antibody treatment altered the cytokine profile of CD4+ ββ T cells (a subset of CD4+ Th2-type cells) from dominant Th2 to Th1 type, resulting in the prevention of mucosal inflammation in TCR-α -/- mice[110]. The treatment of peripheral blood mononuclear cells form active UC and CD patients with IL-4 in vitro resulted in significant decrease of the vascular endothelial growth factor (VEGF) production of these cells, which suggests that the known defective immunosuppressive role of IL-4 in IBD may contribute to the pathogenesis of inflammation by VEGF mediated mechanisms[111].
Similarly to UC, the IL-1 system plays an important role in the pathogenesis of CD. The IL-1/IL-1Ra ratio is in line with the activity of CD[7]. In a recent study using phage display technology, a short peptide (TCP-353) was identified from the blood mononuclear cells of CD patients which specifically binds to CD sera and stimulates the pro-inflammatory responses (IL-1β, IL-6 and TNF-α) of CD mononuclear cells[112]. This novelty may have diagnostic, pathogenic and therapeutic significance with regard to the treatment of CD.
IL-18, another member of the IL-1 family, was originally described as an important Th1 cell polarizing cytokine[113]. The level of IL-18 is increased in the inflamed mucosa of a subgroup of CD patients[114,115]. The balance between this pleiotropic pro-inflammatory cytokine and its natural inhibitor, IL-18-binding protein (IL-18BP), may contribute to the pathogenesis of IBD[116]. IL-18 is localized to lamina proprial cells and intestinal epithelial cells, suggesting that both groups of cells may be involved in the complex events occurring in CD[114]. In the presence of IL-18, mucosal T cells from active CD have been shown to produce less IL-10 than control tissue[117]. Recombinant IL-18 alone is able to induce a significant proliferative response in mucosal lymphocytes of active CD, moreover a synergy between IL-18 and IL-12 in macrophages may regulate driving of mucosal lymphocytes toward a Th1 response[118,119].
Leach et al[116] found that IL-18, produced in the colons of children with CD, contributes to the local inflammatory changes. They showed that systemic IL-18 level is a possible and useful indicator of disease activity. Furthermore, free IL-18 was found to be greatly elevated in CD children, suggesting that compensatory increases in IL-18BP are insufficient. Further exploration of the role of IL-18 in the pathogenesis of CD is now required.
The role of TNF-α in CD has been widely investigated[120-122]. Binding TNF-α to serum soluble TNF receptor 1 and 2 (sTNFR1 and 2) initiates pro-inflammatory signaling. The levels of sTNFR1 and 2 are elevated in CD sera compared to both UC and normal controls, hence it can be used as a marker for disease activity and discriminatory factor between the two subtypes of IBD[123,124]. It was recently demonstrated that TNFR1-signaling cascade in colonic myeloid lineage cells contributes to the suppression of acute damage-associated mortality presumably by controlling colonic epithelial cell homeostasis[125].
The central pro-inflammatory role of TNF-α has substantiated the use of anti-TNF-α antibodies in the treatment of CD[126].
The TL1A/DR3 system is also involved in the pathogenesis of CD[32]. The macrophages of the lamina propria are a major producer of TL1A, which expression is markedly enhanced in CD compared with UC or normal colon[127]. Kamada et al[127] found that TL1A and IL-23 synergistically promotes the production of IFN-γ and IL-17 by mucosal T cells, while TL1A alone does not induce cytokine production. Furthermore, they have also shown that TL1A promotes Th17 differentiation from naïve T cells by mucosal macrophages; however, IL-23 did not show any synergistic effects on Th17 differentiation.
TNF ligand superfamily, member 14 (TNFSF14, also known as LIGHT) is a type II membrane protein that forms a biologically active homotrimer, which can be cleaved into a soluble form or exist in an intracellular form with deleted transmembrane region and not displayed on the cell surface[128,129]. The human intestinal mucosa may be a primary site for LIGHT-mediated pro-inflammatory activity, which shows a correlation with disease activity[128]. In CD, it was shown that IFN-producing CD4+ lamina propria T cells express LIGHT mediating a Th1 response[128]. As several data from transgenic mouse models[130,131] indicated that LIGHT-dependent inflammation selectively targeted the intestine, the mucosal specificity of LIGHT-mediated inflammation could have significant pathological implications in human CD, which needs further investigation.
The IL-6/STAT3 signaling system plays a key role in the pathogenesis of CD. The circulating levels of IL-6 and sIL-6R is in correlation with the activity of the disease[132]. The pathogenic role of IL-6-sIL-6R system in mediating the resistance of T cells to apoptosis in CD was proved by blocking IL-6 trans-signaling[133].
Subepithelial myofibroblasts can also be a source of mucosal IL-6 in CD. It was recently demonstrated that the increased production of IL-6 synthesis related to the oxidative state, suggesting redox regulation with the involvement of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase activation[134]. Based on these data, IL-6 may have an influence not just on the chronic inflammatory process, but on relapses occurring in the pathology of CD.
As it is in UC, the expression of IL-12 is up-regulated in active CD mucosa as well, and its level is in correlation with disease activity[50]. Because of the elevated levels of IL-12p40 and IL-12Rβ2 in the early phase of CD, it is suggested that IL-12 is primarily involved in the early induction of Th1 polarization of naïve T cells[135]. However, the expansion and maintenance of Th1 cell response in the colon requires additional signals. The IL-12-dependent synthesis of IFN-γ of the mucosal T cells can be enhanced by cytokines that signal through the common γ-chain receptor (i.e., IL-7, IL-15, IL-21)[136].
IL-23 also has an elevated mucosal level in CD[137]. Based on the results from mouse studies with targeted deletion of either the IL-12/p35 or IL-23/p19 subunit, it is suggested that IL-23 and not IL-12 is essential for manifestation of intestinal inflammation occurring in IL-10-deficient mice[138]. The IL-23-driven intestinal inflammation seems to be mediated by IL-17 and IL-6 production. It needs to be further investigated whether the harmful effect of IL-23 on the ongoing mucosal inflammation occurs only in the absence of IL-10-related regulatory effects.
IL-27 is a newly described, heterodimeric member of the IL-12 family[47]. It was proved by in vitro studies that IL-27 is mainly produced by activated monocytes and dendritic cells, it induces the proliferation of naïve CD4+ T cells and synergizes with IL-12 for IFN-γ production[47].
The mucosal expression of IL-27p28 was shown to correlate with the activity of disease in both UC and CD[137]. Particularly, IL-27p28 and EBI3 transcripts have shown to be significantly elevated only in active CD[137].
In humans, the mucosal level of IL-17 levels is highly elevated in active CD[78]. Recently, it has been shown that in CD patients increased numbers of circulating IL-17 and IFN-γ-producing CD161+ memory cells are present, and these cells constitute a high percentage of colonic mucosal cells[139]. In addition, CD patients have increased numbers of circulating IL-23R expressing T cells, which respond to IL-23 with increased production of IL-17, IL-22 and IFN-γ, which is further increased by the presence of IL-1β. Moreover, these cells express gut homing receptors CCR6 and β7-integrin, which makes them to be programmed to recruit into the lamina propria during inflammation[140]. Based on these results, Th17 cells producing both IL-17 and IFN-γ are identified as important elements in the inflammatory response in CD.
Dendritic cells are crucial in inducing acquired immunity. In CD, dendritic cells of myeloid origin were found to produce a higher amount of IL-23 and a lower amount of IL-10, when stimulated with exogenous bacterial derivative, moreover they induced a dysregulated Th1/Th17 immune response in mixed lymphocyte reaction than it is in UC and normal control[78].
Similarly to UC, different results were obtained from different studies; therefore it will be important to clarify the source and function of IL-17 in the pathogenesis of CD.
IL-21 is significantly overexpressed in CD mucosa[141]. IL-21 is generated mainly by CD4+IFN-γ-producing T cells[84]. In contrast, only a small fraction of IL-21 producing CD4+ T-cells co-express IL-17A, thus indicating that, in humans, IL-21 is produced preferentially by Th1 rather than Th17 cells. Activation of CD4+ T-lymphocytes from normal colon with anti-CD3 antibody and exogenous IL-12 increases the proportion of IL-21-secreting Th1 cells, whereas blockade of endogenous IL-12 in CD mucosal cell cultures significantly reduces IL-21 production[142]. On the other hand, blocking IL-21 in cells from CD with antibodies or soluble receptor fusion proteins inhibits IL-17A and IFN-γ production[142].
It was also found that intestinal epithelial cells and subepithelial fibroblasts constitutively express IL-21R and respond to IL-21 by inflammatory molecule secretion. Following IL-21 stimulation, colonic fibroblasts secrete large amounts of matrix metalloproteinase 1 and 3, enzymes involved in mucosal injury of CD[143,144].
IL-10 plays a pivotal anti-inflammatory role in CD. An inactivation of IL-10 resulted in an increased production of the pro-inflammatory IL-12 and IFN-γ in mice[145]. In humans, the inflamed mucosa and granulomas of CD show low IL-10 levels[146]. It was also recently described that endogenous IL-10 constrains Th17 cell development through the control of dendritic cells’ IL-1 production, which reaffirms the crucial anti-inflammatory role of IL-10 in patients with CD[147].
On the contrary, the level of IL-22 is elevated in CD mucosa and serum[98]. It was shown that IL-23R genotypes have an effect the serum concentrations of IL-22, which links genetic CD susceptibility to Th17 cell function[97].
Regarding IL-22, a new regulatory pathway was recently described in CD[148]. The aryl hydrocarbon receptor (AhR) may represent a link between the environment and the mucosal immune system. AhR is a transcription factor which is activated by a large number of environmental factors[148]. It has been recently shown that mucosal T cells and NK cells isolated from active CD biopsies express low levels of AhR and respond to AhR ligands with decreasing pro-inflammatory cytokine production and up-regulating IL-22[149]. Hereby, the changing mucosal cytokine profile promotes mucosal healing.
TGF-β is thought to be an inhibitory key cytokine of immunological homeostasis and inflammatory responses. On the other hand, TGF-β is also a potent profibrogenic agent inducing collagen synthesis and regulating the balance between matrix-degrading matrix metalloproteinases and their inhibitors[150]. It has a role in CD-related fibrosis, as changes in TGF-β signaling and matrix metalloproteinase production were identified in the mucosa overlying strictures[150].
It was also shown that TGF-β induces IL-13 expression and epithelial-to-mesenchymal transition of intestinal epithelial cells, while IL-13 promotes the expression of genes associated with cell invasion[151]. Based on these data, it seems that TGF-β and IL-13 play a synergistic role in the pathogenesis of CD-associated fistulae[151], which has therapeutic consequences.
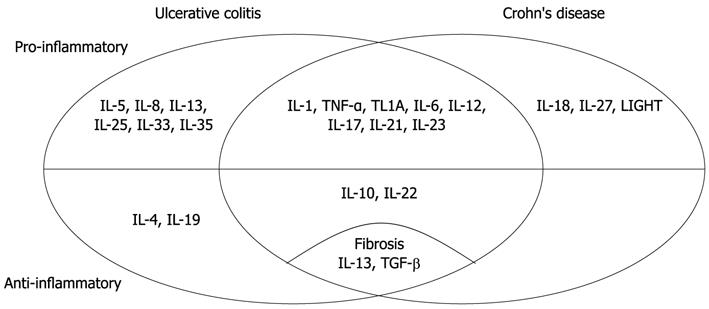
Cytokines have important and complex role in the pathogenesis of IBD (Table 1 and Figure 1). There are several different biologic therapies directed to cytokines or their receptors which have possibilities in the treatment of IBD.
| Ulcerative colitis | Crohn's disease | ||
| Interleukin-1 family | IL-1 | Inflammation induction | |
| IL-18 | NI | Mucosal T cell IL-10 secretion ↓ | |
| Th1 response ↑ | |||
| IL-33 | Reconstitute mucosal barrier defence | NI | |
| Epithelial mucus secretion ↑ | |||
| Th2-response ↑ | |||
| Wound healing and angiogenesis ↑ | |||
| Tumor necrosis factor superfamily | TNF-α | Adhesion molecules expression ↑ | Initiates pro-inflammatory signaling |
| Fibroblast proliferation ↑ | |||
| Procoagulant factors level ↑ | |||
| Initiation of cytotoxic, apoptotic, and acute-phase responses | |||
| IL-1β, IL-6, IL-33 production ↑ | |||
| Modulates epithelial cell ST2 expression | |||
| TL1A | IFN-γ secretion ↑ | IFN-γ, IL-17 production ↑ | |
| Modulates epithelial cell apoptosis | Th17 differentiation ↑ | ||
| LIGHT | NI | Mediates Th1 response and mucosa specific inflammation | |
| Interleukin-6 family | IL-6 | Involved in colitis-associated carcinogenesis | Mediates T cell resistance to apoptosis |
| Possible role in extraintestinal manifestations | Influences of disease relapse | ||
| Interleukin-8 | IL-8 | Mediates the activation and migration of neutrophils | NI |
| Interleukin-12 family | IL-12 | Modulates macrophage activity | Early induction of Th1 polarization of naïve T cells |
| IFN-γ of the mucosal T cells | |||
| IL-23 | Promotes Th17 cell differentiation | IL-17 and IL-6 mediated intestinal inflammation | |
| Controlling Th17 response | |||
| Influences Treg cell development | |||
| Number of mucosal Treg cells ↓ | |||
| IL-27 | Proliferation of naïve CD4+ T cells ↑ | ||
| IFN-γ production ↑ | |||
| IL-35 | Possible controlling of T-cell dependent inflammation | NI | |
| Interleukin-13 | IL-13 | Induces cellular cytotoxicity against colonic epithelium | NI |
| Epithelial cell apoptosis ↑ | |||
| Tight junction integrity ↓ | |||
| Epithelial restitution velocity ↓ | |||
| Colonic wall fibrosis ↑ | |||
| Interleukin 17 family | IL-17 | Pro- and anti-inflammatory effects | IL-17, IL-22, IFN-γ production ↑ |
| Enhance T cell recruitment into the lamina propria | |||
| IL-25 | Possible inhibition of IL-12 secretion | NI | |
| Possible promotion of Th1-driven inflammation | |||
| Interleukin-21 | IL-21 | Maintainer of Th1-mediated inflammation | Required for IL-17A and IFN-γ production |
| Inhibits Treg cell differentiation | Fibroblasts MMP secretion ↑ | ||
| CD4+ T cell resistance to Treg-suppression ↑ | |||
| Interleukin-5 | IL-5 | Activates eosinophil function and migration | NI |
| Interleukin-10 family | IL-10 | Inhibits antigen presentation | Constrains Th17 cell development |
| Pro-inflammatory cytokine release ↓ | |||
| IL-19 | TNF-α, IL-6, IL-12 secretion ↓ | NI | |
| IL-22 | Goblet cell hyperplasia ↑ | Promotes mucosal healing | |
| Mucus production ↑ | |||
| Interleukin-4 | IL-4 | TNF-α, IL-1 production ↓ | NI |
| Humoral immune response ↑ | |||
| Mucosal healing ↑ | |||
| Monocyte/macrophage VEGF production ↓ | |||
| Transforming growth factor-β | TGF-β | Possible suppression of IL-33 production | Collagen synthesis ↑ |
| Regulates the balance between matrix-degrading MMPs and their inhibitors | |||
| IL-13 expression ↑ | |||
| EMT ↑ | |||
Some anti-TNF-α antibodies are currently being used to treat CD and UC. Although these molecules dramatically improved the treatment of patients, sometimes severe side effects or the development of anti-drug antibodies limits their application.
Neutralizing antibodies targeting other pathways of the immune response have been developed and tested[152]. Antibodies targeting the IL-12 and IL-23 pathways, or pro-inflammatory cytokines (i.e., IFN-γ, IL-2, IL-6, IL-17A) initially showed a promising result, but for none of their efficacy has undoubtedly been established[153]. Administration of the regulatory cytokines, namely IL-10 and IL-11, also failed to induce reproducible clinical effects[152].
Accordingly to the complex effects and regulation of cytokines in IBD, the cytokine-based therapies of the future must have higher specificity and lower toxicity.
We thank Tiana M Germann for her assistance in English language editing.
| 1. | Casini-Raggi V, Kam L, Chong YJ, Fiocchi C, Pizarro TT, Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154:2434-2440. [PubMed] |
| 2. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3416] [Article Influence: 179.8] [Reference Citation Analysis (12)] |
| 3. | Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261-1270. [PubMed] |
| 4. | Bamias G, Nyce MR, De La Rue SA, Cominelli F. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143:895-904. [PubMed] |
| 5. | Dinarello CA. Interleukin-1beta and the autoinflammatory diseases. N Engl J Med. 2009;360:2467-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1-13. [PubMed] |
| 7. | Dionne S, D'Agata ID, Hiscott J, Vanounou T, Seidman EG. Colonic explant production of IL-1and its receptor antagonist is imbalanced in inflammatory bowel disease (IBD). Clin Exp Immunol. 1998;112:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | McAlindon ME, Hawkey CJ, Mahida YR. Expression of interleukin 1 beta and interleukin 1 beta converting enzyme by intestinal macrophages in health and inflammatory bowel disease. Gut. 1998;42:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Baekkevold ES, Roussigné M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 377] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 10. | Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2564] [Cited by in RCA: 2956] [Article Influence: 140.8] [Reference Citation Analysis (6)] |
| 11. | Wood IS, Wang B, Trayhurn P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun. 2009;384:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008;3:e3331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 829] [Cited by in RCA: 963] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 13. | Matsuba-Kitamura S, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Taki Y, Muto T, Ikeda T, Mimura O, Nakanishi K. Contribution of IL-33 to induction and augmentation of experimental allergic conjunctivitis. Int Immunol. 2010;22:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469-6477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 581] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 15. | Palmer G, Talabot-Ayer D, Lamacchia C, Toy D, Seemayer CA, Viatte S, Finckh A, Smith DE, Gabay C. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009;60:738-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Beltrán CJ, Núñez LE, Díaz-Jiménez D, Farfan N, Candia E, Heine C, López F, González MJ, Quera R, Hermoso MA. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1097-1107. [PubMed] |
| 17. | Kobori A, Yagi Y, Imaeda H, Ban H, Bamba S, Tsujikawa T, Saito Y, Fujiyama Y, Andoh A. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 18. | Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M, Fiocchi C, Vecchi M, Pizarro TT. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA. 2010;107:8017-8022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 361] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 19. | Seidelin JB, Bjerrum JT, Coskun M, Widjaya B, Vainer B, Nielsen OH. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett. 2010;128:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Pastorelli L, De Salvo C, Cominelli MA, Vecchi M, Pizarro TT. Novel cytokine signaling pathways in inflammatory bowel disease: insight into the dichotomous functions of IL-33 during chronic intestinal inflammation. Therap Adv Gastroenterol. 2011;4:311-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Pushparaj PN, Tay HK, H'ng SC, Pitman N, Xu D, McKenzie A, Liew FY, Melendez AJ. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci USA. 2009;106:9773-9778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 799] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 23. | Küchler AM, Pollheimer J, Balogh J, Sponheim J, Manley L, Sorensen DR, De Angelis PM, Scott H, Haraldsen G. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol. 2008;173:1229-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 24. | Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2679] [Cited by in RCA: 2914] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 25. | Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech. 2000;50:184-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1424] [Cited by in RCA: 1421] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 27. | Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 388] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1942] [Cited by in RCA: 1884] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 29. | Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280-4288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 440] [Cited by in RCA: 532] [Article Influence: 29.6] [Reference Citation Analysis (11)] |
| 30. | Begue B, Wajant H, Bambou JC, Dubuquoy L, Siegmund D, Beaulieu JF, Canioni D, Berrebi D, Brousse N, Desreumaux P. Implication of TNF-related apoptosis-inducing ligand in inflammatory intestinal epithelial lesions. Gastroenterology. 2006;130:1962-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Reimund JM, Wittersheim C, Dumont S, Muller CD, Baumann R, Poindron P, Duclos B. Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn's disease. J Clin Immunol. 1996;16:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Bamias G, Martin C, Marini M, Hoang S, Mishina M, Ross WG, Sachedina MA, Friel CM, Mize J, Bickston SJ. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171:4868-4874. [PubMed] |
| 33. | Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243-1254. [PubMed] |
| 34. | Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Boulanger MJ, Bankovich AJ, Kortemme T, Baker D, Garcia KC. Convergent mechanisms for recognition of divergent cytokines by the shared signaling receptor gp130. Mol Cell. 2003;12:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Li Y, de Haar C, Chen M, Deuring J, Gerrits MM, Smits R, Xia B, Kuipers EJ, van der Woude CJ. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 37. | Mitsuyama K, Toyonaga A, Sasaki E, Ishida O, Ikeda H, Tsuruta O, Harada K, Tateishi H, Nishiyama T, Tanikawa K. Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut. 1995;36:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 167] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Kusugami K, Fukatsu A, Tanimoto M, Shinoda M, Haruta J, Kuroiwa A, Ina K, Kanayama K, Ando T, Matsuura T. Elevation of interleukin-6 in inflammatory bowel disease is macrophage- and epithelial cell-dependent. Dig Dis Sci. 1995;40:949-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Daig R, Rogler G, Aschenbrenner E, Vogl D, Falk W, Gross V, Schölmerich J, Andus T. Human intestinal epithelial cells secrete interleukin-1 receptor antagonist and interleukin-8 but not interleukin-1 or interleukin-6. Gut. 2000;46:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Wang L, Walia B, Evans J, Gewirtz AT, Merlin D, Sitaraman SV. IL-6 induces NF-kappa B activation in the intestinal epithelia. J Immunol. 2003;171:3194-3201. [PubMed] |
| 41. | Salmi M, Jalkanen S. Human leukocyte subpopulations from inflamed gut bind to joint vasculature using distinct sets of adhesion molecules. J Immunol. 2001;166:4650-4657. [PubMed] |
| 42. | Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007;13:1016-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 43. | Hull J, Ackerman H, Isles K, Usen S, Pinder M, Thomson A, Kwiatkowski D. Unusual haplotypic structure of IL8, a susceptibility locus for a common respiratory virus. Am J Hum Genet. 2001;69:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Rodríguez-Perlvárez ML, García-Sánchez V, Villar-Pastor CM, González R, Iglesias-Flores E, Muntane J, Gómez-Camacho F. Role of serum cytokine profile in ulcerative colitis assessment. Inflamm Bowel Dis. 2012;18:1864-1871. [PubMed] [DOI] [Full Text] |
| 45. | Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 421] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 46. | Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2046] [Cited by in RCA: 2138] [Article Influence: 82.2] [Reference Citation Analysis (7)] |
| 47. | Larousserie F, Bardel E, Pflanz S, Arnulf B, Lome-Maldonado C, Hermine O, Brégeaud L, Perennec M, Brousse N, Kastelein R. Analysis of interleukin-27 (EBI3/p28) expression in Epstein-Barr virus- and human T-cell leukemia virus type 1-associated lymphomas: heterogeneous expression of EBI3 subunit by tumoral cells. Am J Pathol. 2005;166:1217-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1324] [Cited by in RCA: 1551] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 49. | Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2687] [Cited by in RCA: 2933] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 50. | Nielsen OH, Kirman I, Rüdiger N, Hendel J, Vainer B. Upregulation of interleukin-12 and -17 in active inflammatory bowel disease. Scand J Gastroenterol. 2003;38:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Kobayashi T, Matsuoka K, Sheikh SZ, Elloumi HZ, Kamada N, Hisamatsu T, Hansen JJ, Doty KR, Pope SD, Smale ST. NFIL3 is a regulator of IL-12 p40 in macrophages and mucosal immunity. J Immunol. 2011;186:4649-4655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 52. | Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 803] [Article Influence: 40.2] [Reference Citation Analysis (1)] |
| 53. | Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 54. | Maul J, Zeitz M. Ulcerative colitis: immune function, tissue fibrosis and current therapeutic considerations. Langenbecks Arch Surg. 2012;397:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 695] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 56. | Becker C, Dornhoff H, Neufert C, Fantini MC, Wirtz S, Huebner S, Nikolaev A, Lehr HA, Murphy AJ, Valenzuela DM. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol. 2006;177:2760-2764. [PubMed] |
| 57. | Shen W, Durum SK. Synergy of IL-23 and Th17 cytokines: new light on inflammatory bowel disease. Neurochem Res. 2010;35:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3769] [Cited by in RCA: 4210] [Article Influence: 210.5] [Reference Citation Analysis (0)] |
| 59. | Műzes G, Molnár B, Sipos F. Regulatory T cells in inflammatory bowel diseases and colorectal cancer. World J Gastroenterol. 2012;In press. |
| 60. | Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1569] [Cited by in RCA: 1572] [Article Influence: 87.3] [Reference Citation Analysis (18)] |
| 61. | Izcue A, Hue S, Buonocore S, Arancibia-Cárcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 328] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 62. | Wirtz S, Billmeier U, Mchedlidze T, Blumberg RS, Neurath MF. Interleukin-35 mediates mucosal immune responses that protect against T-cell-dependent colitis. Gastroenterology. 2011;141:1875-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 63. | Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 582] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 64. | Inoue S, Matsumoto T, Iida M, Mizuno M, Kuroki F, Hoshika K, Shimizu M. Characterization of cytokine expression in the rectal mucosa of ulcerative colitis: correlation with disease activity. Am J Gastroenterol. 1999;94:2441-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 919] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 66. | Fuss IJ, Strober W. The role of IL-13 and NK T cells in experimental and human ulcerative colitis. Mucosal Immunol. 2008;1 Suppl 1:S31-S33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 67. | Mannon PJ, Hornung RL, Yang Z, Yi C, Groden C, Friend J, Yao M, Strober W, Fuss IJ. Suppression of inflammation in ulcerative colitis by interferon-β-1a is accompanied by inhibition of IL-13 production. Gut. 2011;60:449-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 68. | Rosen MJ, Frey MR, Washington MK, Chaturvedi R, Kuhnhein LA, Matta P, Revetta FL, Wilson KT, Polk DB. STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflamm Bowel Dis. 2011;17:2224-2234. [PubMed] [DOI] [Full Text] |
| 69. | Bamias G, Kaltsa G, Ladas SD. Cytokines in the pathogenesis of ulcerative colitis. Discov Med. 2011;11:459-467. [PubMed] |
| 70. | Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 729] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 71. | Fichtner-Feigl S, Fuss IJ, Young CA, Watanabe T, Geissler EK, Schlitt HJ, Kitani A, Strober W. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859-5870. [PubMed] |
| 72. | Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2372] [Cited by in RCA: 2518] [Article Influence: 125.9] [Reference Citation Analysis (4)] |
| 73. | Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 925] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 74. | Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36-39. [PubMed] |
| 75. | Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Iwakura Y, Okanoue T. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008;377:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 76. | Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 369] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 77. | O'Connor W, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 642] [Cited by in RCA: 651] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 78. | Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 79. | Caruso R, Sarra M, Stolfi C, Rizzo A, Fina D, Fantini MC, Pallone F, MacDonald TT, Monteleone G. Interleukin-25 inhibits interleukin-12 production and Th1 cell-driven inflammation in the gut. Gastroenterology. 2009;136:2270-2279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 80. | Hom JT, Estridge T. Antigen-induced recruitment of eosinophils: importance of CD4+ T cells, IL5, and mast cells. Clin Immunol Immunopathol. 1994;73:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Denis MA, Louis R, Malaise M, Belaiche J, Louis E. Increased response of blood eosinophils to various chemotactic agents in quiescent Crohn disease. Scand J Gastroenterol. 2001;36:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Lampinen M, Carlson M, Håkansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy. 2004;59:793-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 83. | Fina D, Caruso R, Pallone F, Monteleone G. Interleukin-21 (IL-21) controls inflammatory pathways in the gut. Endocr Metab Immune Disord Drug Targets. 2007;7:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | Sarra M, Monteleone I, Stolfi C, Fantini MC, Sileri P, Sica G, Tersigni R, Macdonald TT, Pallone F, Monteleone G. Interferon-gamma-expressing cells are a major source of interleukin-21 in inflammatory bowel diseases. Inflamm Bowel Dis. 2010;16:1332-1339. [PubMed] |
| 85. | De Nitto D, Sarra M, Pallone F, Monteleone G. Interleukin-21 triggers effector cell responses in the gut. World J Gastroenterol. 2010;16:3638-3641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081-2095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2007] [Cited by in RCA: 2104] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 87. | Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121:1108-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 88. | Paul G, Khare V, Gasche C. Inflamed gut mucosa: downstream of interleukin-10. Eur J Clin Invest. 2012;42:95-109. [PubMed] [DOI] [Full Text] |
| 89. | Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev. 2007;59:1073-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 368] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 90. | Melgar S, Yeung MM, Bas A, Forsberg G, Suhr O, Oberg A, Hammarstrom S, Danielsson A, Hammarstrom ML. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol. 2003;134:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 92. | Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 474] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 93. | Anderson CA, Massey DC, Barrett JC, Prescott NJ, Tremelling M, Fisher SA, Gwilliam R, Jacob J, Nimmo ER, Drummond H. Investigation of Crohn's disease risk loci in ulcerative colitis further defines their molecular relationship. Gastroenterology. 2009;136:523-9.e3. [PubMed] |
| 94. | Azuma YT, Nakajima H, Takeuchi T. IL-19 as a potential therapeutic in autoimmune and inflammatory diseases. Curr Pharm Des. 2011;17:3776-3780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Chang C, Magracheva E, Kozlov S, Fong S, Tobin G, Kotenko S, Wlodawer A, Zdanov A. Crystal structure of interleukin-19 defines a new subfamily of helical cytokines. J Biol Chem. 2003;278:3308-3313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Yamamoto-Furusho JK, Álvarez-León E, Fragoso JM, Gozalishvilli A, Vallejo M, Vargas-Alarcón G. Protective role of interleukin-19 gene polymorphisms in patients with ulcerative colitis. Hum Immunol. 2011;72:1029-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 97. | Schmechel S, Konrad A, Diegelmann J, Glas J, Wetzke M, Paschos E, Lohse P, Göke B, Brand S. Linking genetic susceptibility to Crohn's disease with Th17 cell function: IL-22 serum levels are increased in Crohn's disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis. 2008;14:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 98. | Shimomura Y, Ogawa A, Kawada M, Sugimoto K, Mizoguchi E, Shi HN, Pillai S, Bhan AK, Mizoguchi A. A unique B2 B cell subset in the intestine. J Exp Med. 2008;205:1343-1355. [PubMed] |
| 99. | Yamamoto-Furusho JK, Miranda-Pérez E, Fonseca-Camarillo G, Sánchez-Muñoz F, Dominguez-Lopez A, Barreto-Zuñiga R. Colonic epithelial upregulation of interleukin 22 (IL-22) in patients with ulcerative colitis. Inflamm Bowel Dis. 2010;16:1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH, Loke P. IL-22+ CD4+ T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med. 2010;2:60ra88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 101. | Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1535] [Cited by in RCA: 1753] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 102. | Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 597] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 103. | Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 104. | Fahlén L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 387] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 105. | Rani R, Smulian AG, Greaves DR, Hogan SP, Herbert DR. TGF-β limits IL-33 production and promotes the resolution of colitis through regulation of macrophage function. Eur J Immunol. 2011;41:2000-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 106. | Souza RF, Lei J, Yin J, Appel R, Zou TT, Zhou X, Wang S, Rhyu MG, Cymes K, Chan O. A transforming growth factor beta 1 receptor type II mutation in ulcerative colitis-associated neoplasms. Gastroenterology. 1997;112:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | West GA, Matsuura T, Levine AD, Klein JS, Fiocchi C. Interleukin 4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology. 1996;110:1683-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 108. | Nielsen OH, Køppen T, Rüdiger N, Horn T, Eriksen J, Kirman I. Involvement of interleukin-4 and -10 in inflammatory bowel disease. Dig Dis Sci. 1996;41:1786-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 109. | Rogy MA, Beinhauer BG, Reinisch W, Huang L, Pokieser P. Transfer of interleukin-4 and interleukin-10 in patients with severe inflammatory bowel disease of the rectum. Hum Gene Ther. 2000;11:1731-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 110. | Iijima H, Takahashi I, Kishi D, Kim JK, Kawano S, Hori M, Kiyono H. Alteration of interleukin 4 production results in the inhibition of T helper type 2 cell-dominated inflammatory bowel disease in T cell receptor alpha chain-deficient mice. J Exp Med. 1999;190:607-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Griga T, Hebler U, Voigt E, Tromm A, May B. Interleukin-4 inhibits the increased production of vascular endothelial growth factor by peripheral blood mononuclear cells in patients with inflammatory bowel disease. Hepatogastroenterology. 2000;47:1604-1607. [PubMed] |
| 112. | Mitsuyama K, Niwa M, Masuda J, Kuwaki K, Yamasaki H, Takedatsu H, Kobayashi T, Sata M. Isolation and characterization of a novel short peptide associated with Crohn's disease. Clin Exp Immunol. 2011;166:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 113. | Dinarello CA. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 615] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 114. | Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Foley E, Moskaluk CA, Bickston SJ, Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829-6835. [PubMed] |
| 115. | Schmidt C, Giese T, Goebel R, Schilling M, Marth T, Ruether A, Schreiber S, Zeuzem S, Meuer SC, Stallmach A. Interleukin-18 is increased only in a minority of patients with active Crohn's disease. Int J Colorectal Dis. 2007;22:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 116. | Leach ST, Messina I, Lemberg DA, Novick D, Rubenstein M, Day AS. Local and systemic interleukin-18 and interleukin-18-binding protein in children with inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 117. | Maerten P, Shen C, Colpaert S, Liu Z, Bullens DA, van Assche G, Penninckx F, Geboes K, Vanham G, Rutgeerts P. Involvement of interleukin 18 in Crohn's disease: evidence from in vitro analysis of human gut inflammatory cells and from experimental colitis models. Clin Exp Immunol. 2004;135:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 118. | Kanai T, Watanabe M, Okazawa A, Sato T, Yamazaki M, Okamoto S, Ishii H, Totsuka T, Iiyama R, Okamoto R. Macrophage-derived IL-18-mediated intestinal inflammation in the murine model of Crohn's disease. Gastroenterology. 2001;121:875-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 119. | Okazawa A, Kanai T, Nakamaru K, Sato T, Inoue N, Ogata H, Iwao Y, Ikeda M, Kawamura T, Makita S. Human intestinal epithelial cell-derived interleukin (IL)-18, along with IL-2, IL-7 and IL-15, is a potent synergistic factor for the proliferation of intraepithelial lymphocytes. Clin Exp Immunol. 2004;136:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Veerappan SG, O'Morain CA, Daly JS, Ryan BM. Review article: the effects of antitumour necrosis factor-α on bone metabolism in inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:1261-1272. [PubMed] [DOI] [Full Text] |
| 121. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2773] [Article Influence: 115.5] [Reference Citation Analysis (3)] |
| 122. | Akobeng AK, Zachos M. Tumor necrosis factor-alpha antibody for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2004;CD003574. [PubMed] |
| 123. | Spoettl T, Hausmann M, Klebl F, Dirmeier A, Klump B, Hoffmann J, Herfarth H, Timmer A, Rogler G. Serum soluble TNF receptor I and II levels correlate with disease activity in IBD patients. Inflamm Bowel Dis. 2007;13:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 124. | Yamamoto-Furusho JK. Innovative therapeutics for inflammatory bowel disease. World J Gastroenterol. 2007;13:1893-1896. [PubMed] |
| 125. | Mizoguchi E, Hachiya Y, Kawada M, Nagatani K, Ogawa A, Sugimoto K, Mizoguchi A, Podolsky DK. TNF receptor type I-dependent activation of innate responses to reduce intestinal damage-associated mortality. Gastroenterology. 2008;134:470-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 126. | Buchner AM, Blonski W, Lichtenstein GR. Update on the management of Crohn's disease. Curr Gastroenterol Rep. 2011;13:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 127. | Kamada N, Hisamatsu T, Honda H, Kobayashi T, Chinen H, Takayama T, Kitazume MT, Okamoto S, Koganei K, Sugita A. TL1A produced by lamina propria macrophages induces Th1 and Th17 immune responses in cooperation with IL-23 in patients with Crohn's disease. Inflamm Bowel Dis. 2010;16:568-575. [PubMed] |
| 128. | Cohavy O, Zhou J, Granger SW, Ware CF, Targan SR. LIGHT expression by mucosal T cells may regulate IFN-gamma expression in the intestine. J Immunol. 2004;173:251-258. [PubMed] |
| 129. | Granger SW, Butrovich KD, Houshmand P, Edwards WR, Ware CF. Genomic characterization of LIGHT reveals linkage to an immune response locus on chromosome 19p13.3 and distinct isoforms generated by alternate splicing or proteolysis. J Immunol. 2001;167:5122-5128. [PubMed] |
| 130. | Shaikh RB, Santee S, Granger SW, Butrovich K, Cheung T, Kronenberg M, Cheroutre H, Ware CF. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol. 2001;167:6330-6337. [PubMed] |
| 131. | Wang J, Lo JC, Foster A, Yu P, Chen HM, Wang Y, Tamada K, Chen L, Fu YX. The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. J Clin Invest. 2001;108:1771-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 132. | Reinisch W, Gasché C, Tillinger W, Wyatt J, Lichtenberger C, Willheim M, Dejaco C, Waldhör T, Bakos S, Vogelsang H. Clinical relevance of serum interleukin-6 in Crohn's disease: single point measurements, therapy monitoring, and prediction of clinical relapse. Am J Gastroenterol. 1999;94:2156-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 138] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 133. | Van Kemseke C, Belaiche J, Louis E. Frequently relapsing Crohn's disease is characterized by persistent elevation in interleukin-6 and soluble interleukin-2 receptor serum levels during remission. Int J Colorectal Dis. 2000;15:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 134. | Catarzi S, Favilli F, Romagnoli C, Marcucci T, Picariello L, Tonelli F, Vincenzini MT, Iantomasi T. Oxidative state and IL-6 production in intestinal myofibroblasts of Crohn's disease patients. Inflamm Bowel Dis. 2011;17:1674-1684. [PubMed] [DOI] [Full Text] |
| 135. | Kugathasan S, Saubermann LJ, Smith L, Kou D, Itoh J, Binion DG, Levine AD, Blumberg RS, Fiocchi C. Mucosal T-cell immunoregulation varies in early and late inflammatory bowel disease. Gut. 2007;56:1696-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 136. | Peluso I, Pallone F, Monteleone G. Interleukin-12 and Th1 immune response in Crohn's disease: pathogenetic relevance and therapeutic implication. World J Gastroenterol. 2006;12:5606-5610. [PubMed] |
| 137. | Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, Meuer SC, Stallmach A. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis. Inflamm Bowel Dis. 2005;11:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 138. | Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1249] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 139. | Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 382] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 140. | Strober W, Zhang F, Kitani A, Fuss I, Fichtner-Feigl S. Proinflammatory cytokines underlying the inflammation of Crohn's disease. Curr Opin Gastroenterol. 2010;26:310-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 141. | Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 889] [Cited by in RCA: 850] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 142. | Monteleone G, Monteleone I, Fina D, Vavassori P, Del Vecchio Blanco G, Caruso R, Tersigni R, Alessandroni L, Biancone L, Naccari GC. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn's disease. Gastroenterology. 2005;128:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 245] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 143. | Sengupta N, MacDonald TT. The role of matrix metalloproteinases in stromal/epithelial interactions in the gut. Physiology (Bethesda). 2007;22:401-409. [PubMed] |
| 144. | Monteleone G, Caruso R, Fina D, Peluso I, Gioia V, Stolfi C, Fantini MC, Caprioli F, Tersigni R, Alessandroni L. Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut. 2006;55:1774-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 145. | Rennick DM, Fort MM. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10(-/-) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2000;278:G829-G833. [PubMed] |
| 146. | Schreiber S, Heinig T, Thiele HG, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995;108:1434-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 263] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 147. | Wilke CM, Wang L, Wei S, Kryczek I, Huang E, Kao J, Lin Y, Fang J, Zou W. Endogenous interleukin-10 constrains Th17 cells in patients with inflammatory bowel disease. J Transl Med. 2011;9:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 148. | Monteleone I, MacDonald TT, Pallone F, Monteleone G. The aryl hydrocarbon receptor in inflammatory bowel disease: linking the environment to disease pathogenesis. Curr Opin Gastroenterol. 2012;28:310-313. [PubMed] |
| 149. | Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237-48, 248.e1. [PubMed] |
| 150. | Di Sabatino A, Jackson CL, Pickard KM, Buckley M, Rovedatti L, Leakey NA, Picariello L, Cazzola P, Monteleone G, Tonelli F. Transforming growth factor beta signalling and matrix metalloproteinases in the mucosa overlying Crohn's disease strictures. Gut. 2009;58:777-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 151. | Scharl M, Frei S, Pesch T, Kellermeier S, Arikkat J, Frei P, Fried M, Weber A, Jehle E, Rühl A. Interleukin-13 and transforming growth factor β synergise in the pathogenesis of human intestinal fistulae. Gut. 2012;Epub ahead of print. [PubMed] |
| 152. | Perrier C, Rutgeerts P. Cytokine blockade in inflammatory bowel diseases. Immunotherapy. 2011;3:1341-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 153. | Zhang Z, Hinrichs DJ, Lu H, Chen H, Zhong W, Kolls JK. After interleukin-12p40, are interleukin-23 and interleukin-17 the next therapeutic targets for inflammatory bowel disease? Int Immunopharmacol. 2007;7:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Peer reviewers: Kazuichi Okazaki, Professor, The Third Department of Internal Medicine, Kansai Medical University, 2-3-1, Shinmachi, Hirakata, Osaka 573-1191, Japan; Raquel Rocha, Professor, Federal University of Bahia, Rua Araujo Pinho 32, Salvador 40110-150, Brazil
S- Editor Gou SX L- Editor A E- Editor Zhang DN